Abstract
Antigen-specific priming of human, naive T cells has been difficult to assess. Owing to the low initial frequency in the naive cell pool of specific T cell precursors, such an analysis has been obscured by the requirements for repeated stimulations and prolonged culture time. In this protocol, we describe how to evaluate antigen-specific priming of CD8+ cells 10 d after a single specific stimulation. The assay provides reference conditions, which result in the expansion of a substantial population of antigen-specific T cells from the naive repertoire. Various conditions and modifications during the priming process (e.g., testing new cytokines, co-stimulators and so on) can now be directly compared with the reference conditions. Factors relevant to achieving effective priming include the dendritic cell preparation, the T cell preparation, the cell ratio at the time of priming, the serum source used for the experiment and the timing of addition and concentration of the cytokines used for expansion. This protocol is relevant for human immunology, vaccine biology and drug development.
Similar content being viewed by others
Introduction
The initial antigen encounter of a naive T cell with its cognate antigen is generally referred to as priming. It should be noted that the term priming has sometimes been used ambiguously to reflect incubation of cells before their activation with cytokines or reagents regardless of the T cell receptor (TCR) trigger, but in the context of this paper we will use priming to reflect the initial activation of naive T cells after an encounter with their respective cognate peptide in the context of a major histocompatibility complex (MHC) molecule.
A successful first encounter, resulting in the generation and expansion of functional T cells, requires a sequence of signals carefully orchestrated in vivo by professional antigen-presenting cells (APCs). Upon stimulation, T cells proliferate and differentiate into effector and memory T cells. The magnitude of this T cell response, as well as the degree and functional characteristics acquired during differentiation are—at least in part—programmed by the signals provided during this initial priming step1. Thus, the priming process shapes the resulting immune response and is key to our understanding of how T cell responses evolve2,3.
Methods for investigating antigen-specific priming
Systematic studies of antigen-specific priming have been hampered by the exceedingly low frequency of each TCR specificity within the vast diversity of the repertoire of naive T cell precursors. Animal models enable analysis of evolving immune responses to infectious model antigens, such as lymphocytic choriomeningitis virus (LCMV) in mice, which simulates effective or dysfunctional T cell responses depending on the viral variant of LCMV4. Furthermore, TCR-transgenic mice, in which virtually all T cells are specific for a defined epitope, have been extremely valuable for our understanding of the basic concepts regarding T cell and tumor immunology5,6,7. However, mouse immunology differs in many aspects from that of humans8, and strategies to validate results from small animal models for translation to human immunobiology are needed to advance current approaches in immunotherapy and vaccine development9. Vaccinologists and virologists have increasingly resorted to testing nonhuman primates, but these studies are rightfully restricted to only very key questions. Thus, for ethical, regulatory and financial reasons, studies in monkeys are limited to a few specialized laboratories10,11.
Developing principles of antigen-specific priming of human T cells in vitro has been hindered by the variability of T cell responses observed not only between individual donors but also, more importantly, in experiments performed from the same individual. This variability is generally attributed to the low and varying T cell precursor frequency. In fact, repetitive stimulation of T cell lines is frequently used as the method required to reach level of detection. However, such repetitive stimulation that requires a prolonged time period has made it almost impossible to draw plausible conclusions about the initial priming process (Fig. 1).
Top, the low frequency of antigen-specific precursor T cells requires repeated stimulations over a prolonged culture time, when suboptimal stimulation conditions are chosen. Factors that can interfere with the results, and that are independent of the initial priming, are: the mode of re-stimulation (APC, peptide concentration), the chosen cytokines for expansion, cell density and serum quality. The need for repeated stimulations renders conclusions about the initial priming step difficult. Bottom, short-term expansion after single peptide stimulation reduces variation within the expansion period, thus allowing conclusions about the initial priming conditions.
In 1994, two groups identified an antigen that was overexpressed in melanoma, which was recognized by a large number of tumor-infiltrating T cells isolated from patients. The gene, officially known as MLANA, was independently termed Melan-A12 or MART-1 (ref. 13) (for simplicity, we will refer to the protein encoded by this gene as Melan-A). An immunogenic peptide, restricted to the human leukocyte antigen (HLA)-A0201 allele, was identified as AAGIGILTV, corresponding to positions 27–35 of the Melan-A protein14, and was the target of the immune-dominant response in HLA-A0201+ individuals. In addition to its use as a major immunogenic target in melanoma15, which we will not discuss in detail, this epitope has become the prototypical antigen for probing naive T cell responses in human T cells.
The peptide epitope Melan-A27−35, or the corresponding decamer from positions 26–35, can be used as a model antigen because the frequency of naive T cells specific for this epitope is about tenfold higher than the frequencies observed against other self- or tumor-associated antigens16,17. Precisely what is responsible for this increased frequency (∼1 in 1,000 naive T cells) is not completely understood, but enhanced positive selection in the thymus18 and/or cross-reactivity of T cells with various other specificities have been described19. In addition to cell phenotype, analyses of TCR excision circles and telomere length have shown that Melan-A–specific T cells in normal persons have characteristics similar to naive T cells, with none or only one potential additional round of expansion detected18. Valmori and colleagues18 discovered that exchange of the alanine to leucine in position 27 greatly increased binding to HLA-A0201, thereby enhancing the immunogenicity of this epitope. Marked chemical and immunological differences between the known epitopes, the nonamer 27–35, the decamer 26–35 and the heteroclitic peptide 26–35A27L, exist. An extensive summary of the biochemical properties, immunogenicity and clinical use of this peptide can be found in Romero et al.15.
Advantages and applications of the current protocol
By using this model antigen (Melan-A 26–35A27L), which has a distinctly high frequency of reactive naive T cells, we developed an in vitro priming system to reliably assess priming conditions for CD8+ T cells20. This method, which we call ACE-CD8 for antigen-specific activation and cytokine-facilitated expansion of naive, human CD8+ T cells, focuses on the encounter of effectively matured, peptide-loaded dendritic cells (DCs) with highly purified naive CD8+ T cells (Fig. 2). ACE-CD8 defines conditions that, after a single stimulation, will lead to the rapid expansion of Melan-A–specific T cells within 10 d of culture. The protocol described here for ACE-CD8 is highly reproducible; thus, the experimental variability frequently reported when using other published protocols is seen to a much lesser extent in this protocol (Table 1 and Fig. 3). ACE-CD8 therefore allows an analysis of the effect of further variables (e.g., new cytokines, chemical compounds or drugs) at the time of priming with an antigen-specific T cell readout providing functional and quantitative data. Even if Melan-A or this specific priming regimen is not the prime focus of an intended experiment, e.g., when assessing the immunogenicity of alternative peptide antigens or proteins, the use of ACE-CD8, including the Melan-A peptide as a reference peptide, provides a validated, internal positive control for the priming and expansion conditions, and should clarify whether the experimental conditions of a specific experiment have been suboptimal.
Start with monocyte isolation on day −4 of culture (Step 1), followed by T cell isolation on day −1 of culture (Step 1B). Initiation of coculture of peptide-pulsed DCs with T cells is termed day 0 (Steps 2–12). Expansion may be extended to more than 10 d, but the phenotype of responding cells may change gradually. Analysis earlier than day 10 is possible, but the variation is bigger and the yield is substantially lower, as increased proliferation is generally seen between day 7 and day 10.
In all, 17 consecutive experiments performed by two technicians were evaluated. Experiments with at least three wells for standard conditions were included, and only the first three wells were counted if more than three wells had been setup. Depicted are the means of these experiments. Bars indicate median and range (left). As an indicator of intra-assay variation, the coefficient of variation was calculated (right, median and range) for each of these experiments.
