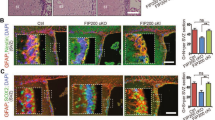
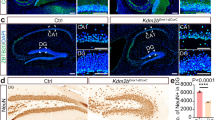
Abstract
Neural stem and progenitor cells (NSCs/NPCs) give rise to neurons, astrocytes and oligodendrocytes. However, the mechanisms underlying the decision of a stem cell to either self-renew or differentiate are incompletely understood. We demonstrate here that Fbw7 (F-box and WD repeat domain containing-7), the substrate recognition component of an SCF (complex of SKP1, CUL1 and F-box protein)-type E3 ubiquitin ligase, is a key regulator of NSC/NPC viability and differentiation. The absence of Fbw7 in the mouse brain caused severely impaired stem cell differentiation and increased progenitor cell death. Fbw7 deficiency resulted in accumulation of two SCF(Fbw7) substrates, the transcription factors active Notch1 and N-terminally phosphorylated c-Jun. Genetic and pharmacological rescue experiments identified c-Jun as a key substrate of Fbw7 in controlling progenitor cell viability, whereas inhibition of Notch signaling alleviated the block in stem cell differentiation. Thus Fbw7 controls neurogenesis by antagonizing Notch and c-Jun N-terminal kinase (JNK)/c-Jun signaling.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Merkle, F.T. & Alvarez-Buylla, A. Neural stem cells in mammalian development. Curr. Opin. Cell Biol. 18, 704–709 (2006).
Götz, M. & Barde, Y.A. Radial glial cells defined and major intermediates between embryonic stem cells and CNS neurons. Neuron 46, 369–372 (2005).
Ho, M.S., Tsai, P.I. & Chien, C.T. F-box proteins: the key to protein degradation. J. Biomed. Sci. 13, 181–191 (2006).
Welcker, M. & Clurman, B.E. FBW7 ubiquitin ligase: a tumour suppressor at the crossroads of cell division, growth and differentiation. Nat. Rev. Cancer 8, 83–93 (2008).
Tan, Y., Sangfelt, O. & Spruck, C. The Fbxw7/hCdc4 tumor suppressor in human cancer. Cancer Lett. 271, 1–12 (2008).
Fuchs, S.Y. Tumor suppressor activities of the Fbw7 E3 ubiquitin ligase receptor. Cancer Biol. Ther. 4, 506–508 (2005).
Behrens, A., Sibilia, M. & Wagner, E.F. Amino-terminal phosphorylation of c-Jun regulates stress-induced apoptosis and cellular proliferation. Nat. Genet. 21, 326–329 (1999).
Besirli, C.G., Wagner, E.F. & Johnson, E.M. Jr. The limited role of NH2-terminal c-Jun phosphorylation in neuronal apoptosis: identification of the nuclear pore complex as a potential target of the JNK pathway. J. Cell Biol. 170, 401–411 (2005).
Raivich, G. et al. The AP-1 transcription factor c-Jun is required for efficient axonal regeneration. Neuron 43, 57–67 (2004).
Yoon, K.J. et al. Mind bomb 1-expressing intermediate progenitors generate notch signaling to maintain radial glial cells. Neuron 58, 519–531 (2008).
Mizutani, K., Yoon, K., Dang, L., Tokunaga, A. & Gaiano, N. Differential Notch signalling distinguishes neural stem cells from intermediate progenitors. Nature 449, 351–355 (2007).
Corbin, J.G. et al. Regulation of neural progenitor cell development in the nervous system. J. Neurochem. 106, 2272–2287 (2008).
Yoon, K. & Gaiano, N. Notch signaling in the mammalian central nervous system: insights from mouse mutants. Nat. Neurosci. 8, 709–715 (2005).
Tetzlaff, M.T. et al. Defective cardiovascular development and elevated cyclin E and Notch proteins in mice lacking the Fbw7 F-box protein. Proc. Natl. Acad. Sci. USA 101, 3338–3345 (2004).
Tsunematsu, R. et al. Mouse Fbw7/Sel-10/Cdc4 is required for notch degradation during vascular development. J. Biol. Chem. 279, 9417–9423 (2004).
