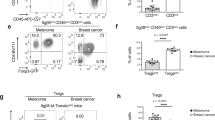
Abstract
Interleukin-9 (IL-9) is a T cell cytokine that acts through a γC-family receptor on target cells and is associated with inflammation and allergy. We determined that T cells from mice deficient in the T helper type 17 (TH17) pathway genes encoding retinoid-related orphan receptor γ (ROR-γ) and IL-23 receptor (IL-23R) produced abundant IL-9, and we found substantial growth inhibition of B16F10 melanoma in these mice. IL-9–blocking antibodies reversed this tumor growth inhibition and enhanced tumor growth in wild-type (WT) mice. Il9r−/− mice showed accelerated tumor growth, and administration of recombinant IL-9 (rIL-9) to tumor-bearing WT and Rag1−/− mice inhibited melanoma as well as lung carcinoma growth. Adoptive transfer of tumor-antigen–specific TH9 cells into both WT and Rag1−/− mice suppressed melanoma growth; this effect was abrogated by treatment with neutralizing antibodies to IL-9. Exogenous rIL-9 inhibited tumor growth in Rag1−/− mice but not in mast-cell–deficient mice, suggesting that the targets of IL-9 in this setting include mast cells but not T or B cells. In addition, we found higher numbers of TH9 cells in normal human skin and blood compared to metastatic lesions of subjects with progressive stage IV melanoma. These results suggest a role for IL-9 in tumor immunity and offer insight into potential therapeutic strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Accession codes
References
Hodi, F.S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Topalian, S.L. et al. Safety, activity, and immune correlates of anti–PD-1 antibody in cancer. N. Engl. J. Med. published online doi:10.1056/NEJMoa1200690 (2 June 2012).
Mumberg, D. et al. CD4+ T cells eliminate MHC class II–negative cancer cells in vivo by indirect effects of IFN-γ. Proc. Natl. Acad. Sci. USA 96, 8633–8638 (1999).
Perez-Diez, A. et al. CD4 cells can be more efficient at tumor rejection than CD8 cells. Blood 109, 5346–5354 (2007).
Quezada, S.A. et al. Tumor-reactive CD4+ T cells develop cytotoxic activity and eradicate large established melanoma after transfer into lymphopenic hosts. J. Exp. Med. 207, 637–650 (2010).
Xie, Y. et al. Naive tumor-specific CD4+ T cells differentiated in vivo eradicate established melanoma. J. Exp. Med. 207, 651–667 (2010).
Mattes, J. et al. Immunotherapy of cytotoxic T cell–resistant tumors by T helper 2 cells: an eotaxin and STAT6-dependent process. J. Exp. Med. 197, 387–393 (2003).
Kryczek, I. et al. Phenotype, distribution, generation, and functional and clinical relevance of Th17 cells in the human tumor environments. Blood 114, 1141–1149 (2009).
Martin-Orozco, N. et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798 (2009).
Muranski, P. et al. Tumor-specific Th17-polarized cells eradicate large established melanoma. Blood 112, 362–373 (2008).
Numasaki, M. et al. Interleukin-17 promotes angiogenesis and tumor growth. Blood 101, 2620–2627 (2003).
Numasaki, M. et al. IL-17 enhances the net angiogenic activity and in vivo growth of human non-small cell lung cancer in SCID mice through promoting CXCR-2–dependent angiogenesis. J. Immunol. 175, 6177–6189 (2005).
Wang, L. et al. IL-17 can promote tumor growth through an IL-6–Stat3 signaling pathway. J. Exp. Med. 206, 1457–1464 (2009).
Ivanov, I.I., Zhou, L. & Littman, D.R. Transcriptional regulation of Th17 cell differentiation. Semin. Immunol. 19, 409–417 (2007).
Jetten, A.M. & Joo, J.H. Retinoid-related orphan receptors (RORs): roles in cellular differentiation and development. Adv. Dev. Biol. 16, 313–355 (2006).
Yang, X.O. et al. T helper 17 lineage differentiation is programmed by orphan nuclear receptors RORα and RORγ. Immunity 28, 29–39 (2008).
Tilley, S.L. et al. Retinoid-related orphan receptor gamma controls immunoglobulin production and Th1/Th2 cytokine balance in the adaptive immune response to allergen. J. Immunol. 178, 3208–3218 (2007).
Kurebayashi, S. et al. Retinoid-related orphan receptor γ (RORγ) is essential for lymphoid organogenesis and controls apoptosis during thymopoiesis. Proc. Natl. Acad. Sci. USA 97, 10132–10137 (2000).
Elyaman, W. et al. IL-9 induces differentiation of TH17 cells and enhances function of FoxP3+ natural regulatory T cells. Proc. Natl. Acad. Sci. USA 106, 12885–12890 (2009).
Schmitt, E. et al. IL-9 production of naive CD4+ T cells depends on IL-2, is synergistically enhanced by a combination of TGF-β and IL-4, and is inhibited by IFN-γ. J. Immunol. 153, 3989–3996 (1994).
Dardalhon, V. et al. IL-4 inhibits TGF-β–induced Foxp3+ T cells and, together with TGF-β, generates IL-9+ IL-10+ Foxp3− effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Angkasekwinai, P., Chang, S.H., Thapa, M., Watarai, H. & Dong, C. Regulation of IL-9 expression by IL-25 signaling. Nat. Immunol. 11, 250–256 (2010).
Wong, M.T. et al. Regulation of human Th9 differentiation by type I interferons and IL-21. Immunol. Cell Biol. 88, 624–631 (2010).
Hung, K. et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188, 2357–2368 (1998).