There are various potential applications of this protocol, depending on the specific research question: ACE-CD8 may be used to test the immunogenicity of defined peptides and altered peptide ligands, and to analyze the antigen-specific T cell repertoire as well as the enumeration of T cell precursor frequencies. Moreover, it is especially suited to screen factors that may affect DC and/or T cell function during the initial priming step. By using ACE-CD8 and slightly modified previous versions of this protocol, we probed the naive T cell repertoire of T cells specific for viral variants of HCV21, assessed the effect of differentially matured DCs on priming22 and identified novel peptide epitopes for the Wilms tumor antigen 1 (ref. 23), a protein overexpressed in leukemia and many other malignancies. Recently, we used ACE-CD8 to analyze the impact of a small molecule drug, dasatinib, on the priming of human T cells24. This drug, which is used clinically as a brc/abl-kinase inhibitor, can also block TCR triggering through inhibition of the src-kinase Lck25. By using ACE-CD8, however, it was observed that dasatinib has a strong stimulatory activity on DCs, synergistically enhancing IL-12 in combination with a toll-like receptor 4 (TLR4) agonist. Thus, by dissecting the priming process into different steps—in this case incubating only the maturing DCs with the drug—it became possible to identify an as-yet unknown mechanism of a widely used drug. Interestingly, when testing mouse and rhesus macaque DCs, we did not see the same effect, possibly owing to different binding properties of the drug24. Therefore, the results emphasize the need for a valid immunologic test system using human cells.
Comparison with other methods for antigen-specific priming of human T cells in vitro
When searching the literature, it quickly becomes clear that most protocols differ from each other in many seemingly small, but in the end, crucial details. In addition to the use of different epitope variants of the Melan-A epitope (the nonamer, the decamer and the heteroclitic variant), many different forms of antigen presentation are being used: Table 1 lists representative studies that use the Melan-A epitope as a model antigen. Differently generated and differently matured DCs, as well as CD40L-activated B cells26 and artificial APCs27 have been used to present the antigen. Even when focusing on the use of only DCs as presenting cells, it has become clear that there are a multitude of ways to induce a functional state called 'mature DCs'. This mature state, however, is ill-defined, as upregulation of co-stimulatory factors, cytokine production, migration, endocytosis and priming of T cells are rarely quantitatively assessed and compared within one set of experiments. In fact, a systematic analysis of differentially matured DCs with regard to their priming capacity of T cells is far from complete. By using ACE-CD8 as a model system, we have begun to decipher the many details in the DC maturation process that impact the resulting T cell response, and this has revealed how variable and versatile this APC remains22.
In the studies described in Table 1, additional factors confound the comparability of the individual analyses. Results from 1–6 rounds of stimulation, different use of cytokines for T cell expansion and variation in the purity of the starting cell population have been reported. All of these factors explain the largely variable results observed across the different studies. In fact, even within the same priming system, results from repeated experiments are rarely reported. Thus, the resulting T cell responses strongly vary from paper to paper, and presumably from experiment to experiment, making the direct comparison of different variables almost impossible.
Artificial APCs have been used as one alternative for analyzing in vitro priming. Immortalized cell lines (such as K562 or fibroblasts) can be transfected with the desired HLA allele along with a set of co-stimulatory molecules27. By using this approach with mouse cells, it was shown that a single brief encounter of the T cell with its cognate antigen is sufficient to set off the expansion and differentiation of the T cell28. For human cells, in the context of stimulation with professional APCs, such as DCs providing additional signals (e.g., IL-12), less information is available.
Instead of genetically modified cells, the alternative approach is to use beads and coat the necessary molecules on their surface. This approach may serve as an off-the-shelf, 'Lego'-type system29,30 to help understand the role of individual molecules for priming. The strength of such a system lies in its modular design as well as in the possibility to process large numbers of T cells, even if functional, autologous DCs are not available. Moreover, defined characteristics of such stimulatory beads are advantageous when re-stimulation conditions after successful priming are in question. Whether these artificial systems really are a match for an optimally activated DC, naturally providing the full set of signals including cytokine production in cis, is unproven.
Priming of T cells by using nonspecific stimuli such as phorbol 12-myristate 13-acetate (PMA), ionomycin or anti-CD3 antibodies has often been reported as a means to analyze bulk T cells within a short period of time. Alternatively, fully HLA-mismatched allogeneic APCs may serve as initial stimulators (a classical mixed leukocyte reaction), especially when assessing CD4 responses, as this increases the percentage of reactive naive T cells from the repertoire. All of these stimuli deliver a supraphysiological signal, and some bypass the need for co-stimulation or additional cytokines. This type of stimulation has been used to answer basic questions about proliferation and polarization characteristics of certain T cell populations31. However, as all cells are stimulated simultaneously, results need to be interpreted with caution, as the magnitude and qualities of the cells concurrently responding most likely have effects on each other, e.g., by producing cytokines and expressing ligands for stimulatory and inhibitory molecules. Therefore, such results differ from a priming event, in which few individual cells are initially activated.
There have also been many reports on the activation of memory T cells by various APCs. Cytomegalovirus (CMV), adenovirus (ADV) or Epstein-Barr virus (EBV) responses in healthy previously infected donors are generally robust, and specific T cells for the respective immunodominant epitopes can be tracked easily by using MHC multimers32. Functional assays, such as antigen-specific proliferation assays, degranulation and cytotoxicity assays, or cytokine production in response to the respective APC are relatively easy to perform. However, memory T cells have different prerequisites for full activation as well as different expansion dynamics after a TCR trigger. Many cell types, not just professional APCs, may trigger a secondary T cell response. Thus, memory responses cannot be used as an alternative method to study T cell priming, but rather to answer questions about the secondary response after in vivo priming.
Experimental design
The ACE-CD8 protocol can be divided in two major parts: first, the preparation of DCs (Step 1), and then the stimulation and expansion of the T cells (Steps 2–26). The following subsections discuss key aspects of the protocol.
DC preparation. The preparation of DCs and the quality of the maturation trigger are crucial to achieving maximal expansion of the antigen-specific T cells. In this protocol, we use monocyte-derived DCs cultured for 72 h in granulocyte macrophage colony-stimulating factor (GM-CSF)- and IL-4-containing medium. The cells are then lifted from the plate, resuspended in fresh medium containing GM-CSF, IL-4 and the maturation reagents lipopolysaccharide (LPS) and interferon (IFN)-γ. At this time, the peptide epitope is added to the culture as well; 16 h later, these cells are used for stimulating the naive T cell preparation.
Plastic adherence is used for enriching monocytes as the initial selection step. This step includes a rinsing step to get rid of the nonadherent fraction. Rinsing the plate with the appropriate pressure requires practice: rinsing with too little pressure will result in a large number of contaminating lymphocytes, rinsing with too much pressure will wash away some monocytes and lead to a low yield. In trained hands, this method gives rise to consistent DC populations with full stimulatory capacity.
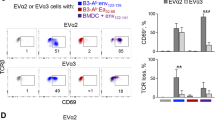
For maturation of the DCs, LPS and IFN-γ are used as the essential reagents to induce IL-12 production. IFN-γ primes the promoter region of IL-12p40 (ref. 33), whereas LPS activates IL-12 production via TLR4 triggering. In accordance with the kinetics of IL-12 induction, maturing DCs need to be used for priming within 16 h, as longer incubation with the maturing reagents only leads to DC exhaustion34. Unfortunately, the widely used maturation cocktail based on a mixture of pro-inflammatory cytokines (IL-1β, TNF-α, IL-6 and PGE2)35 does not lead to IL-12 production by DCs, as IL-12p35 is dose-dependently inhibited by prostaglandin E2 (ref. 36), and, as a consequence, such DCs show only limited priming capacity (Fig. 4)24.
DCs were matured differently (three wells per group) and the resulting T cell responses were evaluated by day 10. (a) Different maturation conditions for DCs are indicated above each plot in this panel, defining each experimental group of T cells. IL-1β/TNF-α/PgE2-matured DCs prime the naive T cells poorly, leading to dysfunctional T cell responses. (a) Representative MHC-multimer staining of T cells. (b) Summary of the fraction of Melan-A–specific T cells in each group and total number of specific T cells in each well. (c) T cells were tested for recognition of a HLA-A0201+, Melan-A+ melanoma cell line at a 2:1 effector:target ratio. The fraction of IFN-γ/TNF-α double-positive cells/MHC multimer+ T cells is indicated. (d) The ratio of T cells and DCs critically influences total expansion of Melan-A–specific cells. (e) IL-21 greatly increases total proliferation of antigen-specific T cells. Experiments shown in d,e were matured under standard conditions. Experiments were performed in triplicate; error bars represent the s.d.
R848, a TLR7/8 agonist, in combination with IFN-γ also induces IL-12 in the DCs, leading to a good specific expansion and full T cell function (Fig. 4a,b). It may therefore serve as an alternative maturation cocktail, although it has not been tested in our hands as extensively as LPS/IFN-γ.
