Key Points
-
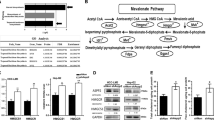
Mevalonate (MVA) pathway metabolites are essential for cancer cell survival and growth.
-
Expression of the genes encoding MVA pathway enzymes is controlled by the sterol regulatory element-binding protein (SREBP) family of transcription factors.
-
In cancer cells, oncogenic signalling pathways deregulate the activity of the SREBP transcription factors and MVA pathway enzymes.
-
Deregulated production of MVA pathway metabolites modulates multiple signalling pathways in cancer cells and contributes to transformation.
-
Clinical trials to evaluate the utility of MVA pathway inhibitors as anticancer agents have shown responses in some, but not all, patients; discovering biomarkers to identify responders and developing combination therapies will further enhance the utility of these inhibitors.
-
Inhibiting the SREBP transcription factors is a promising strategy to increase the efficacy of MVA pathway inhibitors as anticancer therapeutics, and also potentially to combat resistance to MVA pathway therapies.
Abstract
The mevalonate (MVA) pathway is an essential metabolic pathway that uses acetyl-CoA to produce sterols and isoprenoids that are integral to tumour growth and progression. In recent years, many oncogenic signalling pathways have been shown to increase the activity and/or the expression of MVA pathway enzymes. This Review summarizes recent advances and discusses unique opportunities for immediately targeting this metabolic vulnerability in cancer with agents that have been approved for other therapeutic uses, such as the statin family of drugs, to improve outcomes for cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Boroughs, L. K. & DeBerardinis, R. J. Metabolic pathways promoting cancer cell survival and growth. Nat. Cell Biol. 17, 351–359 (2015).
Son, J. et al. Glutamine supports pancreatic cancer growth through a KRAS-regulated metabolic pathway. Nature 496, 101–105 (2013).
Possemato, R. et al. Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 (2011).
Comerford, S. A. et al. Acetate dependence of tumors. Cell 159, 1591–1602 (2014).
Mashimo, T. et al. Acetate is a bioenergetic substrate for human glioblastoma and brain metastases. Cell 159, 1603–1614 (2014).
Mardis, E. R. et al. Recurring mutations found by sequencing an acute myeloid leukemia genome. N. Engl. J. Med. 361, 1058–1066 (2009).
Parsons, D. W. et al. An integrated genomic analysis of human glioblastoma multiforme. Science 321, 1807–1812 (2008).
Christofk, H. R. et al. The M2 splice isoform of pyruvate kinase is important for cancer metabolism and tumour growth. Nature 452, 230–233 (2008).
Patra, K. C. et al. Hexokinase 2 is required for tumor initiation and maintenance and its systemic deletion is therapeutic in mouse models of cancer. Cancer Cell 24, 213–228 (2013).
Adam, J., Yang, M., Soga, T. & Pollard, P. J. Rare insights into cancer biology. Oncogene 33, 2547–2556 (2014).
Goldstein, J. L. & Brown, M. S. Familial hypercholesterolemia: identification of a defect in the regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity associated with overproduction of cholesterol. Proc. Natl Acad. Sci. USA 70, 2804–2808 (1973). This manuscript was the first to suggest that a genetic abnormality could lead to the deregulation of HMGCR and could result in a defect in the regulation of cholesterol synthesis. It contributed to Goldstein and Brown winning the Nobel Prize in Physiology or Medicine in 1985.
Clendening, J. W. & Penn, L. Z. Targeting tumor cell metabolism with statins. Oncogene 31, 4967–4978 (2012).
Pietrocola, F., Galluzzi, L., Bravo-San Pedro, J. M., Madeo, F. & Kroemer, G. Acetyl coenzyme A: a central metabolite and second messenger. Cell Metab. 21, 805–821 (2015).
Schug, Z. T. et al. Acetyl-CoA synthetase 2 promotes acetate utilization and maintains cancer cell growth under metabolic stress. Cancer Cell 27, 57–71 (2015).