Knoops, L. & Renauld, J.C. IL-9 and its receptor: from signal transduction to tumorigenesis. Growth Factors 22, 207–215 (2004).
Kearley, J. et al. IL-9 governs allergen-induced mast cell numbers in the lung and chronic remodeling of the airways. Am. J. Respir. Crit. Care Med. 183, 865–875 (2011).
Forbes, E.E. et al. IL-9– and mast cell–mediated intestinal permeability predisposes to oral antigen hypersensitivity. J. Exp. Med. 205, 897–913 (2008).
Oldford, S.A. et al. A critical role for mast cells and mast cell–derived IL-6 in TLR2-mediated inhibition of tumor growth. J. Immunol. 185, 7067–7076 (2010).
Yang, X.R. et al. Identification of modifier genes for cutaneous malignant melanoma in melanoma-prone families with and without CDKN2A mutations. Int. J. Cancer 125, 2912–2917 (2009).
Smith, S.E., Hoelzinger, D.B., Dominguez, A.L., Van Snick, J. & Lustgarten, J. Signals through 4–1BB inhibit T regulatory cells by blocking IL-9 production enhancing antitumor responses. Cancer Immunol. Immunother. 60, 1775–1787 (2011).
Atkins, M.B., Kunkel, L., Sznol, M. & Rosenberg, S.A. High-dose recombinant interleukin-2 therapy in patients with metastatic melanoma: long-term survival update. Cancer J. Sci. Am. 6 (suppl. 1), S11–S14 (2000).
Dougan, M. & Dranoff, G. Immune therapy for cancer. Annu. Rev. Immunol. 27, 83–117 (2009).
Ma, H.L. et al. IL-21 activates both innate and adaptive immunity to generate potent antitumor responses that require perforin but are independent of IFN-γ. J. Immunol. 171, 608–615 (2003).
Restifo, N.P., Dudley, M.E. & Rosenberg, S.A. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat. Rev. Immunol. 12, 269–281 (2012).
Amos, S.M. et al. Adoptive immunotherapy combined with intratumoral TLR agonist delivery eradicates established melanoma in mice. Cancer Immunol. Immunother. 60, 671–683 (2011).
Zou, W. & Restifo, N.P. TH17 cells in tumour immunity and immunotherapy. Nat. Rev. Immunol. 10, 248–256 (2010).
Schumacher, T.N. & Restifo, N.P. Adoptive T cell therapy of cancer. Curr. Opin. Immunol. 21, 187–189 (2009).
Muranski, P. & Restifo, N.P. Adoptive immunotherapy of cancer using CD4+ T cells. Curr. Opin. Immunol. 21, 200–208 (2009).
Steenwinckel, V. et al. IL-13 mediates in vivo IL-9 activities on lung epithelial cells but not on hematopoietic cells. J. Immunol. 178, 3244–3251 (2007).
Purwar, R. et al. Resident memory T cells (TRM) are abundant in human lung: diversity, function, and antigen specificity. PLoS ONE 6, e16245 (2011).
Clark, R.A. et al. A novel method for the isolation of skin resident T cells from normal and diseased human skin. J. Invest. Dermatol. 126, 1059–1070 (2006).
O'Leary, F.M. et al. Injury-induced GR-1+ macrophage expansion and activation occurs independently of CD4 T-cell influence. Shock 36, 162–169 (2011).
Acknowledgements
This research was supported by grants from US National Institutes of Health to T.S.K. (R01 AI-041707, R01 AI-097128 and P50 CA-093683), R.A.C. (R01-AR-056720 and R03-MH-095529) and A.M.J. (Z01-ES-101586). R.P. received a Research Grant Award from The Skin Cancer Foundation. The authors thank K. Gerrish (US National Institutes of Health) with his help with the microarray analysis. J.-C. Renauld39 (Ludwig Institute, Belgium) provided Il9r−/− mice and the corresponding control mice (Il9r+/−). Neutralizing antibodies to IL-9 (MM9C1) were a kind gift from J.v. Snick (Ludwig Institute, Belgium). Salary support for C.S. was provided by the Swiss National Science Foundation and the Foundation Rene Touraine and for R.A.C. from a Damon Runyon Clinical Investigator Award.
Author information
Authors and Affiliations
Contributions
R.P. designed the study, performed and analyzed the experiments, and wrote the manuscript. S.X. and H.S.K. performed experiments. W.E., A.M.J., S.J.K. and V.K.K. discussed the data, provided reagents and edited the manuscript. X.J. provided reagents. C.S., R.A.C. and R.C.F. performed the human T cell experiments, discussed the data and edited the manuscript. T.S.K. designed the study, analyzed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 (PDF 681 kb)
Rights and permissions
About this article
Cite this article
Purwar, R., Schlapbach, C., Xiao, S. et al. Robust tumor immunity to melanoma mediated by interleukin-9–producing T cells. Nat Med 18, 1248–1253 (2012). https://doi.org/10.1038/nm.2856
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.2856
This article is cited by
-
Distinct gene expression signatures comparing latent tuberculosis infection with different routes of Bacillus Calmette-Guérin vaccination
Nature Communications (2023)
-
IL-1β neutralization prevents diastolic dysfunction development, but lacks hepatoprotective effect in an aged mouse model of NASH
Scientific Reports (2023)
-
CD4+ T cells in cancer
Nature Cancer (2023)
-
Identification of excretory and secretory proteins from Haemonchus contortus inducing a Th9 immune response in goats
Veterinary Research (2022)
-
Mitochondrial fission induces immunoescape in solid tumors through decreasing MHC-I surface expression
Nature Communications (2022)