IL-12 production has been, in our hands, the only factor that correlated with fully functional T helper cell 1 (TH1)-primed T cell populations. Thus, quality controls on DC preparation should include assessment of IL-12 either by ELISA (for IL-12p70) or by intracellular cytokine staining after a 16-h stimulation in the presence of brefeldin A. Note that there are marked differences between the staining quality of different antibodies. We routinely use the Alexa Fluor 488–labeled IL-12p40 antibody from Affymetrix/eBiosciences (Clone: C8.6, order no. 53-7129). Surface marker expression has not been sufficiently reliable to distinguish between optimal (IL-12 producing) and suboptimal (IL-12 nonproducing) DC populations.
The matured DCs are irradiated (30 Gy) before co-incubation with T cells for priming. This prevents contamination with natural killer (NK) cells or non-naive T cells present in the DC preparation. Unless this fraction is irradiated, memory and NK cells may proliferate vigorously to IL-15, thus competing with the primed T cells. In fact, outgrowth of non-CD8 cells (usually NK cells) is still sometimes observed compromising the experiment results. For DCs, effects of irradiation have been controversially discussed37,38. Treatment with mitomycin C (followed by extensive washing) may be an alternative method, but it has not been validated in our hands so far.
Purity of the T cell preparation. There are several reasons why highly purified naive T cells are a more suitable starting population than total CD8 preparations or whole peripheral blood leukocytes (PBLs). First, the fraction of naive T cells within the CD8 compartment varies from donor to donor. Therefore, unless purified naive T cells are used, the number of naive cells in the starting well will vary from donor to donor. Moreover, the purity of naive T cells is crucial, as effector memory T cells may be cross-reactive and therefore may interfere with T cell priming. Furthermore, memory T cells have a lower activation threshold and may be triggered solely by the cytokines added to the culture39, and therefore could compete for cytokines as well as alter the relative ratio (percentage) of antigen-specific T cells. The same argument holds true for contaminating NK cells, which proliferate quickly in response to IL-15. Regulatory T cells would also be present if using PBLs, and the influence of such cells on the initial priming may add to the variability between individuals40. Each T cell preparation is therefore evaluated for the purity of the preparation by using CD62L and CCR7 as positive markers for naive T cells, and CD45RO and CD57 as exclusion markers. We generally demand that the CD62L−CCR7− fraction and/or the CD57+ fraction and/or CD45RO+ fraction be less than 10%, indicating a naive population of more than 90% (Fig. 5).
(a) Example of the evaluation of the initial population of naive T cells (gating strategy and panel). (b) Representative results of an experiment set up in five parallel wells per tested peptide. The tumor-associated peptide antigens STEAP1292−300(I293L) and gp100209−217(T210M), and the CMV-related peptide antigen pp65(495−503) were evaluated by using the same naive T cell preparation. (c) Functional testing of the lines depicted in row 3 of b, using peptide-loaded autologous monocytes as APCs (bottom); top, monocytes without peptide were used as control stimulators. Numbers in brackets indicate the percentage of cytokine+ T cells in relation to MHC multimer+ cells in the respective well. T cells were stimulated for 5 h in the presence of brefeldin A. FSC, forward scatter; SSC, side scatter.
Initial cell numbers and cell ratios. ACE-CD8 starts with a T cell number of 5 × 105 naive T cells per well in 0.5 ml of complete medium in a 48-well plate. Given the approximate frequency of 0.1% Melan-A–specific T cells within the naive T cell repertoire of a healthy donor, ∼500 specific precursor T cells will be present in each well. The T cell:DC ratio is adjusted to 4:1. Note that ACE-CD8 is validated for these conditions: changing the number of input cells, the size and volume of each well or interfering with the T cell:DC ratio may change the expansion rate markedly and requires side-by-side comparison (Fig. 4d). If alternative peptide epitopes are investigated, the precursor frequency is likely to be lower than that for Melan-A. Nevertheless, we recommend using the same input numbers, but increasing the number of parallel wells and evaluating them separately, as with lower precursor numbers there will likely be some negative wells and a few positive cultures at the end of the 10-d period. Thus, this microculture approach enables the detection of rare cells by limiting the number of irrelevant cells per well (the rare events follow a Poisson distribution) (Fig. 5)41.
Note that the setup of a reliable experiment requires relatively large amounts of PBLs as a starting cell population. Downscaling to very few cells, in our hands, negatively affects the overall experimental outcome, but it may become feasible when using a 96-well format at the initial priming step. Upscaling this procedure for clinical use is an ongoing project. It is possible to switch to larger flasks by using higher cell numbers, but careful re-evaluation is needed regarding the initial culture volume, cell density and so on.
Cytokines: timing and concentration. For the initial stage of priming, IL-21 is the only exogenous cytokine added to the culture. The advantage of using IL-21 at this initial step was first shown by Li et al.42. All other cytokines required for sufficient priming are produced by the DCs or by the T cells themselves. Formation of small proliferative clusters by day 3 of culture suggests that, within these clusters, secreted cytokines (e.g., IL-12, IL-2 and/or IL-15) or cell contact may provide a local advantage for the responding antigen-specific cells. In fact, when gently washing away un-clustered cells, enrichment of antigen-specific T cells can already be observed in the clustered fraction (personal observation). IL-21 maintains the proliferative capacity of the T cells and, to some extent, maintains the expression of a favorable phenotype (e.g., expression of CD28)43. For robust and strong expansion, addition of this cytokine is essential (Fig. 4d). Adding IL-7 and IL-15 in a relatively low concentration (5 ng/ml each), 3 d after the initial priming step (and subsequently every 2 d), is based on the observation that T cell responses to cognate antigen are boosted by these cytokines39. During the first 3 d of culture in the absence of IL-7 and IL-15, a proliferative advantage is provided to cells receiving a TCR stimulus, whereas nonspecific cells may be 'starved' during this period44. Indeed, total cell numbers per well decrease by day 3 and dying/fading cells may be observed. Note that cytokine concentrations are kept intentionally low to limit cytokine-driven, nonspecific homeostatic proliferation45.
Moreover, addition of IL-2, which is used in many protocols, is not necessary in this protocol, as it is known to drive cells toward terminal differentiation46. Note that for extended culture, beyond day 15,the addition of IL-2 is beneficial.
Serum and culture medium. The quality of the AB serum used for culture is critical for the robustness and the strength of the proliferative response. We have noted considerable serum batch-to-batch variations with regard to total proliferation of the cells. This has great implications, as sometimes several batches need to be tested: ideally, a priming experiment is set up in which the new serum batch is used in parallel to the old, validated serum batch. Once a suitable batch has been identified, purchase of a large amount of serum can be costly and requires room for safe storage. However, having the same serum batch available throughout a whole set of experiments markedly reduces inter-assay variation. One way to start the comparison of different serum batches is to include autologous serum off the clot from the healthy donor of the PBLs. This may not necessarily be the best serum batch, but it qualifies as a baseline. For the analysis of patient cells, autologous serum is generally not recommended, as interference with disease-related factors in the serum (drugs, inhibitory cytokines and so on) cannot be excluded47. Also, note that the serum concentration in the medium has a substantial effect, which may not necessarily be reflected in the percentage of antigen-specific T cells but rather in the total T cell proliferation (e.g., using 3% (vol/vol) human serum (HS) rather than the standard 5% (vol/vol) HS resulted in a 66% decrease in the absolute number of Melan-A–specific T cells (Table 2)). A frequently asked question is whether medium x, y or z may be equally suitable for expansion. We have only tested a certain number of different culture media and found the CellGenix DC medium to be the best in terms of expansion of specific cells and total proliferative capacity of the T cells. Side-by-side comparisons should be performed when introducing a new medium (Table 2).
