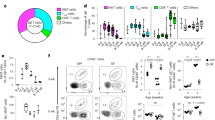
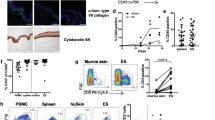
Abstract
Dendritic cells (DCs) are key regulators of immune responses that activate naive antigen-specific T lymphocytes. In draining lymph nodes, antigen-bearing DCs are reported to be rare and short-lived. How such small numbers of short-lived DCs can activate rare antigen-specific T cells is unclear. Here we show that after immunization of mouse skins by gene gun, the number of antigen-bearing DCs that migrate to draining lymph node is 100-fold higher than previously estimated and that they persist for approximately 2 weeks. The substantial frequency and longevity of DCs in situ ensures ample antigen presentation and stimulation for the rare antigen-specific T cells in draining lymph nodes.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Banchereau, J. & Steinman, R.M. Dendritic cells and the control of immunity. Nature 392, 245–252 (1998).
Banchereau, J. et al. Immunobiology of dendritic cells. Annu. Rev. Immunol. 18, 767–811 (2000).
Lanzavecchia, A. & Sallusto, F. Regulation of T cell immunity by dendritic cells. Cell 106, 263–266 (2001).
Mellman, I. & Steinman, R.M. Dendritic cells: specialized and regulated antigen processing machines. Cell 106, 255–258 (2001).
Pulendran, B., Palucka, K. & Banchereau, J. Sensing pathogens and tuning immune responses. Science 293, 253–256 (2001).
Liu, Y.J. Dendritic cell subsets and lineages, and their functions in innate and adaptive immunity. Cell 106, 259–262 (2001).
Lipscomb, M.F. & Masten, B.J. Dendritic cells: immune regulators in health and disease. Physiol. Rev. 82, 97–130 (2002).
Porgador, A. et al. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. J. Exp. Med. 188, 1075–1082 (1998).
Condon, C., Watkins, S.C., Celluzzi, C.M., Thompson, K. & Falo, L.D. DNA-based immunization by in vivo transfection of dendritic cells. Nat. Med. 2, 1122–1128 (1996).
Bouloc, A., Walker, P., Grivel, J.C., Vogel, J.C. & Katz, S.I. Immunization through dermal delivery of protein-encoding DNA: a role for migratory dendritic cells. Eur. J. Immunol. 29, 446–454 (1999).
Macatonia, S.E., Knight, S.C., Edwards, A.J., Griffiths, S. & Fryer, P. Localization of antigen on lymph node dendritic cells after exposure to the contact sensitizer fluorescein isothiocyanate. Functional and morphological studies. J. Exp. Med. 166, 1654–1667 (1987).
Ruedl, C., Koebel, P., Bachmann, M., Hess, M. & Karjalainen, K. Anatomical origin of dendritic cells determines their life span in peripheral lymph nodes. J. Immunol. 165, 4910–4916 (2000).
Ruedl, C., Koebel, P. & Karjalainen, K. In vivo-matured Langerhans cells continue to take up and process native proteins unlike in vitro-matured counterparts. J. Immunol. 166, 7178–7182 (2001).
Kamath, A.T., Henri, S., Battye, F., Tough, D.F. & Shortman, K. Developmental kinetics and lifespan of dendritic cells in mouse lymphoid organs. Blood 100, 1734–1741 (2002).
Sauer, B. Manipulation of transgenes by site-specific recombination: use of Cre recombinase. Meth. Enzymol. 225, 890–900 (1993).
Soriano, P. Generalized lacZ expression with the ROSA26 Cre reporter strain. Nat. Genet. 21, 70–71 (1999).
Zambrowicz, B.P. et al. Disruption of overlapping transcripts in the ROSA β geo 26 gene trap strain leads to widespread expression of β-galactosidase in mouse embryos and hematopoietic cells. Proc. Natl. Acad. Sci. USA 94, 3789–3794 (1997).
Nolan, G.P., Fiering, S., Nicolas, J.F. & Herzenberg, L.A. Fluorescence-activated cell analysis and sorting of viable mammalian cells based on β-D-galactosidase activity after transduction of Escherichia coli lacZ. Proc. Natl. Acad. Sci. USA 85, 2603–2607 (1988).
Stingl, G. Dendritic cells of the skin. Dermatol. Clin. 8, 673–679 (1990).
Henri, S. et al. The dendritic cell populations of mouse lymph nodes. J. Immunol. 167, 741–748 (2001).
Pulendran, B., Banchereau, J., Maraskovsky, E. & Maliszewski, C. Modulating the immune response with dendritic cells and their growth factors. Trends Immunol. 22, 41–47 (2001).
