Abstract
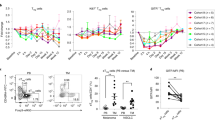
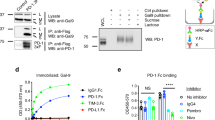
T cell stimulation via glucocorticoid-induced tumor necrosis factor receptor (TNFR)-related protein (GITR) elicits antitumor activity in various tumor models; however, the underlying mechanism of action remains unclear. Here we demonstrate a crucial role for interleukin (IL)-9 in antitumor immunity generated by the GITR agonistic antibody DTA-1. IL-4 receptor knockout (Il4ra−/−) mice, which have reduced expression of IL-9, were resistant to tumor growth inhibition by DTA-1. Notably, neutralization of IL-9 considerably impaired tumor rejection induced by DTA-1. In particular, DTA-1–induced IL-9 promoted tumor-specific cytotoxic T lymphocyte (CTL) responses by enhancing the function of dendritic cells in vivo. Furthermore, GITR signaling enhanced the differentiation of IL-9–producing CD4+ T-helper (TH9) cells in a TNFR-associated factor 6 (TRAF6)- and NF-κB–dependent manner and inhibited the generation of induced regulatory T cells in vitro. Our findings demonstrate that GITR co-stimulation mediates antitumor immunity by promoting TH9 cell differentiation and enhancing CTL responses and thus provide a mechanism of action for GITR agonist–mediated cancer immunotherapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Melero, I., Hervas-Stubbs, S., Glennie, M., Pardoll, D.M. & Chen, L. Immunostimulatory monoclonal antibodies for cancer therapy. Nat. Rev. Cancer 7, 95–106 (2007).
Peggs, K.S., Quezada, S.A., Korman, A.J. & Allison, J.P. Principles and use of anti-CTLA4 antibody in human cancer immunotherapy. Curr. Opin. Immunol. 18, 206–213 (2006).
Maio, M., Di Giacomo, A.M., Robert, C. & Eggermont, A.M. Update on the role of ipilimumab in melanoma and first data on new combination therapies. Curr. Opin. Oncol. 25, 166–172 (2013).
Topalian, S.L., Drake, C.G. & Pardoll, D.M. Targeting the PD-1–B7-H1(PD-L1) pathway to activate antitumor immunity. Curr. Opin. Immunol. 24, 207–212 (2012).
Tone, M. et al. Mouse glucocorticoid-induced tumor necrosis factor receptor ligand is costimulatory for T cells. Proc. Natl. Acad. Sci. USA 100, 15059–15064 (2003).
Croft, M. Costimulatory members of the TNFR family: keys to effective T cell immunity? Nat. Rev. Immunol. 3, 609–620 (2003).
Schaer, D.A., Murphy, J.T. & Wolchok, J.D. Modulation of GITR for cancer immunotherapy. Curr. Opin. Immunol. 24, 217–224 (2012).
Zhou, P., L'Italien, L., Hodges, D. & Schebye, X.M. Pivotal roles of CD4+ effector T cells in mediating agonistic anti-GITR mAb–induced immune activation and tumor immunity in CT26 tumors. J. Immunol. 179, 7365–7375 (2007).
Ramirez-Montagut, T. et al. Glucocorticoid-induced TNF receptor family–related gene activation overcomes tolerance/ignorance to melanoma differentiation antigens and enhances antitumor immunity. J. Immunol. 176, 6434–6442 (2006).
Nishikawa, H. et al. Regulatory T cell–resistant CD8+ T cells induced by glucocorticoid-induced tumor necrosis factor receptor signaling. Cancer Res. 68, 5948–5954 (2008).
Zhou, P. et al. Mature B cells are critical to T cell–mediated tumor immunity induced by an agonist anti-GITR monoclonal antibody. J. Immunother. 33, 789–797 (2010).
Ko, K. et al. Treatment of advanced tumors with agonistic anti-GITR mAb and its effects on tumor-infiltrating Foxp3+CD25+CD4+ regulatory T cells. J. Exp. Med. 202, 885–891 (2005).
Cohen, A.D. et al. Agonist anti-GITR monoclonal antibody induces melanoma tumor immunity in mice by altering regulatory T cell stability and intra-tumor accumulation. PLoS ONE 5, e10436 (2010).
Shimizu, J., Yamazaki, S., Takahashi, T., Ishida, Y. & Sakaguchi, S. Stimulation of CD25+CD4+ regulatory T cells through GITR breaks immunological self-tolerance. Nat. Immunol. 3, 135–142 (2002).
Muranski, P. & Restifo, N.P. Adoptive immunotherapy of cancer using CD4+ T cells. Curr. Opin. Immunol. 21, 200–208 (2009).
Dunn, G.P., Koebel, C.M. & Schreiber, R.D. Interferons, immunity and cancer immunoediting. Nat. Rev. Immunol. 6, 836–848 (2006).
