Abstract
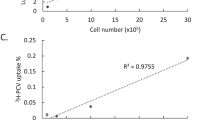
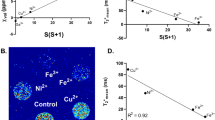
Ferritin, the iron storage protein, was recently suggested to be a candidate reporter for the detection of gene expression by magnetic resonance imaging (MRI). Here we report the generation of TET:EGFP-HAferritin (tet-hfer) transgenic mice, in which tissue-specific inducible transcriptional regulation of expression of the heavy chain of ferritin could be detected in vivo by MRI. We show organ specificity by mating the tet-hfer mice with transgenic mice expressing tetracycline transactivator (tTA) in liver hepatocytes and in vascular endothelial cells. Tetracycline-regulated overexpression of ferritin resulted in specific alterations of the transverse relaxation rate (R2) of water. Transgene-dependent changes in R2 were detectable by MRI in adult mice, and we also found fetal developmental induction of transgene expression in utero. Thus, the tet-hfer MRI reporter mice provide a new transgenic mouse platform for in vivo molecular imaging of reporter gene expression by MRI during both embryonic and adult life.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
Accession codes
References
Yang, M., Baranov, E., Moossa, A.R., Penman, S. & Hoffman, R.M. Visualizing gene expression by whole-body fluorescence imaging. Proc. Natl. Acad. Sci. USA 97, 12278–12282 (2000).
Contag, C.H. & Bachmann, M.H. Advances in in vivo bioluminescence imaging of gene expression. Annu. Rev. Biomed. Eng. 4, 235–260 (2002).
Ray, P., De, A., Min, J.J., Tsien, R.Y. & Gambhir, S.S. Imaging tri-fusion multimodality reporter gene expression in living subjects. Cancer Res. 64, 1323–1330 (2004).
Louie, A.Y. et al. In vivo visualization of gene expression using magnetic resonance imaging. Nat. Biotechnol. 18, 321–325 (2000).
Yu, J., Liu, L., Kodibagkar, V.D., Cui, W. & Mason, R.P. Synthesis and evaluation of novel enhanced gene reporter molecules: detection of beta-galactosidase activity using 19F NMR of trifluoromethylated aryl beta-D-galactopyranosides. Bioorg. Med. Chem. 14, 326–333 (2006).
Li, Z. et al. Creatine kinase, a magnetic resonance-detectable marker gene for quantification of liver-directed gene transfer. Hum. Gene Ther. 16, 1429–1438 (2005).
Walter, G., Barton, E.R. & Sweeney, H.L. Noninvasive measurement of gene expression in skeletal muscle. Proc. Natl. Acad. Sci. USA 97, 5151–5155 (2000).
Koretsky, A.P., Lin, Y.J., Schorle, H. & Jaenisch, R. Genetic control of MRI contrast by expression of the transferrin receptor. Proc. Int. Soc. for Magn. Reson. in Med. 4, 69 (1996).
Moore, A., Josephson, L., Bhorade, R.M., Basilion, J.P. & Weissleder, R. Human transferrin receptor gene as a marker gene for MR imaging. Radiology 221, 244–250 (2001).
Alfke, H. et al. In vitro MR imaging of regulated gene expression. Radiology 228, 488–492 (2003).
Weissleder, R. et al. MR imaging and scintigraphy of gene expression through melanin induction. Radiology 204, 425–429 (1997).
Cohen, B., Dafni, H., Meir, G., Harmelin, A. & Neeman, M. Ferritin as an endogenous MRI reporter for noninvasive imaging of gene expression in C6 glioma tumors. Neoplasia 7, 109–117 (2005).
Deans, A.E. et al. Cellular MRI contrast via coexpression of transferrin receptor and ferritin. Magn. Reson. Med. 56, 51–59 (2006).
Genove, G., DeMarco, U., Xu, H., Goins, W.F. & Ahrens, E.T. A new transgene reporter for in vivo magnetic resonance imaging. Nat. Med. 11, 450–454 (2005).
Gilad, A.A et al. Artificial reporter gene providing MRI contrast based on proton exchange. Nat. Biotechnol. 25, 217–219 (2007).
