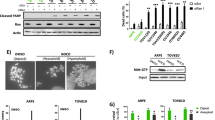
Abstract
The gene encoding ARID1A, a chromatin remodeler, shows one of the highest mutation rates across many cancer types. Notably, ARID1A is mutated in over 50% of ovarian clear cell carcinomas, which currently have no effective therapy. To date, clinically applicable targeted cancer therapy based on ARID1A mutational status has not been described. Here we show that inhibition of the EZH2 methyltransferase acts in a synthetic lethal manner in ARID1A-mutated ovarian cancer cells and that ARID1A mutational status correlated with response to the EZH2 inhibitor. We identified PIK3IP1 as a direct target of ARID1A and EZH2 that is upregulated by EZH2 inhibition and contributed to the observed synthetic lethality by inhibiting PI3K–AKT signaling. Importantly, EZH2 inhibition caused regression of ARID1A-mutated ovarian tumors in vivo. To our knowledge, this is the first data set to demonstrate a synthetic lethality between ARID1A mutation and EZH2 inhibition. Our data indicate that pharmacological inhibition of EZH2 represents a novel treatment strategy for cancers involving ARID1A mutations.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Garraway, L.A. & Lander, E.S. Lessons from the cancer genome. Cell 153, 17–37 (2013).
Lawrence, M.S. et al. Discovery and saturation analysis of cancer genes across 21 tumour types. Nature 505, 495–501 (2014).
Wilson, B.G. & Roberts, C.W. SWI/SNF nucleosome remodellers and cancer. Nat. Rev. Cancer 11, 481–492 (2011).
Wiegand, K.C. et al. ARID1A mutations in endometriosis-associated ovarian carcinomas. N. Engl. J. Med. 363, 1532–1543 (2010).
Jones, S. et al. Frequent mutations of chromatin remodeling gene ARID1A in ovarian clear cell carcinoma. Science 330, 228–231 (2010).
Anglesio, M.S. et al. Type-specific cell line models for type-specific ovarian cancer research. PLoS ONE 8, e72162 (2013).
Cao, R. & Zhang, Y. The functions of E(Z)/EZH2-mediated methylation of lysine 27 in histone H3. Curr. Opin. Genet. Dev. 14, 155–164 (2004).
Li, H., Cai, Q., Godwin, A.K. & Zhang, R. Enhancer of zeste homolog 2 promotes the proliferation and invasion of epithelial ovarian cancer cells. Mol. Cancer Res. 8, 1610–1618 (2010).
McCabe, M.T. et al. EZH2 inhibition as a therapeutic strategy for lymphoma with EZH2-activating mutations. Nature 492, 108–112 (2012).
Knutson, S.K. et al. A selective inhibitor of EZH2 blocks H3K27 methylation and kills mutant lymphoma cells. Nat. Chem. Biol. 8, 890–896 (2012).
Qi, W. et al. Selective inhibition of EZH2 by a small molecule inhibitor blocks tumor cells proliferation. Proc. Natl. Acad. Sci. USA 109, 21360–21365 (2012).
Guan, B., Gao, M., Wu, C.H., Wang, T.L. & Shih Ie, M. Functional analysis of in-frame indel ARID1A mutations reveals new regulatory mechanisms of its tumor suppressor functions. Neoplasia 14, 986–993 (2012).
Yamada, K.M. & Cukierman, E. Modeling tissue morphogenesis and cancer in 3D. Cell 130, 601–610 (2007).
Jenuwein, T. The epigenetic magic of histone lysine methylation. FEBS J. 273, 3121–3135 (2006).
Guan, B., Wang, T.L. & Shih Ie, M. ARID1A, a factor that promotes formation of SWI/SNF-mediated chromatin remodeling, is a tumor suppressor in gynecologic cancers. Cancer Res. 71, 6718–6727 (2011).
Konze, K.D. et al. An orally bioavailable chemical probe of the lysine methyltransferases EZH2 and EZH1. ACS Chem. Biol. 8, 1324–1334 (2013).
Kennison, J.A. & Tamkun, J.W. Dosage-dependent modifiers of polycomb and antennapedia mutations in Drosophila. Proc. Natl. Acad. Sci. USA 85, 8136–8140 (1988).
Li, H. et al. ALDH1A1 is a novel EZH2 target gene in epithelial ovarian cancer identified by genome-wide approaches. Cancer Prev. Res. (Phila.) 5, 484–491 (2012).
Stany, M.P. et al. Identification of novel therapeutic targets in microdissected clear cell ovarian cancers. PLoS ONE 6, e21121 (2011).
He, X. et al. PIK3IP1, a negative regulator of PI3K, suppresses the development of hepatocellular carcinoma. Cancer Res. 68, 5591–5598 (2008).
Zhu, Z. et al. PI3K is negatively regulated by PIK3IP1, a novel p110 interacting protein. Biochem. Biophys. Res. Commun. 358, 66–72 (2007).
Yamamoto, S., Tsuda, H., Takano, M., Tamai, S. & Matsubara, O. Loss of ARID1A protein expression occurs as an early event in ovarian clear-cell carcinoma development and frequently coexists with PIK3CA mutations. Mod. Pathol. 25, 615–624 (2012).