Experimental variation. One major issue is reproducibility, including intra-assay and inter-assay variation. As a measure of inter-assay variation, we plotted representative data from 17 independent experiments, performed by two technicians within the last 1.5 years (Fig. 3). The mean of the first three wells of each experiment from the standard expansion group is presented (Fig. 3a). The coefficient of variation (CV), indicating the ratio of s.d. and mean in percent, is plotted in Figure 3b to illustrate the spread of the data. Except for three outliers with higher CVs, the majority of experiments showed a CV between 3% and 20%. Given the nature of a biological assay, which extends over a 10-d period, and the small n (three wells per group), we consider this CV as an indicator for acceptably low inter-assay variability. These data may also be used to define a lower cutoff for the percentage of Melan-A multimer+ T cell lines: the fifth percentile of this experimental series is 24%. Thus, in any given experiment, we expect at least 24% Melan-A–specific T cells within the experimental standard group (mean results out of three wells), which serve as a positive control. From a few donor leukapheresis specimens, we observed a particularly high cell expansion (e.g., compare absolute numbers in Fig. 4d with those in Fig. 4e). Whether such 'super donors' reflect biological characteristics (e.g., HLA-A02 homozygosity, polymorphism, etc.) or depend on certain technical issues at the time of collection (apheresis, freezing) has not been studied in detail. If different factors are to be analyzed using ACE-CD8 (e.g., novel cytokines or drugs), evaluation for each individual experimental group should be based on several separate wells per group. For screening assays, e.g., testing new compounds, the number of wells per experimental group may be reduced (for pilot experiments, we often reduce the standard group to two parallel wells). For in-depth evaluation of certain conditions, or for testing new peptide epitopes with an unknown precursor frequency, higher numbers of parallel wells (depending on the statistical considerations) are beneficial.
Experimenter variability is an important factor for observed inter-assay variability and robustness. Adherence to the protocol should greatly reduce such experimenter variability. The biggest variation may occur at the time of removing nonadherent cells from the monocyte layer (see above and Step 1A(v)). Washing intensity varies from person to person and with respect to training, and frequent controls based on microscopic analysis are required. In our laboratory, students with basic cell culture experience easily pick up the protocol details, allowing them to get valid results from the very first experiment.
When assessing T cell responses against different epitopes, experimental variation stems mostly from the sporadic events representing the clonal expansion of a single cell. Peptides differ in their response rates, and systematic evaluation of the T cell repertoire of a specific donor is possible, revealing differences in the immunogenicity of different peptide antigens. For example, peptide epitopes reliably leading to a T cell response well above the detection limit are STEAP1292−300(I293L) (ref. 48), PRAME435−443 (ref. 49), gp100209−217(T210M) or MELOE1(36−44) (ref. 50). In contrast, responses to glioma-associated antigens EphA2(288−891) or IL13Rα(345−353) have been sporadic at best.
At very low precursor frequencies, the experimental setup is similar to classical limiting dilution assays in which each positive well indicates at least one specific T cell precursor51. Thus, the minimum precursor frequency may be estimated by the number of positive wells divided by the number of total input cells. For example, the frequency of gp100-specific T cells in the donor shown in Figure 6 must be at least 3/2.5 × 106 (three positive wells, three in 5 × 5 × 105 naive T cells). Distribution of such rare events follows a Poisson distribution, as has been discussed for limiting dilution assays51. Precision will be increased in tests of a larger number of wells (e.g., 20 parallel wells). Note that such calculations are estimates only; more elaborate statistical analyses are required for an exact determination of the precursor frequency52,53. For exact quantification of single precursors only, MHC multimer–guided enrichment starting with a large quantity of CD8+ T cells may provide a more direct approach to quantifying very low frequency cell numbers16. In contrast, advantages of ACE-CD8 include obtaining sufficient specific cell numbers for functional characterization and having the means to dissect the priming process into different steps.
(a) Melan-A–specific T cells were expanded by using the ACE-CD8 procedure and purified on day 10 of culture with Melan-A dextramer as outlined in Box 1. Gating strategy and purity of the antigen-specific T cells. (b) Gating strategy for the caspase-3 assay: large cells according to forward scatter (FSC)/side scatter (SSC) are included and further analyzed for CD8 and CD45 staining (right). (c) CD8-CD45- tumor cells are included in the analysis. The upper left panel shows activated caspase 3 in the Melan-A+, HLA-A0201+ melanoma cell line FM55 (gift from J. Becker, Würzburg). As a positive control for maximum caspase activation achievable within 4 h, peptide-loaded melanoma cells are routinely used (right). Note that this maximum control differs from the 'maximum lysis control' in classical chromium-release assays (which usually shows lysis of targets induced by a lysing reagent). The lower left image shows spontaneous caspase-3 activation in tumor (Tu) cells without T cells. An HLA-A2 negative, Melan-A negative tumor cell line remains unaltered by the T cells (lower right).
Experimental readout
ACE-CD8 is designed to provide sufficient numbers of antigen-specific T cells for quantitative, phenotypic and functional characterization. The expanded T cells have an expansion history of 10 d under strong TH1-driven culture conditions. This has to be kept in mind, when interpreting the data. The T cell status immediately after priming remains difficult to assess owing to a lack of sufficient antigen-specific T cells. Detection and analysis of T cells by using MHC multimers can be done for Melan-A as early as 5 d after priming, but only few cells will be available at this early time point. Questions regarding, e.g., TCR triggering and subsequent signaling during the priming event cannot be answered properly owing to the low number of specific T cells at the time of priming. Ultimately the outcome of the priming process is of interest here, e.g., when introducing a new variable at the time of priming, and may be assessed after 10 d of culture.
Each experimental group is started in multiple wells (3–6, depending on the analyses required) and analysis of each of the resulting cell lines separately adds to the validity of the data. Pooling cells from different wells within the experimental groups may be necessary for assays that require large cell numbers, but evaluation of the individual wells by MHC-multimer staining and cell counts before the pooling step is highly recommended. It should be noted that the timing of the assay with respect to the last medium-cytokine exchange may critically influence the functionality of the T cells: the presence of high cytokine concentrations on the day before the assay may increase nonspecific activation, whereas failure to refresh the medium for longer than 72 h increases the fraction of apoptotic or dysfunctional cells. T cells may be kept in culture for up to 21 d without re-stimulation. After this time point, re-stimulation is necessary to maintain sufficient cell numbers. Note that the T cells tested later than day 18 of culture may already display a different degree of differentiation. For cytotoxicity assays (Fig. 6), selection of Melan-A–specific T cells using MHC multimers and magnetic beads allows differential comparison of generated T cell lines even if the fraction of Melan-A–specific T cells from a certain condition greatly differs from the lines generated under standard conditions (Box 1). Note that to avoid interference of the multimer with the TCR at the time of the assay, this purification is recommended to be performed 2 d prior to the assay.
Materials
REAGENTS
-
Peripheral blood leukocytes, see Reagent Setup
-
Biocoll, density 1,077 g/ml (Merck Millipore, cat. no L6113)
-
CellGro dendritic cell medium (CellGenix, cat. no. 2005)
-
Penicillin-streptomycin (PAA, cat. no. P11-010)
-
Dulbecco's PBS (PAA, cat. no. H15-002)
-
Human serum type AB
Critical
We have used PAA, cat. no. C15-021. However, this serum is no longer available. We recently tested Biochrom's AB serum (no. S 01049.2-0.1; lot no. 0672B) with good results.