Theil, E.C. Ferritin: at the crossroads of iron and oxygen metabolism. J. Nutr. 133, 1549S–1553S (2003).
Gottesfeld, Z. & Neeman, M. Ferritin effect on the transverse relaxation of water: NMR microscopy at 9.4 T. Magn. Reson. Med. 35, 514–520 (1996).
Vymazal, J., Brooks, R.A., Bulte, J.W., Gordon, D. & Aisen, P. Iron uptake by ferritin: NMR relaxometry studies at low iron loads. J. Inorg. Biochem. 71, 153–157 (1998).
Vymazal, J., Zak, O., Bulte, J.W., Aisen, P. & Brooks, R.A. T1 and T2 of ferritin solutions: effect of loading factor. Magn. Reson. Med. 36, 61–65 (1996).
Gossuin, Y., Muller, R.N. & Gillis, P. Relaxation induced by ferritin: a better understanding for an improved MRI iron quantification. NMR Biomed. 17, 427–432 (2004).
Wood, J.C., Fassler, J.D. & Meade, T. Mimicking liver iron overload using liposomal ferritin preparations. Magn. Reson. Med. 51, 607–611 (2004).
Hardy, P.A. et al. Correlation of R2 with total iron concentration in the brains of rhesus monkeys. J. Magn. Reson. Imaging 21, 118–127 (2005).
Haacke, E.M. et al. Imaging iron stores in the brain using magnetic resonance imaging. Magn. Reson. Imaging 23, 1–25 (2005).
Bauminger, E.R. et al. Iron (II) oxidation and early intermediates of iron-core formation in recombinant human H-chain ferritin. Biochem. J. 296, 709–719 (1993).
Ferreira, C. et al. Early embryonic lethality of H ferritin gene deletion in mice. J. Biol. Chem. 275, 3021–3024 (2000).
Thompson, K. et al. Mouse brains deficient in H-ferritin have normal iron concentration but a protein profile of iron deficiency and increased evidence of oxidative stress. J. Neurosci. Res. 71, 46–63 (2003).
Orino, K. et al. Ferritin and the response to oxidative stress. Biochem. J. 357, 241–247 (2001).
Pham, C.G. et al. Ferritin heavy chain upregulation by NF-kappaB inhibits TNFalpha-induced apoptosis by suppressing reactive oxygen species. Cell 119, 529–542 (2004).
Giordani, A. et al. Contrasting effects of excess ferritin expression on the iron-mediated oxidative stress induced by tert-butyl hydroperoxide or ultraviolet-A in human fibroblasts and keratinocytes. J. Photochem. Photobiol. B 54, 43–54 (2000).
Kakhlon, O., Gruenbaum, Y. & Cabantchik, Z.I. Repression of the heavy ferritin chain increases the labile iron pool of human K562 cells. Biochem. J. 356, 311–316 (2001).
Wilkinson IV, J. et al. Tissue-specific expression of ferritin H regulates cellular iron homeostasis in vivo. Biochem. J. 395, 501–507 (2006).
Sun, J.F. et al. Microvascular patterning is controlled by fine-tuning the Akt signal. Proc. Natl. Acad. Sci. USA 102, 128–133 (2005).
Gory, S. et al. The vascular endothelial-cadherin promoter directs endothelial-specific expression in transgenic mice. Blood 93, 184–192 (1999).
Kistner, A. et al. Doxycycline-mediated quantitative and tissue-specific control of gene expression in transgenic mice. Proc. Natl. Acad. Sci. USA 93, 10933–10938 (1996).
Cozzi, A. et al. Overexpression of wild type and mutated human ferritin H-chain in HeLa cells: in vivo role of ferritin ferroxidase activity. J. Biol. Chem. 275, 25122–25129 (2000).
Haacke, E.M., Xu, Y., Cheng, Y.C. & Reichenbach, J.R. Susceptibility weighted imaging (SWI). Magn. Reson. Med. 52, 612–618 (2004).