Samartzis, E.P., Noske, A., Dedes, K.J., Fink, D. & Imesch, P. ARID1A mutations and PI3K/AKT pathway alterations in endometriosis and endometriosis-associated ovarian carcinomas. Int. J. Mol. Sci. 14, 18824–18849 (2013).
Chandler, R.L. et al. ARID1a-DNA interactions are required for promoter occupancy by SWI/SNF. Mol. Cell. Biol. 33, 265–280 (2013).
Davidovich, C., Zheng, L., Goodrich, K.J. & Cech, T.R. Promiscuous RNA binding by polycomb repressive complex 2. Nat. Struct. Mol. Biol. 20, 1250–1257 (2013).
Cho, K.R. & Shih Ie, M. Ovarian cancer. Annu. Rev. Pathol. 4, 287–313 (2009).
Helming, K.C. et al. ARID1B is a specific vulnerability in ARID1A-mutant cancers. Nat. Med. 20, 251–254 (2014).
Wilson, B.G. et al. Epigenetic antagonism between polycomb and SWI/SNF complexes during oncogenic transformation. Cancer Cell 18, 316–328 (2010).
Knutson, S.K. et al. Durable tumor regression in genetically altered malignant rhabdoid tumors by inhibition of methyltransferase EZH2. Proc. Natl. Acad. Sci. USA 110, 7922–7927 (2013).
Hargreaves, D.C. & Crabtree, G.R. ATP-dependent chromatin remodeling: genetics, genomics and mechanisms. Cell Res. 21, 396–420 (2011).
Debnath, J., Muthuswamy, S.K. & Brugge, J.S. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods 30, 256–268 (2003).
Ye, X. et al. Downregulation of Wnt signaling is a trigger for formation of facultative heterochromatin and onset of cell senescence in primary human cells. Mol. Cell 27, 183–196 (2007).
Tu, Z. et al. Oncogenic RAS regulates BRIP1 expression to induce dissociation of BRCA1 from chromatin, inhibit DNA repair, and promote senescence. Dev. Cell 21, 1077–1091 (2011).
Zhang, S. A comprehensive evaluation of SAM, the SAM R-package and a simple modification to improve its performance. BMC Bioinformatics 8, 230 (2007).
Storey, J.D. & Tibshirani, R. Statistical significance for genomewide studies. Proc. Natl. Acad. Sci. USA 100, 9440–9445 (2003).
Langmead, B. & Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 9, 357–359 (2012).
Kim, D. et al. TopHat2: accurate alignment of transcriptomes in the presence of insertions, deletions and gene fusions. Genome Biol. 14, R36 (2013).
Bitler, B.G. et al. Wnt5a suppresses epithelial ovarian cancer by promoting cellular senescence. Cancer Res. 71, 6184–6194 (2011).
Li, H. et al. SUZ12 promotes human epithelial ovarian cancer by suppressing apoptosis via silencing HRK. Mol. Cancer Res. 10, 1462–1472 (2012).
Acknowledgements
We thank D. Altieri, M. Murphy and R. Shiekhattar for critical comments and X. Hua and Y. Park for technical assistance. This work was supported by grants from the US National Institutes of Health/National Cancer Institute (R01CA160331 and R01CA163377 to R.Z.), a US Department of Defense Ovarian Cancer Academy award (OC093420 to R.Z.) and an Ovarian Cancer Research Fund Program project (to R.Z.). R.Z. is an Ovarian Cancer Research Fund Liz Tilberis Scholar. B.G.B. is supported by an American Cancer Society postdoctoral fellowship (PF-13-058-01-TBE). K.M.A. is supported by a training grant from the US National Institutes of Health/National Cancer Institute (T32CA9171-35). Support of Core Facilities was provided by Cancer Center Support grant CA010815 to the Wistar Institute.
Author information
Authors and Affiliations
Contributions
B.G.B. designed and performed all the experiments, analyzed data and wrote the manuscript. K.M.A. contributed to Figure 5g–i and manuscript writing. A.G. contributed to Figure 2b,c. H.L. contributed to Supplementary Figure 4g. M.A. contributed to Supplementary Figure 2c,f. A.V.K. performed the analysis presented in Figure 4a,c. D.C.S. contributed to the epigenetic-set construction. Q.L. contributed to statistical design and analysis. I.-M.S. contributed key reagents. J.R.C.-G. and D.W.S. participated in the experimental design. R.Z. conceived the study and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6 and Supplementary Tables 1–3. (PDF 13397 kb)
Rights and permissions
About this article
Cite this article
Bitler, B., Aird, K., Garipov, A. et al. Synthetic lethality by targeting EZH2 methyltransferase activity in ARID1A-mutated cancers. Nat Med 21, 231–238 (2015). https://doi.org/10.1038/nm.3799
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3799
This article is cited by
-
ARID1A loss activates MAPK signaling via DUSP4 downregulation
Journal of Biomedical Science (2023)
-
Treatment for ovarian clear cell carcinoma with combined inhibition of WEE1 and ATR
Journal of Ovarian Research (2023)
-
The effects of ARID1A mutation in gastric cancer and its significance for treatment
Cancer Cell International (2023)
-
Methylation across the central dogma in health and diseases: new therapeutic strategies
Signal Transduction and Targeted Therapy (2023)
-
Chromatin and noncoding RNA-mediated mechanisms of gastric tumorigenesis
Experimental & Molecular Medicine (2023)