Valladeau, J. et al. Langerin, a novel C-type lectin specific to Langerhans cells, is an endocytic receptor that induces the formation of Birbeck granules. Immunity 12, 71–81 (2000).
Stoitzner, P. et al. Visualization and characterization of migratory Langerhans cells in murine skin and lymph nodes by antibodies against Langerin/CD207. J. Invest. Dermatol. 120, 266–274 (2003).
Takahara, K. et al. Identification and expression of mouse Langerin (CD207) in dendritic cells. Int. Immunol. 14, 433–444 (2002).
Williams, I.R. et al. IL-7 overexpression in transgenic mouse keratinocytes causes a lymphoproliferative skin disease dominated by intermediate TCR cells: evidence for a hierarchy in IL-7 responsiveness among cutaneous T cells. J. Immunol. 159, 3044–3056 (1997).
Sasaki, S., Amara, R.R., Oran, A.E., Smith, J.M. & Robinson, H.L. Apoptosis-mediated enhancement of DNA-raised immune responses by mutant caspases. Nat. Biotechnol. 19, 543–547 (2001).
Barnden, M.J., Allison, J., Heath, W.R. & Carbone, F.R. Defective TCR expression in transgenic mice constructed using cDNA-based α- and β-chain genes under the control of heterologous regulatory elements. Immunol. Cell Biol. 76, 34–40 (1998).
Fiering, S.N. et al. Improved FACS-Gal: flow cytometric analysis and sorting of viable eukaryotic cells expressing reporter gene constructs. Cytometry 12, 291–301 (1991).
Josien, R. et al. TRANCE, a tumor necrosis factor family member, enhances the longevity and adjuvant properties of dendritic cells in vivo. J. Exp. Med. 191, 495–502 (2000).
Nopora, A. & Brocker, T. Bcl-2 controls dendritic cell longevity in vivo. J. Immunol. 169, 3006–3014 (2002).
Bousso, P. & Robey, E. Dynamics of CD8+ T cell priming by dendritic cells in intact lymph nodes. Nat. Immunol. 4, 579–585 (2003).
Parker, I., Wei, S.H. & Miller, M.J. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. Nat. Rev. Immunol. 2, 872–880 (2002).
Rauer, H. et al. Two-photon imaging of lymphocyte motility and antigen response in intact lymph node. J. Biol. Chem. 276, 12249–12256 (2001).
Stoll, S. & Germain, R.N. Dynamic imaging of T cell-dendritic cell interactions in lymph nodes. Immunol. Rev. 189, 51–63 (2002).
Torres, C.A., Iwasaki, A., Barber, B.H. & Robinson, H.L. Differential dependence on target site tissue for gene gun and intramuscular DNA immunizations. J. Immunol. 158, 4529–4532 (1997).
Ross, T.M., Xu, Y., Bright, R.A. & Robinson, H.L. C3d enhancement of antibodies to hemagglutinin accelerates protection against influenza virus challenge. Nat. Immunol. 1, 127–131 (2000).
Acknowledgements
The authors thank R. Germain for advice; B. Pulendran and members of the Jacob laboratory for discussions; and I. Williams and P. Lambeth for human keratinocyte transgenic construct. J.A.K. was supported by grants from the Foundation Fighting Blindness, the National Eye Institute (National Institutes of Health; EY13459 and P30 EYO06360), and a gift from M. and M. Powell. J.W. was supported by a National Research Service Award grant (F32 EY06985) from the National Eye Institute. J.J. was supported by National Institutes of Health grant AI RO1524751.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Garg, S., Oran, A., Wajchman, J. et al. Genetic tagging shows increased frequency and longevity of antigen-presenting, skin-derived dendritic cells in vivo. Nat Immunol 4, 907–912 (2003). https://doi.org/10.1038/ni962
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ni962
This article is cited by
-
Immune synapse formation promotes lipid peroxidation and MHC-I upregulation in licensed dendritic cells for efficient priming of CD8+ T cells
Nature Communications (2023)
-
CRISPRa-mediated FOXP3 gene upregulation in mammalian cells
Cell & Bioscience (2019)
-
A randomized controlled phase II trial of vaccination with lysate-loaded, mature dendritic cells integrated into standard radiochemotherapy of newly diagnosed glioblastoma (GlioVax): study protocol for a randomized controlled trial
Trials (2018)
-
An innovative immunotherapeutic strategy for ovarian cancer: CLEC10A and glycomimetic peptides
Journal for ImmunoTherapy of Cancer (2018)
-
DNA vaccine elicits an efficient antitumor response by targeting the mutant Kras in a transgenic mouse lung cancer model
Gene Therapy (2014)