Carmeliet, P. & Collen, D. Molecular basis of angiogenesis. Role of VEGF and VE-cadherin. Ann. NY Acad. Sci. 902, 249–262 (2000).
Kim, I., Yilmaz, O.H. & Morrison, S.J. CD144 (VE-cadherin) is transiently expressed by fetal liver hematopoietic stem cells. Blood 106, 903–905 (2005).
Grabill, C., Silva, A.C., Smith, S.S., Koretsky, A.P. & Rouault, T.A. MRI detection of ferritin iron overload and associated neuronal pathology in iron regulatory protein-2 knockout mice. Brain Res. 971, 95–106 (2003).
Kato, J. et al. A mutation, in the iron-responsive element of H ferritin mRNA, causing autosomal dominant iron overload. Am. J. Hum. Genet. 69, 191–197 (2001).
Furth, P.A. et al. Temporal control of gene expression in transgenic mice by a tetracycline-responsive promoter. Proc. Natl. Acad. Sci. USA 91, 9302–9306 (1994).
Plaks, V., Kalchenko, V., Dekel, N. & Neeman, M. MRI analysis of angiogenesis during mouse embryo implantation. Magn. Reson. Med. 55, 1013–1022 (2006).
Sheehan, D.C. & Hrapchak, B.B. Theory and Practice of Histotechnology 2nd edn, 217 (Mosby, St Louis, 1980).
Acknowledgements
We would like to acknowledge helpful discussions with A. Koretsky, P. Bendel, E. Keshet and Y. Dor. We would like to thank G. Damari and E. Ino for their help in generation and breeding of the transgenic mice. This work was supported by the Israel Science Foundation 391/02 and by the Minerva Foundation (to M.N.).
Author information
Authors and Affiliations
Contributions
B.C. generated the construct and derived the transgenic mice; K.Z., V.P. and M.N. performed the MRI experiments and conducted the data analyses; K.Z., V.P., B.C., T.I., V.K. and A.H. contributed to the ex vivo fluorescence and histological analysis, L.E.B. provided the VE-cadherin mice; M.N., B.C., K.Z. and V.P. wrote the manuscript and M.N. supervised the project.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Fig. 1
Histological analysis of multiple organs in male VE cadherin h-fer mice. (PDF 136 kb)
Supplementary Fig. 2
Ferritin expression during embryonic development. (PDF 301 kb)
Supplementary Fig. 3
Prussian Blue staining of ferritin iron. (PDF 147 kb)
Supplementary Video 1
Contrast enhanced 3D gradient echo MRI of E13.5 pregnant mouse. The dataset was acquired at the end of the spin echo measurement of R2 relaxation, after intravenous administration of biotin-BSA-GdDTPA. Hyperintense areas show the maternal vasculature including maternal circulation in the placenta (same mouse as Figure 3). (AVI 885 kb)
Supplementary Video 2
Maximal intensity projection revealing the placenta of E13.5 pregnancy. Movie was derived from the 3D contrast enhanced gradient echo dataset of movie S3. (AVI 652 kb)
Rights and permissions
About this article
Cite this article
Cohen, B., Ziv, K., Plaks, V. et al. MRI detection of transcriptional regulation of gene expression in transgenic mice. Nat Med 13, 498–503 (2007). https://doi.org/10.1038/nm1497
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nm1497
This article is cited by
-
Computationally designed dual-color MRI reporters for noninvasive imaging of transgene expression
Nature Biotechnology (2022)
-
Sialic acid-engineered mesoporous polydopamine dual loaded with ferritin gene and SPIO for achieving endogenous and exogenous synergistic T2-weighted magnetic resonance imaging of HCC
Journal of Nanobiotechnology (2021)
-
Redesigned reporter gene for improved proton exchange-based molecular MRI contrast
Scientific Reports (2020)
-
The Continuing Evolution of Molecular Functional Imaging in Clinical Oncology: The Road to Precision Medicine and Radiogenomics (Part II)
Molecular Diagnosis & Therapy (2019)
-
Molecular Imaging of CXCL12 Promoter-driven HSV1-TK Reporter Gene Expression
Biotechnology and Bioprocess Engineering (2018)