Kennedy, R. & Celis, E. Multiple roles for CD4+ T cells in antitumor immune responses. Immunol. Rev. 222, 129–144 (2008).
Hong, S. et al. Roles of idiotype-specific T cells in myeloma cell growth and survival: TH1 and CTL cells are tumoricidal while TH2 cells promote tumor growth. Cancer Res. 68, 8456–8464 (2008).
De Monte, L. et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 208, 469–478 (2011).
Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nat. Rev. Immunol. 6, 295–307 (2006).
Purwar, R. et al. Robust tumor immunity to melanoma mediated by interleukin-9–producing T cells. Nat. Med. 18, 1248–1253 (2012).
Lu, Y. et al. TH9 cells promote antitumor immune responses in vivo. J. Clin. Invest. 122, 4160–4171 (2012).
Patel, M. et al. Glucocorticoid-induced TNFR family–related protein (GITR) activation exacerbates murine asthma and collagen-induced arthritis. Eur. J. Immunol. 35, 3581–3590 (2005).
Motta, A.C. et al. GITR signaling potentiates airway hyperresponsiveness by enhancing TH2 cell activity in a mouse model of asthma. Respir. Res. 10, 93 (2009).
van der Werf, N. et al. TH2 responses to helminth parasites can be therapeutically enhanced by, but are not dependent upon, GITR–GITR ligand costimulation in vivo. J. Immunol. 187, 1411–1420 (2011).
Zhu, J., Yamane, H. & Paul, W.E. Differentiation of effector CD4 T cell populations. Annu. Rev. Immunol. 28, 445–489 (2010).
Veldhoen, M. et al. Transforming growth factor–β 'reprograms' the differentiation of T helper 2 cells and promotes an interleukin 9–producing subset. Nat. Immunol. 9, 1341–1346 (2008).
Dardalhon, V. et al. IL-4 inhibits TGF-β–induced Foxp3+ T cells and, together with TGF-β, generates IL-9+IL-10+Foxp3− effector T cells. Nat. Immunol. 9, 1347–1355 (2008).
Végran, F. et al. The transcription factor IRF1 dictates the IL-21–dependent anticancer functions of TH9 cells. Nat. Immunol. 15, 758–766 (2014).
Takami, M., Love, R.B. & Iwashima, M. TGF-β converts apoptotic stimuli into the signal for TH9 differentiation. J. Immunol. 188, 4369–4375 (2012).
Perumal, N.B. & Kaplan, M.H. Regulating Il9 transcription in T helper cells. Trends Immunol. 32, 146–150 (2011).
Chang, H.C. et al. The transcription factor PU.1 is required for the development of IL-9–producing T cells and allergic inflammation. Nat. Immunol. 11, 527–534 (2010).
Staudt, V. et al. Interferon-regulatory factor 4 is essential for the developmental program of T helper 9 cells. Immunity 33, 192–202 (2010).
Jabeen, R. et al. TH9 cell development requires a BATF-regulated transcriptional network. J. Clin. Invest. 123, 4641–4653 (2013).
Stassen, M. et al. IL-9 and IL-13 production by activated mast cells is strongly enhanced in the presence of lipopolysaccharide: NF-κB is decisively involved in the expression of IL-9. J. Immunol. 166, 4391–4398 (2001).
Jash, A. et al. Nuclear factor of activated T cells 1 (NFAT1)-induced permissive chromatin modification facilitates nuclear factor–kappaB (NF-kappaB)-mediated interleukin-9 (IL-9) transactivation. J. Biol. Chem. 287, 15445–15457 (2012).
Xiao, X. et al. OX40 signaling favors the induction of TH9 cells and airway inflammation. Nat. Immunol. 13, 981–990 (2012).
Ruffell, B., Affara, N.I. & Coussens, L.M. Differential macrophage programming in the tumor microenvironment. Trends Immunol. 33, 119–126 (2012).
Kitajima, M. et al. Memory type 2 helper T cells induce long-lasting antitumor immunity by activating natural killer cells. Cancer Res. 71, 4790–4798 (2011).
Hung, K. et al. The central role of CD4+ T cells in the antitumor immune response. J. Exp. Med. 188, 2357–2368 (1998).
Mattes, J. et al. Immunotherapy of cytotoxic T cell–resistant tumors by T helper 2 cells: an eotaxin- and STAT6-dependent process. J. Exp. Med. 197, 387–393 (2003).
Stephens, G.L. et al. Engagement of glucocorticoid-induced TNFR family–related receptor on effector T cells by its ligand mediates resistance to suppression by CD4+CD25+ T cells. J. Immunol. 173, 5008–5020 (2004).
Bulliard, Y. et al. Activating Fc γ receptors contribute to the antitumor activities of immunoregulatory receptor-targeting antibodies. J. Exp. Med. 210, 1685–1693 (2013).