-
Recombinant human IL-4 (CellGenix, cat. no. 1403; alternative source: Peprotech)
-
Recombinant human GM-CSF (Gentaur, cat. no. 04-RHUGM-CSF-300MCG)
-
Human IFN-γ (Peprotech, cat. no. 300-02)
-
Human IL-7 (CellGenix, cat. no. 1410; alternative source: Peprotech)
-
Human IL-15 (CellGenix, cat. no. 1413; alternative source: Peprotech)
-
Human IL-21 (CellGenix, cat. no. 1419; alternative source: Peprotech)
-
Melan-A (26-35(A27L), peptide, immunograde (ELAGIGILTV; JPT)
-
CD8+ T cell isolation kit, human (Miltenyi Biotec, cat. no. 130-094-156)
-
CD45RO beads (Miltenyi Biotec, cat. no. 130-046-001)
-
CD57 beads (Miltenyi Biotec, cat. no. 130-092-073)
-
LD column (Miltenyi Biotec, cat. no. 130-042-901)
-
LS+ column (Miltenyi Biotec, cat. no. 130-042-401)
-
Countess cell-counting chambers (Invitrogen, cat. no. C10228)
Antibodies and staining reagents
-
Anti-human CD8-α PerCp-Cy5.5 (BioLegend, cat. no. 300928)
-
Anti-human CD57-FITC (BioLegend, cat. no. 322306)
-
Anti-human CD45RO-PE (BioLegend, cat. no. 304206)
-
Anti-human CD62L-FITC (eBioscience, cat. no. 11-0629-42)
-
Anti-human CCR7-PE (R&D Systems, cat. no. FAB197P)
-
MelanA dextramer (Immudex, cat. no. WB2162-APC)
-
Additional antibodies for final analysis, depending on the desired panel
Plasticware
-
Plate, 48 wells, sterile (GBO, cat. no. 677 180)
-
Plate, 12 wells, sterile (GBO, cat. no. 655 180)
-
Plate, six wells, sterile (GBO, cat. no. 657 160)
Equipment
-
Countess cell counter (Invitrogen) or Neubauer hemocytometer
-
Microscope (Leica DM IL)
-
FACS instrument (two lasers minimum)
-
MACS MultiStand (Miltenyi Biotec, cat. no. 130-042-303)
-
QuadroMACS separator (Miltenyi Biotec, cat. no. 130-090-976)
-
Cell incubator (temperature 37 °C, CO2 level 5%)
-
Temperature-controlled freezing container (Mr. Frosty)
REAGENT SETUP
PBLs
-
We obtain these from unstimulated apheresis products from HLA-A2+ healthy donors. We immediately process apheresis products (no overnight storage). PBLs are separated with a Ficoll (Biocoll) gradient and subsequently stored as aliquots in vials containing 3 × 108 or 1 × 108 cells. Freezing medium contains CellGro medium (50%), autologous plasma (40%) and DMSO (10%). Cells are then placed in a temperature-controlled freezing container (Mr. Frosty) and stored at −80 °C overnight before they are transferred to liquid nitrogen. To thaw cells, vials are quickly placed in a water bath and transferred to cold PBS/5% (vol/vol) HS (20 ml) immediately after thawing. PBLs are spun down (5 min, 400g, 4 °C) and resuspended once more in PBS/1% (vol/vol) HS, spun again and then used for further assays. Frozen PBLs are best used within 1 year of cryoconservation, as viability will decrease with longer storage.
Caution
Apheresis products and cells are blood products. Universal safety precautions apply, such as the use of gloves and protective eyewear.
Caution
Experiments must conform to all relevant institutional and governmental ethics regulations, and appropriate informed consent must be obtained for the proposed use of human blood.
Critical
To be able to use the Melan-A26-35-peptide, expression of HLA-A201 is a requirement. Candidate donors can be screened by flow cytometry using an antibody against HLA-A2 (e.g., Affymetrix/eBioscience, clone BB7.2, cat. no. 17-9876-41).
Human AB serum
-
We purchase the serum in frozen flasks of 500 ml and store them directly at −20 °C. Individual flasks are slowly thawed (at 4 °C overnight), and serum aliquots are prepared in 50-ml tubes and frozen again (see note regarding differences in serum quality). The serum can be stored for up to 2 years at −20 °C.
DC medium
-
For DC preparation, supplement CellGro DC medium with 1% (vol/vol) penicillin-streptomycin and 1% (vol/vol) human AB serum. Store this medium at 4 °C.
T cell medium
-
For T cell expansion, supplement CellGro DC medium with penicillin-streptomycin (1% (vol/vol)) and 5% (vol/vol) human AB serum.
Peptides
-
Dissolve peptides in DMSO to a concentration of 5 μg/μl. For the Melan-A26−35(A27L) peptide, which has a molecular weight of 985 g/mol, this stock solution thus equals 5 mM. Store the aliquots at −80 °C. Note that a peptide's stability in DMSO is limited to ∼2 months, depending also on the specific peptide sequence.
Cytokines
-
Many cytokine concentrations are indicated in IU/ml, which is the correct form for comparison on the basis of functional activity. However, for some cytokines, depending on the vendor, this information is not available. Values can differ greatly from company to company, suggesting that the measurement of activity may have its challenges. We therefore provide information in ng/μl, referring to specific cytokines of specific companies. Adjustments need to be made when cytokines from different vendors are being used. The biggest difference between this protocol and the values published in many other protocols is the concentration of IL-4. Originally, concentrations of 1,000 IU/ml were reported for DC differentiation35. We titrated down this concentration and found 10 ng/ml, which equals 50 IU/ml (with our cytokine batch), to be sufficient to support DC differentiation. We dilute all the following cytokines in PBS/0.1% HS and store them in aliquots at −20 °C. Cytokines (prepared in stock solution as mentioned above) can be stored at 4 °C for no longer than 4 weeks. The concentrations vary, as indicated in the following table:
Table 4
Cytokine
Stock concentration
IL-4
10 ng (50 IU)/μl
GM-CSF
86 ng (800 IU)/μl
IL-7
10 ng/μl
IL-15
10 ng/μl
IL-21
30 ng/μl
IFN-γ
10 ng/μl
Procedure
Generation of monocyte-derived DCs and naive T cells
-
1
Generate monocyte-derived DCs and prepare naive T cells as described in options A and B, respectively. Both options must be performed. Option A should be started first, and option B should be started 1 d before T cell stimulation (Step 12) and on the same day that DC maturation is induced (Step 1A(x), denoted as day −1). PBLs from the same HLA-A2+ donor must be used for both options A and B.
-
A
Generation of monocyte-derived DCs
-
i
Thaw a vial of PBLs rapidly by diluting the cells in at least 20 ml of cold wash buffer. Count the cells and perform a routine spin. (A routine spin as mentioned throughout this protocol consists of 5 min of centrifugation at 400g and 4 °C.) For standard-sized assays, start with at least 1 × 108 PBLs.
-
ii
Resuspend the cells in prewarmed Cellgro DC medium/1% HS to achieve a concentration of 1 × 107 cells per ml.
-
iii
Pipette 2 ml of this cell suspension into each well of a six-well plate. Prepare at least 2–5 six-well plates.
-
iv
Incubate the cells for 2–3 h at 37 °C to allow adherence to plastic.
-
v
Gently resuspend nonadherent cells by using a blue 1-ml tip. Allow the medium to run over the bottom of the well to wash nonadherent cells off. Also rinse the corners of each well.
Critical Step
Applying the correct pressure for washing the cells off takes practice. Check results frequently with a microscope. Ideally, a monolayer of larger cells can be seen, with little round, small lymphocytes remaining as contaminants. Plastic adherence of monocytes differs depending on the plate manufacturer. If plates from other manufacturers are used, adherence and DC recovery may vary.
-
vi
Add 3 ml of warm Cellgro DC medium supplemented with 1% (vol/vol) HS and 10 ng/ml of IL-4 and 800 IU/ml of GM-CSF.
-
vii
Incubate the cells for 2 d at 37 °C.
-
viii
Add 1.5 ml of fresh Cellgro DC medium supplemented with 10 ng/ml of IL-4 and 1,600 IU/ml of GM-CSF.
-
ix
Incubate the cells for an additional 24 h in the cell incubator.
-
x
Collection of immature DCs. Collect 3 ml of the supernatant first, then vigorously resuspend the remaining medium to remove all cells. Check under the microscope and, if necessary, wash the plate again with cold wash buffer. Pool the cells from all the wells and count.
-
xi
Spin the cells and resuspend them in prewarmed Cellgro DC medium, supplemented with 1% (vol/vol) HS, GM-CSF (800 IU/ml), IL4 (10 ng/ml), LPS (10 ng/ml) and IFN-γ (100 IU/ml). Calculate the volume depending on the cell number and the number of groups needed for the assay. A minimum of 5 × 105 cells per well (optimal is ∼2 × 106 cells per well; 2 ml per well) is necessary to obtain sufficient numbers of semi-mature DCs.
-
xii
Add the peptide (2.5 μg/ml = 2.5 μM) to each well individually. (Alternatively, if a large batch of DCs is required to be loaded with the same peptide, peptide can be added to the initial Cellgro DC medium mix).
Critical Step
The assay is validated for DCs that have been pulsed with single peptides for each individual well. Peptide mixtures can be used, but interference with peptide binding between peptides with high and low binding affinities is theoretically possible.
-
xiii
Incubate the cells at 37 °C for 16 h.
-
i
-
B
Preparation of naive T cells
-
i
Thaw PBLs as described in Step 1A(i). For a normal-sized experiment, 1–2 × 108 PBLs are required.
Critical Step
For each experiment, naive T cells are isolated freshly from PBLs. We do not recommend freezing purified naive T cells.
-
ii
Resuspend the thawed cells in cold wash buffer; count and spin.