Kramer, E.R. et al. Cooperation between GDNF/Ret and ephrinA/EphA4 signals for motor-axon pathway selection in the limb. Neuron 50, 35–47 (2006).
Hartfuss, E., Galli, R., Heins, N. & Götz, M. Characterization of CNS precursor subtypes and radial glia. Dev. Biol. 229, 15–30 (2001).
Reynolds, B.A. & Rietze, R.L. Neural stem cells and neurospheres–re-evaluating the relationship. Nat. Methods 2, 333–336 (2005).
Nateri, A.S., Riera-Sans, L., Da Costa, C. & Behrens, A. The ubiquitin ligase SCFFbw7 antagonizes apoptotic JNK signaling. Science 303, 1374–1378 (2004).
Bossy-Wetzel, E., Bakiri, L. & Yaniv, M. Induction of apoptosis by the transcription factor c-Jun. EMBO J. 16, 1695–1709 (1997).
Whitfield, J., Neame, S.J., Paquet, L., Bernard, O. & Ham, J. Dominant-negative c-Jun promotes neuronal survival by reducing BIM expression and inhibiting mitochondrial cytochrome c release. Neuron 29, 629–643 (2001).
Ma, C. et al. dp5/HRK is a c-Jun target gene and required for apoptosis induced by potassium deprivation in cerebellar granule neurons. J. Biol. Chem. 282, 30901–30909 (2007).
Angel, P., Hattori, K., Smeal, T. & Karin, M. The jun proto-oncogene is positively autoregulated by its product, Jun/AP1. Cell 55, 875–885 (1988).
Gaiano, N., Nye, J.S. & Fishell, G. Radial glial identity is promoted by Notch1 signaling in the murine forebrain. Neuron 26, 395–404 (2000).
Anthony, T.E., Mason, H.A., Gridley, T., Fishell, G. & Heintz, N. Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev. 19, 1028–1033 (2005).
Radtke, F. et al. Deficient T cell fate specification in mice with an induced inactivation of Notch1. Immunity 10, 547–558 (1999).
Selkoe, D. & Kopan, R. Notch and Presenilin: regulated intramembrane proteolysis links development and degeneration. Annu. Rev. Neurosci. 26, 565–597 (2003).
Anthony, T.E., Klein, C., Fishell, G. & Heintz, N. Radial glia serve as neuronal progenitors in all regions of the central nervous system. Neuron 41, 881–890 (2004).
Patten, B.A., Peyrin, J.M., Weinmaster, G. & Corfas, G. Sequential signaling through Notch1 and erbB receptors mediates radial glia differentiation. J. Neurosci. 23, 6132–6140 (2003).
Coffey, E.T. et al. c-Jun N-terminal protein kinase (JNK) 2/3 is specifically activated by stress, mediating c-Jun activation, in the presence of constitutive JNK1 activity in cerebellar neurons. J. Neurosci. 22, 4335–4345 (2002).
Wang, X. et al. Targeted deletion of the mitogen-activated protein kinase kinase 4 gene in the nervous system causes severe brain developmental defects and premature death. Mol. Cell. Biol. 27, 7935–7946 (2007).
Chang, L., Jones, Y., Ellisman, M.H., Goldstein, L.S. & Karin, M. JNK1 is required for maintenance of neuronal microtubules and controls phosphorylation of microtubule-associated proteins. Dev. Cell 4, 521–533 (2003).
Ohtsuka, T. et al. Hes1 and Hes5 as notch effectors in mammalian neuronal differentiation. EMBO J. 18, 2196–2207 (1999).
de la Pompa, J.L. et al. Conservation of the Notch signalling pathway in mammalian neurogenesis. Development 124, 1139–1148 (1997).
Nelson, B.R., Hartman, B.H., Georgi, S.A., Lan, M.S. & Reh, T.A. Transient inactivation of Notch signaling synchronizes differentiation of neural progenitor cells. Dev. Biol. 304, 479–498 (2007).
Hojo, M. et al. Glial cell fate specification modulated by the bHLH gene Hes5 in mouse retina. Development 127, 2515–2522 (2000).