Angkasekwinai, P., Chang, S.H., Thapa, M., Watarai, H. & Dong, C. Regulation of IL-9 expression by IL-25 signaling. Nat. Immunol. 11, 250–256 (2010).
Elyaman, W. et al. Notch receptors and Smad3 signaling cooperate in the induction of interleukin-9–producing T cells. Immunity 36, 623–634 (2012).
Kaplan, M.H. TH9 cells: differentiation and disease. Immunol. Rev. 252, 104–115 (2013).
Yao, W. et al. Interleukin-9 is required for allergic airway inflammation mediated by the cytokine TSLP. Immunity 38, 360–372 (2013).
Lin, G.H., Snell, L.M., Wortzman, M.E., Clouthier, D.L. & Watts, T.H. GITR-dependent regulation of 4-1BB expression: implications for T cell memory and anti–4-1BB–induced pathology. J. Immunol. 190, 4627–4639 (2013).
Haribhai, D. et al. Regulatory T cells dynamically control the primary immune response to foreign antigen. J. Immunol. 178, 2961–2972 (2007).
King, C.G. et al. TRAF6 is a T cell–intrinsic negative regulator required for the maintenance of immune homeostasis. Nat. Med. 12, 1088–1092 (2006).
Vanneman, M. & Dranoff, G. Combining immunotherapy and targeted therapies in cancer treatment. Nat. Rev. Cancer 12, 237–251 (2012).
Johnson, L. et al. Somatic activation of the K-ras oncogene causes early-onset lung cancer in mice. Nature 410, 1111–1116 (2001).
Kim, W.J. et al. Glucocorticoid-induced tumour necrosis factor receptor family related protein (GITR) mediates inflammatory activation of macrophages that can destabilize atherosclerotic plaques. Immunology 119, 421–429 (2006).
Acknowledgements
We thank J.-O. Kim (International Vaccine Institute) for CD45.1 congenic mice, D.H. Chung (Seoul National University College of Medicine) for IL-4–deficient mice, Y.-K. Kim (Ewha Institute of Convergence Medicine) for IL-4Ra–deficient mice, T.H. Watts (University of Toronto) and P.P. Pandolfi (Harvard Medical School) for GITR-deficient mice, C.D. Surh (Pohang University of Science and Technology) and T.A. Chatila (Harvard Medical School) for Foxp3GFP reporter mice, Y. Choi (University of Pennsylvania School of Medicine) for Traf6fl/fl mice, G. Lozano (MD Anderson Cancer Center) for K-Ras transgenic mice, S. Sakaguchi (Osaka University) for the antibody to GITR (DTA-1), J.v. Snick (Ludwig Institute) for the antibody to IL-9 (MM9C1), S.-I. Nishikawa (RIKEN Center for Developmental Biology) for the antibody to c-kit (ACK2), H.-W. Yum (Seoul National University) and D.-H. Kim (Seoul National University) for assistance in setting up a colorectal cancer model, and members of the Kang laboratory for technical support. This work was supported by grants from the Public Welfare & Safety Research Program (20100020843; C.-Y.K.) and the National R&D Program for Cancer Control, Ministry of Health and Welfare (no. 0720500; C.-Y.K.).
Author information
Authors and Affiliations
Contributions
I.-K.K., B.-S.K., Y.C. and C.-Y.K. designed the study; I.-K.K., B.-S.K., C.-H.K., J.-W.S., J.-S.P., K.-S.S., E.-A.B., G.-E.L. and H.J. performed experiments; I.-K.K., B.-S.K., C.-H.K., J.-W.S., Y.C. and C.-Y.K. analyzed data; J.C., Y.J., D.H., B.S.K. and H.-Y.L. provided animals and reagents; I.-K.K., Y.C. and C.-Y.K. wrote the manuscript; C.-Y.K. supervised the study.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–15 and uncropped gels for western blots in main text. (PDF 3738 kb)
Source data
Rights and permissions
About this article
Cite this article
Kim, IK., Kim, BS., Koh, CH. et al. Glucocorticoid-induced tumor necrosis factor receptor–related protein co-stimulation facilitates tumor regression by inducing IL-9–producing helper T cells. Nat Med 21, 1010–1017 (2015). https://doi.org/10.1038/nm.3922
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm.3922
This article is cited by
-
IL-9-producing T cells: potential players in allergy and cancer
Nature Reviews Immunology (2021)
-
Interleukins in cancer: from biology to therapy
Nature Reviews Cancer (2021)
-
Flow cytometry analysis of peripheral Tregs in patients with multiple myeloma under lenalidomide maintenance
International Journal of Hematology (2021)
-
IL-9 and IL-9-producing cells in tumor immunity
Cell Communication and Signaling (2020)
-
STAT5 promotes accessibility and is required for BATF-mediated plasticity at the Il9 locus
Nature Communications (2020)