-
iii
Use the CD8 untouched isolation kit (Miltenyi) (Box 2, Steps 1–11). CD8-negative cells should be retained on the LS column, whereas CD8+ cells should be selected in the flow-through.
-
iv
Directly continue with CD45RO/CD57 depletion (Box 2, Steps 12–22).
Critical Step
CD57 depletion is necessary, as it depletes most of the terminally differentiated cells, which may be CD45RO−, CD45RA+ (TEMRA). The size of this subset greatly varies from donor to donor31.
-
v
Count the cells, remove an aliquot for FACS analysis, and spin the remaining cells.
-
vi
Resuspend cells in Cellgro DC medium supplemented with 5% (vol/vol) HS and 5 ng/ml of IL-7. The final cell concentration should be 3 × 106 per ml.
Critical Step
Preincubation at this cell density in IL-7-containing medium allows for optimal stimulation.
-
vii
Transfer cells to six-well plates, 2 ml per well, and incubate overnight.
-
viii
Perform FACS staining. Stain T cells for CD62L, CD45RA, CD45RO, CD57 and CD8. If desired, stain for contaminating NK cells (CD56). The purity of the naive cell population should be higher than 90% (Fig. 5a). Check the purity for each experiment, as variations in the final results could be due to initial impurities in cell preparation.
Critical Step
This option should be done on the day when DC maturation is induced (Step 1A(x)).
-
i
-
A
Start of DC/T cell coculture
-
2
Collect DCs (from Step 1A) by vigorously resuspending the cells. Empty the wells and add cold wash buffer. Incubate for 20 min on ice and check adherence frequently by using a microscope. Ensure that all DCs are collected by pooling the initial culture medium and the wash buffer.
Critical Step
In contrast to the classical cytokine-based maturation cocktail (IL-1β, TNF-α, IL-6, PGE2), DCs matured with LPS/IFN-γ may be much more adherent to the plastic, displaying a very different morphology (mostly stretched out). Flushing cells after incubation on ice usually leads to good recovery of viable DCs.
-
3
Spin the cells and resuspend in 10 ml of wash buffer.
-
4
Count the DCs.
-
5
Irradiate the DCs with 30 Gy.
Critical Step
Ensure that the cells are kept cool during transport to the radiation source.
Critical Step
Irradiation may have an additional influence on DC maturation. However, the crucial issue is to block potential proliferation of contaminating cells during the prolonged culture. NK cells may respond strongly to IL-15, which is introduced later in the protocol and may therefore compete with the proliferating antigen-specific T cells. Alternative approaches to block proliferation (such as the use of mitomycin C) have not been validated for this assay.
-
6
Spin and resuspend the DCs in warm Cellgro DC medium/5% (vol/vol) HS at a concentration of 5 × 105 cells per ml.
-
7
Collect the T cells (from Step 1B); count and spin.
-
8
Resuspend the T cells in CG-DC medium/5% (vol/vol) HS at a concentration of 2 × 106 cells per ml.
-
9
Add IL-21, 60 ng/ml, to the T cell fraction. (This will result in a final concentration of 30 ng/ml after the addition of the DCs).
-
10
Mix the DCs with T cells at a 1:1 (vol/vol) ratio (resulting in a 4:1 T cell:DC ratio): calculate for at least 3–5 wells per group (condition). If alternative peptides are to be tested, always include a preparation of Melan-A–pulsed DCs, matured under standard conditions, as an internal positive control (provided that the experiment is performed for HLA-A2–restricted peptides). Always mix the cells for each group first in one larger tube. Do not add T cells and DCs separately to the individual wells, as variation owing to pipetting errors will increase. Groups consisting of a single well do not provide sufficient data on the variation within your experiment.
-
11
Transfer 500 μl of the cell mix into individual wells of a 48-well plate.
-
12
Incubate at 37 °C for 72 h.
First feeding
-
13
Check cells under the microscope. When using Melan-A as a model antigen, it should be expected that a few proliferative clusters of cells will be evident. Calculate the required amount of medium to give 0.5 ml per well.
-
14
Make up warm Cellgro DC medium containing 5% (vol/vol) HS and a 5 ng/ml concentration each of IL-15 and IL-7 (referring to the final concentration in the culture medium).
-
15
Add 500 μl of this cytokine-supplemented medium to each well.
-
16
Incubate the cells for 72 h.
Second feeding
-
17
Calculate the required amount of medium to give 1 ml per well.
-
18
Make up warm Cellgro DC medium containing 5% (vol/vol) HS and a 5 ng/ml concentration each of IL-15 and IL-7 (referring to the final concentration in the culture medium).
-
19
Add 1 ml of this cytokine-supplemented medium to each well of a 12-well plate.
-
20
Transfer the cells and medium from each well of the old plate (from Step 16) to the new 12-well plate.
Critical Step
Transfer of cells allows more room for expansion, but also should reduce the number of residual (plastic-adherent) myeloid cells from the DC preparation, which are no longer required.
-
21
Incubate the cells for 48 h.
Third feeding
-
22
Calculate the required amount of medium to give 2 ml per well.
-
23
Make up warm Cellgro DC medium containing 5% (vol/vol) HS and a 10 ng/ml concentration each of IL-15 and IL-7 (referring to the final concentration in the culture medium). Note that now the cytokine concentration is doubled.
-
24
Add 2 ml of this cytokine-supplemented, freshly prepared medium to each well of a six-well plate.
-
25
Transfer the cells and medium from each individual well of the 12-well plate (from Step 21) to individual wells of the new 6-well plate. Incubate for 72 h.
Analysis on day 10 of culture
-
26
Analyze cells. The T cells are routinely analyzed on day 10 of culture. Analysis may vary depending on the research question. We briefly describe the methods that we routinely use for evaluation in Box 3. If T cells are to be evaluated for cytotoxic activity, purification by MHC multimers is recommended (Fig. 6 and Box 1). In Box 4, we present a protocol for one of these methods, a FACS-based cytotoxicity assay. We routinely evaluate each well individually. Especially when low frequency responses are expected, wells without antigen-specific responses may be present (because there were no antigen-specific T cells in the starting well). However, if the wells are pooled before analysis, the specific T cell lines will become diluted with nonspecific T cells from the negative wells.
Troubleshooting
Troubleshooting advice can be found in Table 3.
Timing
The protocol as described here stretches over a total experimental time of 14 d, up until day 10 of T cell culture. For convenience, starting with a DC preparation on a Monday (Step 1) and priming T cells on Friday (Steps 2–12) makes it possible to leave cultures unattended over the weekends. Day 10 of culture then again is a Monday, and all necessary experiments for a functional readout can then be planned in this week.
Note that IL-12 production is highest in the hours after the TLR trigger, and therefore the maturation step should not be extended for more than 16 h.
The following breakdown describes how much time is required on specific days:
Step 1A(i–vii), day −4: 3 h plus 3 h of incubation
Step 1A(viii, ix), day −3: 30 min
Step 1A(x–xiii), day −1: 1 h
Step 1B(i–viii), day −1: 3 h
Steps 2–12, day 0: 3 h
Steps 13–16, day 3: 30 min
Steps 17–21, day 5: 1 h
Steps 22–25, day 7: 1 h
Anticipated results
Examples for typical results of ACE-CD8 are shown in Figures 4,5,6. Figure 4 highlights important aspects of this protocol. The choice of DC maturation stimulus may not only influence the percentage of specific cells but also the total expansion of these cells. More importantly, functionality is dependent on the initial priming conditions: T cells primed with IL-12 nonproducing DCs and matured with a cytokine cocktail including PgE2, only poorly recognize endogenously presented antigen (Fig. 4c). Other factors crucial to optimal priming are the T cell:DC ratio (Fig. 4d) and the presence of IL-21 at the initial priming step (Fig. 4e).
On day 10–12 of culture, T cells display an early effector memory cell type. They are CD45RO+, but still express CD45RA to some level. Moreover, the T cells still express CD62L and, to some degree, CCR7, which gradually declines further as the cells expand. Co-stimulatory molecules such as CD28 and CD27 are still expressed at variable degree. The phenotype after a single stimulation and expansion in IL-7/IL-15 resembles 'young' effector memory cells. For adoptive T cell therapy, such T cells have been shown to be favorable in terms of efficacy and longevity after transfer in vivo54,55. In fact, a recent clinical trial in leukemia patients showed persistence of WT1-specific T cells only, if cells had been cultured in the presence of IL-21 (ref. 56). In this case, this was observed even after a prolonged culture time due to the cloning of individual cells and their massive expansion.