Jeon, S. M., Chandel, N. S. & Hay, N. AMPK regulates NADPH homeostasis to promote tumour cell survival during energy stress. Nature 485, 661–665 (2012).
Anastasiou, D. et al. Inhibition of pyruvate kinase M2 by reactive oxygen species contributes to cellular antioxidant responses. Science 334, 1278–1283 (2011).
Sharpe, L. J. & Brown, A. J. Controlling cholesterol synthesis beyond 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR). J. Biol. Chem. 288, 18707–18715 (2013).
Hart, T. et al. High-resolution CRISPR screens reveal fitness genes and genotype-specific cancer liabilities. Cell 163, 1515–1526 (2015). MVA pathway genes were found to be essential in multiple cancer cell types, highlighting the dependency of cancer cells on the MVA pathway.
Wang, T. et al. Identification and characterization of essential genes in the human genome. Science 350, 1096–1101 (2015).
Blomen, V. A. et al. Gene essentiality and synthetic lethality in haploid human cells. Science 350, 1092–1096 (2015).
Clendening, J. W. et al. Exploiting the mevalonate pathway to distinguish statin-sensitive multiple myeloma. Blood 115, 4787–4797 (2010).
Goard, C. A. et al. Identifying molecular features that distinguish fluvastatin-sensitive breast tumor cells. Breast Cancer Res. Treat. 143, 301–312 (2014).
Keyomarsi, K., Sandoval, L., Band, V. & Pardee, A. B. Synchronization of tumor and normal cells from G1 to multiple cell cycles by lovastatin. Cancer Res. 51, 3602–3609 (1991).
Dimitroulakos, J. et al. Microarray and biochemical analysis of lovastatin-induced apoptosis of squamous cell carcinomas. Neoplasia 4, 337–346 (2002).
Dimitroulakos, J. et al. Increased sensitivity of acute myeloid leukemias to lovastatin-induced apoptosis: a potential therapeutic approach. Blood 93, 1308–1318 (1999).
Larson, R. A. & Yachnin, S. Mevalonic acid induces DNA synthesis in chronic lymphocytic leukemia cells. Blood 64, 257–262 (1984).
Clendening, J. W. et al. Dysregulation of the mevalonate pathway promotes transformation. Proc. Natl Acad. Sci. USA 107, 15051–15056 (2010). This study was the first to show that a flux-controlling enzyme of the MVA pathway, HMGCR, can promote transformation.
Duncan, R. E., El-Sohemy, A. & Archer, M. C. Mevalonate promotes the growth of tumors derived from human cancer cells in vivo and stimulates proliferation in vitro with enhanced cyclin-dependent kinase-2 activity. J. Biol. Chem. 279, 33079–33084 (2004).
Bloch, K. The biological synthesis of cholesterol. Science 150, 19–28 (1965).
Goldstein, J. L. & Brown, M. S. The low-density lipoprotein pathway and its relation to atherosclerosis. Annu. Rev. Biochem. 46, 897–930 (1977).
Pike, L. J. The challenge of lipid rafts. J. Lipid Res. 50 (Suppl.), S323–S328 (2009).
Mollinedo, F. & Gajate, C. Lipid rafts as major platforms for signaling regulation in cancer. Adv. Biol. Regul. 57, 130–146 (2015).
Ray, S., Kassan, A., Busija, A. R., Rangamani, P. & Patel, H. H. The plasma membrane as a capacitor for energy and metabolism. Am. J. Physiol. Cell Physiol. 310, C181–C192 (2015).
York, A. G. et al. Limiting cholesterol biosynthetic flux spontaneously engages type I IFN signaling. Cell 163, 1716–1729 (2015).
Li, H. Y., Appelbaum, F. R., Willman, C. L., Zager, R. A. & Banker, D. E. Cholesterol-modulating agents kill acute myeloid leukemia cells and sensitize them to therapeutics by blocking adaptive cholesterol responses. Blood 101, 3628–3634 (2003).