Thompson, B.J. et al. Control of hematopoietic stem cell quiescence by the E3 ubiquitin ligase Fbw7. J. Exp. Med. 205, 1395–1408 (2008).
Mao, J.H. et al. Fbxw7/Cdc4 is a p53-dependent, haploinsufficient tumour suppressor gene. Nature 432, 775–779 (2004).
Welcker, M., Orian, A., Grim, J.E., Eisenman, R.N. & Clurman, B.E. A nucleolar isoform of the Fbw7 ubiquitin ligase regulates c-Myc and cell size. Curr. Biol. 14, 1852–1857 (2004).
Sangfelt, O., Cepeda, D., Malyukova, A., van Drogen, F. & Reed, S.I. Both SCFCdc4α and SCFCdc4γ are required for cyclin E turnover in cell lines that do not overexpress cyclin E. Cell Cycle 7, 1075–1082 (2008).
van Drogen, F. et al. Ubiquitylation of cyclin E requires the sequential function of SCF complexes containing distinct hCdc4 isoforms. Mol. Cell 23, 37–48 (2006).
Matsuoka, S. et al. Fbxw7 acts as a critical fail-safe against premature loss of hematopoietic stem cells and development of T-ALL. Genes Dev. 22, 986–991 (2008).
Pollard, S.M., Conti, L., Sun, Y., Goffredo, D. & Smith, A. Adherent neural stem (NS) cells from fetal and adult forebrain. Cereb. Cortex 16 (suppl. 1): i112–i120 (2006).
Behrens, A. et al. Impaired intervertebral disc formation in the absence of Jun. Development 130, 103–109 (2003).
Nateri, A.S., Spencer-Dene, B. & Behrens, A. Interaction of phosphorylated c-Jun with TCF4 regulates intestinal cancer development. Nature 437, 281–285 (2005).
Acknowledgements
We are grateful to the Animal Unit, Equipment Park, FACS Lab and the Experimental Pathology Lab at the London Research Institute for technical help and advice on histology. We are grateful to F. Radtke (Swiss Institute for Experimental Cancer Research, Lausanne, Switzerland) for floxed Notch1 mice. We thank R. Sancho for the data on Fbxw7 isoform expression in various tissues. We thank C.R. Da Costa, S. Marino and A. Martin-Villalba for critical reading of the manuscript. S.M.B. is supported by a Federation of the Societies of Biochemistry and Molecular Biology (FEBS) Long Term Fellowship. Part of this work is supported by an MRC project grant (85704) to A.B. The London Research Institute is funded by Cancer Research UK.
Author information
Authors and Affiliations
Contributions
J.D.H. designed and conducted the majority of the experiments, did the data analyses and co-wrote the manuscript. A.J. generated the Fbxw7f/f mice and performed the radioactive in situ hybridization. S.M.B. performed the experiments on adherent NSC cultures. E.N. did the IHC stainings. B.S.-D. performed the nonradioactive in situ hybridization. S.B. gave advice on neurosphere sectioning. A.B. supervised the project, directed the experiments and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–19 (PDF 4489 kb)
Rights and permissions
About this article
Cite this article
Hoeck, J., Jandke, A., Blake, S. et al. Fbw7 controls neural stem cell differentiation and progenitor apoptosis via Notch and c-Jun. Nat Neurosci 13, 1365–1372 (2010). https://doi.org/10.1038/nn.2644
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/nn.2644
This article is cited by
-
Transplantation of MiR-28-5p-Modified BMSCs Promotes Functional Recovery After Spinal Cord Injury
Molecular Neurobiology (2024)
-
Myelin regulatory factor is a target of individual and interactive effects of HIV-1 Tat and morphine in the striatum and pre-frontal cortex
Journal of NeuroVirology (2023)
-
Function of SYDE C2-RhoGAP family as signaling hubs for neuronal development deduced by computational analysis
Scientific Reports (2022)
-
Notch activation suppresses endothelial cell migration and sprouting via miR-223-3p targeting Fbxw7
In Vitro Cellular & Developmental Biology - Animal (2022)
-
Advances in the understanding of the role of type-H vessels in the pathogenesis of osteoporosis
Archives of Osteoporosis (2020)