Figure 5 provides data on a representative experiment targeting other peptide antigens with lower precursor frequency. First, the quality of the naive T cell preparation was evaluated (Fig. 5a). The experiment was subsequently set up in five parallel wells for each peptide tested, and MHC-multimer staining was performed for each individual well (Fig. 5b), with the percentage of antigen-specific T cells by day 10 of culture shown. Note that some wells for the gp100-epitope were negative, which emphasizes the importance in low-frequency responses of using small numbers of cells in each starting well and testing multiple wells in parallel. In addition, selected wells were analyzed functionally (Fig. 5c). T cells expanded over only a short time period remain reactive against endogenously presented antigen. Figure 6 shows data on a representative cytotoxicity assay using purified Melan-A–specific T cells at a 2:1 ratio. Details of the assay are explained in Box 4. Finally, Figure 7 illustrates an experiment with suboptimal results: although differences between the experimental groups were observed, cells using standard conditions did not expand properly; thus, the experimental results are not validated. Repeat experiments indicated that, in this particular case, the leukapheresis obtained was suboptimal. Whether this is due to donor-specific characteristics or to difficulties in the preparation of the leukapheresis product has yet to be determined. Additional experiments are required to determine whether IL-21 throughout the culture may increase the T cell yield, although previous reports suggest otherwise42.
Pilot data from an experiment assessing the effect of additional supplementation with IL-21 on days 3, 5 and 7. Although a positive effect can be observed in the experimental group with additional IL-21, the experiment does not meet the validation criteria as the mean percentage of Melan-A–specific T cells under standard conditions did not meet the predefined criteria of >24% MHC multimer+ T cells. In addition, the assay had not been performed in triplicate, which would have been desirable. Repeat experiments revealed a problem with this particular leukapheresis.
References
Kaech, S.M. & Ahmed, R. Memory CD8+ T cell differentiation: initial antigen encounter triggers a developmental program in naive cells. Nat. Immunol. 2, 415–422 (2001).
Kaech, S.M., Wherry, E.J. & Ahmed, R. Effector and memory T-cell differentiation: implications for vaccine development. Nat. Rev. Immunol. 2, 251–262 (2002).
Arens, R. & Schoenberger, S.P. Plasticity in programming of effector and memory CD8 T-cell formation. Immunol. Rev. 235, 190–205 (2010).
Ahmed, R. & Oldstone, M.B. Organ-specific selection of viral variants during chronic infection. J. Exp. Med. 167, 1719–1724 (1988).
Dissanayake, D., Gronski, M.A., Lin, A., Elford, A.R. & Ohashi, P.S. Immunological perspective of self versus tumor antigens: insights from the RIP-gp model. Immunol. Rev. 241, 164–179 (2011).
Jensen, E.R., Shen, H., Wettstein, F.O., Ahmed, R. & Miller, J.F. Recombinant Listeria monocytogenes as a live vaccine vehicle and a probe for studying cell-mediated immunity. Immunol. Rev. 158, 147–157 (1997).
Ohlen, C. et al. CD8+ T cell tolerance to a tumor-associated antigen is maintained at the level of expansion rather than effector function. J. Exp. Med. 195, 1407–1418 (2002).
Seok, J. et al. Genomic responses in mouse models poorly mimic human inflammatory diseases. Proc. Natl. Acad. Sci. USA 110, 3507–3512 (2013).
Davis, M.M. A prescription for human immunology. Immunity 29, 835–838 (2008).
Donahue, R.E. & Dunbar, C.E. Update on the use of nonhuman primate models for preclinical testing of gene therapy approaches targeting hematopoietic cells. Hum. Gene Therapy 12, 607–617 (2001).
Haigwood, N.L. Predictive value of primate models for AIDS. AIDS Rev. 6, 187–198 (2004).
Coulie, P.G. et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 180, 35–42 (1994).
Kawakami, Y. et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. USA 91, 3515–3519 (1994).
Kawakami, Y. et al. Identification of the immunodominant peptides of the MART-1 human melanoma antigen recognized by the majority of HLA-A2-restricted tumor infiltrating lymphocytes. J Exp. Med. 180, 347–352 (1994).
Romero, P. et al. Antigenicity and immunogenicity of Melan-A/MART-1 derived peptides as targets for tumor reactive CTL in human melanoma. Immunol. Rev. 188, 81–96 (2002).
Alanio, C., Lemaitre, F., Law, H.K., Hasan, M. & Albert, M.L. Enumeration of human antigen-specific naive CD8+ T cells reveals conserved precursor frequencies. Blood 115, 3718–3725 (2010).
Pittet, M.J. et al. High frequencies of naive Melan-A/MART-1-specific CD8+ T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J. Exp. Med. 190, 705–715 (1999).
Zippelius, A. et al. Thymic selection generates a large T cell pool recognizing a self-peptide in humans. J. Exp. Med. 195, 485–494 (2002).
Dutoit, V. et al. Degeneracy of antigen recognition as the molecular basis for the high frequency of naive A2/Melan-A peptide multimer+ CD8+ T cells in humans. J. Exp. Med. 196, 207–216 (2002).
Wolfl, M. et al. Primed tumor-reactive multifunctional CD62L+ human CD8+ T cells for immunotherapy. Cancer Immunol. Immunotherapy 60, 173–186 (2011).
Wolfl, M. et al. Hepatitis C virus immune escape via exploitation of a hole in the T cell repertoire. J. Immunol. 181, 6435–6446 (2008).
Pufnock, J.S. et al. Priming CD8+ T cells with dendritic cells matured using TLR4 and TLR7/8 ligands together enhances generation of CD8+ T cells retaining CD28. Blood 117, 6542–6551 (2011).
Wolfl, M. et al. Activation-induced expression of CD137 permits detection, isolation, and expansion of the full repertoire of CD8+ T cells responding to antigen without requiring knowledge of epitope specificities. Blood 110, 201–210 (2007).
Wolfl, M. et al. Src-kinase inhibitors sensitize human cells of myeloid origin to Toll-like-receptor-induced IL12 synthesis. Blood 122, 1203–1213 (2013).
Schade, A.E. et al. Dasatinib, a small-molecule protein tyrosine kinase inhibitor, inhibits T-cell activation and proliferation. Blood 111, 1366–1377 (2008).
von Bergwelt-Baildon, M.S. et al. Human primary and memory cytotoxic T lymphocyte responses are efficiently induced by means of CD40-activated B cells as antigen-presenting cells: potential for clinical application. Blood 99, 3319–3325 (2002).
Suhoski, M.M. et al. Engineering artificial antigen-presenting cells to express a diverse array of co-stimulatory molecules. Mol. Therapy 15, 981–988 (2007).
van Stipdonk, M.J., Lemmens, E.E. & Schoenberger, S.P. Naive CTLs require a single brief period of antigenic stimulation for clonal expansion and differentiation. Nat. Immunol. 2, 423–429 (2001).
Oelke, M. & Schneck, J.P. Overview of a HLA-Ig based 'Lego-like system' for T cell monitoring, modulation and expansion. Immunolog. Res. 47, 248–256 (2010).
Oelke, M. et al. Ex vivo induction and expansion of antigen-specific cytotoxic T cells by HLA-Ig-coated artificial antigen-presenting cells. Nat. Med. 9, 619–624 (2003).
Geginat, J., Lanzavecchia, A. & Sallusto, F. Proliferation and differentiation potential of human CD8+ memory T-cell subsets in response to antigen or homeostatic cytokines. Blood 101, 4260–4266 (2003).
Altman, J.D. et al. Phenotypic analysis of antigen-specific T lymphocytes. Science 274, 94–96 (1996).
Ma, X. et al. The interleukin 12 p40 gene promoter is primed by interferon-γ in monocytic cells. J. Exp. Med. 183, 147–157 (1996).
Langenkamp, A., Messi, M., Lanzavecchia, A. & Sallusto, F. Kinetics of dendritic cell activation: impact on priming of TH1, TH2 and nonpolarized T cells. Nat. Immunol. 1, 311–316 (2000).
Jonuleit, H. et al. Pro-inflammatory cytokines and prostaglandins induce maturation of potent immunostimulatory dendritic cells under fetal calf serum-free conditions. European Journal of Immunology 27, 3135–3142 (1997).