Novak, A. et al. Cholesterol masks membrane glycosphingolipid tumor-associated antigens to reduce their immunodetection in human cancer biopsies. Glycobiology 23, 1230–1239 (2013).
Ko, Y. J. & Balk, S. P. Targeting steroid hormone receptor pathways in the treatment of hormone dependent cancers. Curr. Pharm. Biotechnol. 5, 459–470 (2004).
Lin, C. Y. & Gustafsson, J. A. Targeting liver X receptors in cancer therapeutics. Nat. Rev. Cancer 15, 216–224 (2015).
Krycer, J. R. & Brown, A. J. Cholesterol accumulation in prostate cancer: a classic observation from a modern perspective. Biochim. Biophys. Acta 1835, 219–229 (2013).
Miziorko, H. M. Enzymes of the mevalonate pathway of isoprenoid biosynthesis. Arch. Biochem. Biophys. 505, 131–143 (2011).
Gruenbacher, G. & Thurnher, M. Mevalonate metabolism in cancer. Cancer Lett. 356, 192–196 (2015).
Thurnher, M. & Gruenbacher, G. T lymphocyte regulation by mevalonate metabolism. Sci. Signal. 8, re4 (2015).
Meraviglia, S. et al. In vivo manipulation of Vγ9Vδ2 T cells with zoledronate and low-dose interleukin-2 for immunotherapy of advanced breast cancer patients. Clin. Exp. Immunol. 161, 290–297 (2010).
Dieli, F. et al. Targeting human γδ T cells with zoledronate and interleukin-2 for immunotherapy of hormone-refractory prostate cancer. Cancer Res. 67, 7450–7457 (2007).
Willumsen, B. M., Christensen, A., Hubbert, N. L., Papageorge, A. G. & Lowy, D. R. The p21 ras C-terminus is required for transformation and membrane association. Nature 310, 583–586 (1984).
Hart, K. C. & Donoghue, D. J. Derivatives of activated H-ras lacking C-terminal lipid modifications retain transforming ability if targeted to the correct subcellular location. Oncogene 14, 945–953 (1997).
Clarke, S., Vogel, J. P., Deschenes, R. J. & Stock, J. Posttranslational modification of the Ha-ras oncogene protein: evidence for a third class of protein carboxyl methyltransferases. Proc. Natl Acad. Sci. USA 85, 4643–4647 (1988).
Moores, S. L. et al. Sequence dependence of protein isoprenylation. J. Biol. Chem. 266, 14603–14610 (1991).
Casey, P. J. & Seabra, M. C. Protein prenyltransferases. J. Biol. Chem. 271, 5289–5292 (1996).
Kang, S., Kim, E. S. & Moon, A. Simvastatin and lovastatin inhibit breast cell invasion induced by H-Ras. Oncol. Rep. 21, 1317–1322 (2009).
Pandyra, A. et al. Immediate utility of two approved agents to target both the metabolic mevalonate pathway and its restorative feedback loop. Cancer Res. 74, 4772–4782 (2014). This study demonstrated the feasibility of targeting SREBP2 to potentiate the anticancer effects of statins.
Wong, W. W. et al. Determinants of sensitivity to lovastatin-induced apoptosis in multiple myeloma. Mol. Cancer Ther. 6, 1886–1897 (2007). This was one of the first studies to show that the isoprenoids GGPP and FPP can reverse statin-induced apoptosis.
Agarwal, B. et al. Mechanism of lovastatin-induced apoptosis in intestinal epithelial cells. Carcinogenesis 23, 521–528 (2002).
Jiang, Z., Zheng, X., Lytle, R. A., Higashikubo, R. & Rich, K. M. Lovastatin-induced up-regulation of the BH3-only protein, Bim, and cell death in glioblastoma cells. J. Neurochem. 89, 168–178 (2004).
Shellman, Y. G. et al. Lovastatin-induced apoptosis in human melanoma cell lines. Melanoma Res. 15, 83–89 (2005).