Kalinski, P., Vieira, P.L., Schuitemaker, J.H., de Jong, E.C. & Kapsenberg, M.L. Prostaglandin E2 is a selective inducer of interleukin-12 p40 (IL-12p40) production and an inhibitor of bioactive IL-12p70 heterodimer. Blood 97, 3466–3469 (2001).
Huang, J. et al. Irradiation enhances human T-cell function by upregulating CD70 expression on antigen-presenting cells in vitro. J. Immunotherapy 34, 327–335 (2011).
Merrick, A. et al. Immunosuppressive effects of radiation on human dendritic cells: reduced IL-12 production on activation and impairment of naive T-cell priming. Br. J. Cancer 92, 1450–1458 (2005).
Geginat, J., Sallusto, F. & Lanzavecchia, A. Cytokine-driven proliferation and differentiation of human naive, central memory, and effector memory CD4+ T cells. J. Exp. Med. 194, 1711–1719 (2001).
Li, Y. & Yee, C. IL-21 mediated Foxp3 suppression leads to enhanced generation of antigen-specific CD8+ cytotoxic T lymphocytes. Blood 111, 229–235 (2008).
Coulie, P.G. et al. Precursor frequency analysis of human cytolytic T lymphocytes directed against autologous melanoma cells. Int. J. Cancer 50, 289–297 (1992).
Li, Y., Bleakley, M. & Yee, C. IL-21 influences the frequency, phenotype, and affinity of the antigen-specific CD8 T cell response. J. Immunol. 175, 2261–2269 (2005).
Alves, N.L., Arosa, F.A. & van Lier, R.A. IL-21 sustains CD28 expression on IL-15-activated human naive CD8+ T cells. J. Immunol. 175, 755–762 (2005).
Ho, W.Y., Nguyen, H.N., Wolfl, M., Kuball, J. & Greenberg, P.D. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J. Immunolog. Methods 310, 40–52 (2006).
Surh, C.D., Boyman, O., Purton, J.F. & Sprent, J. Homeostasis of memory T cells. Immunolog. Rev. 211, 154–163 (2006).
Kamimura, D. & Bevan, M.J. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J. Exp. Med. 204, 1803–1812 (2007).
Wolfl, M., Langhammer, F., Wiegering, V., Eyrich, M. & Schlegel, P.G. Dasatinib medication causing profound immunosuppression in a patient after haploidentical SCT: functional assays from whole blood as diagnostic clues. Bone Marrow Transplant. 48, 875–877 (2013).
Rodeberg, D.A., Nuss, R.A., Elsawa, S.F. & Celis, E. Recognition of six-transmembrane epithelial antigen of the prostate-expressing tumor cells by peptide antigen-induced cytotoxic T lymphocytes. Clin. Cancer Res. 11, 4545–4552 (2005).
Quintarelli, C. et al. High-avidity cytotoxic T lymphocytes specific for a new PRAME-derived peptide can target leukemic and leukemic-precursor cells. Blood 117, 3353–3362 (2011).
Godet, Y. et al. MELOE-1 is a new antigen overexpressed in melanomas and involved in adoptive T cell transfer efficiency. J. Exp. Med. 205, 2673–2682 (2008).
Sharrock, C.E., Kaminski, E. & Man, S. Limiting dilution analysis of human T cells: a useful clinical tool. Immunol. Today 11, 281–286 (1990).
Bonnefoix, T., Bonnefoix, P., Callanan, M., Verdiel, P. & Sotto, J.J. Graphical representation of a generalized linear model-based statistical test estimating the fit of the single-hit Poisson model to limiting dilution assays. J. Immunol. 167, 5725–5730 (2001).
Bonnefoix, T. & Callanan, M. Accurate hematopoietic stem cell frequency estimates by fitting multicell Poisson models substituting to the single-hit Poisson model in limiting dilution transplantation assays. Blood 116, 2472–2475 (2010).
Klebanoff, C.A. et al. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc. Natl. Acad. Sci. USA 102, 9571–9576 (2005).
Berger, C. et al. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J. Clin. Invest. 118, 294–305 (2008).
Chapuis, A.G. et al. Transferred WT1-reactive CD8+ T cells can mediate antileukemic activity and persist in post-transplant patients. Sci. Transl. Med. 5, 174ra127 (2013).
Oelke, M. et al. Generation and purification of CD8+ melan-A–specific cytotoxic T lymphocytes for adoptive transfer in tumor immunotherapy. Clin. Cancer Res. 6, 1997–2005 (2000).
Blanchet, J.S. et al. A new generation of Melan-A/MART-1 peptides that fulfill both increased immunogenicity and high resistance to biodegradation: implication for molecular anti-melanoma immunotherapy. J. Immunol. 167, 5852–5861 (2001).
Xu, S. et al. Rapid high efficiency sensitization of CD8+ T cells to tumor antigens by dendritic cells leads to enhanced functional avidity and direct tumor recognition through an IL-12-dependent mechanism. J. Immunol. 171, 2251–2261 (2003).
Hirano, N. et al. Efficient presentation of naturally processed HLA class I peptides by artificial antigen-presenting cells for the generation of effective antitumor responses. Clin. Cancer Res. 12, 2967–2975 (2006).
Rudolf, D. et al. Potent costimulation of human CD8 T cells by anti-4-1BB and anti-CD28 on synthetic artificial antigen presenting cells. Cancer Immunol. Immunotherapy 57, 175–183 (2008).
Dubsky, P. et al. IL-15-induced human DC efficiently prime melanoma-specific naive CD8+ T cells to differentiate into CTL. Eur. J. Immunol. 37, 1678–1690 (2007).
Dauer, M. et al. FastDC derived from human monocytes within 48 h effectively prime tumor antigen-specific cytotoxic T cells. J. Immunolog. Methods 302, 145–155 (2005).
Chauvin, J.M. et al. HLA anchor optimization of the melan-A-HLA-A2 epitope within a long peptide is required for efficient cross-priming of human tumor-reactive T cells. J. Immunol. 188, 2102–2110 (2012).
Hervas-Stubbs, S. et al. CD8 T cell priming in the presence of IFN-α renders CTLs with improved responsiveness to homeostatic cytokines and recall antigens: important traits for adoptive T cell therapy. J. Immunol. 189, 3299–3310 (2012).
Lissina, A. et al. Protein kinase inhibitors substantially improve the physical detection of T-cells with peptide-MHC tetramers. J. Immunolog. Methods 340, 11–24 (2009).
Acknowledgements
We thank the Würzburg-Team for providing experimental data (namely M.L. Reβ, S. Schwinn and M. Braun) and vital structural support (especially P.G. Schlegel). We thank J. Becker, Department of Dermatology, Würzburg, for providing the HLA-A2+ melanoma cell line FM55. M.W. was largely supported and funded by the parent initiative 'Aktion Regenbogen für leukämie- und tumorkranke Kinder Main-Tauber e.V.' and by a program project grant from BayImmuNet (F2-F5121.7.1.1/13/1/2009). Initial support was also provided by the Deutsche Krebshilfe (to M.W.) and by the US National Institutes of Health (CA 18029, CA 33084) and the Bill and Melinda Gates Foundation (to P.D.G.).
Author information
Authors and Affiliations
Contributions
M.W. selected and evaluated the experimental data. M.W. and P.D.G. wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Rights and permissions
About this article
Cite this article
Wölfl, M., Greenberg, P. Antigen-specific activation and cytokine-facilitated expansion of naive, human CD8+ T cells. Nat Protoc 9, 950–966 (2014). https://doi.org/10.1038/nprot.2014.064
Published:
Issue Date:
DOI: https://doi.org/10.1038/nprot.2014.064
This article is cited by
-
T cell receptor therapeutics: immunological targeting of the intracellular cancer proteome
Nature Reviews Drug Discovery (2023)
-
Tumor RNA transfected DCs derived from iPS cells elicit cytotoxicity against cancer cells induced from colorectal cancer patients in vitro
Scientific Reports (2022)
-
Nanoparticle-based modulation of CD4+ T cell effector and helper functions enhances adoptive immunotherapy
Nature Communications (2022)
-
Immunogenicity and therapeutic targeting of a public neoantigen derived from mutated PIK3CA
Nature Medicine (2022)
-
T cells specific for α-myosin drive immunotherapy-related myocarditis
Nature (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.