Xia, Z. et al. Blocking protein geranylgeranylation is essential for lovastatin-induced apoptosis of human acute myeloid leukemia cells. Leukemia 15, 1398–1407 (2001).
Stirewalt, D. L., Appelbaum, F. R., Willman, C. L., Zager, R. A. & Banker, D. E. Mevastatin can increase toxicity in primary AMLs exposed to standard therapeutic agents, but statin efficacy is not simply associated with ras hotspot mutations or overexpression. Leuk. Res. 27, 133–145 (2003).
Hentschel, A., Zahedi, R. P. & Ahrends, R. Protein lipid modifications—more than just a greasy ballast. Proteomics 16, 759–782 (2016).
Berndt, N., Hamilton, A. D. & Sebti, S. M. Targeting protein prenylation for cancer therapy. Nat. Rev. Cancer 11, 775–791 (2011). This review comprehensively summarizes the feasibility and efficacy of targeting protein prenylation in cancer.
Cox, A. D., Der, C. J. & Philips, M. R. Targeting RAS membrane association: back to the future for anti-RAS drug discovery? Clin. Cancer Res. 21, 1819–1827 (2015).
Cox, A. D., Fesik, S. W., Kimmelman, A. C., Luo, J. & Der, C. J. Drugging the undruggable RAS: mission possible? Nat. Rev. Drug Discov. 13, 828–851 (2014).
Swanson, K. M. & Hohl, R. J. Anti-cancer therapy: targeting the mevalonate pathway. Curr. Cancer Drug Targets 6, 15–37 (2006).
Wiemer, A. J., Wiemer, D. F. & Hohl, R. J. Geranylgeranyl diphosphate synthase: an emerging therapeutic target. Clin. Pharmacol. Ther. 90, 804–812 (2011).
Tsimberidou, A. M., Chandhasin, C. & Kurzrock, R. Farnesyltransferase inhibitors: where are we now? Expert Opin. Investig. Drugs 19, 1569–1580 (2010).
Martin, N. E. et al. A phase I trial of the dual farnesyltransferase and geranylgeranyltransferase inhibitor L-778,123 and radiotherapy for locally advanced pancreatic cancer. Clin. Cancer Res. 10, 5447–5454 (2004).
Ullah, N., Mansha, M. & Casey, P. J. Protein geranylgeranyltransferase type 1 as a target in cancer. Curr. Cancer Drug Targets https://dx.doi.org/10.2174/1568009616666151203224603 (2015).
Chojnacki, T. & Dallner, G. The biological role of dolichol. Biochem. J. 251, 1–9 (1988).
Carlberg, M. et al. Mevalonic acid is limiting for N-linked glycosylation and translocation of the insulin-like growth factor-1 receptor to the cell surface. Evidence for a new link between 3-hydroxy-3- methylglutaryl-coenzyme a reductase and cell growth. J. Biol. Chem. 271, 17453–17462 (1996).
Pinho, S. S. & Reis, C. A. Glycosylation in cancer: mechanisms and clinical implications. Nat. Rev. Cancer 15, 540–555 (2015). This review summarizes the role of aberrant glycosylation in cancer development and progression.
Cheng, C. et al. Glucose-mediated N-glycosylation of SCAP is essential for SREBP-1 activation and tumor growth. Cancer Cell 28, 569–581 (2015). This study links glucose metabolism to the MVA pathway via the N -glycosylation of SCAP.
Ernster, L. & Dallner, G. Biochemical, physiological and medical aspects of ubiquinone function. Biochim. Biophys. Acta 1271, 195–204 (1995).
Maiuri, M. C. & Kroemer, G. Essential role for oxidative phosphorylation in cancer progression. Cell Metab. 21, 11–12 (2015).
Tan, A. S. et al. Mitochondrial genome acquisition restores respiratory function and tumorigenic potential of cancer cells without mitochondrial DNA. Cell Metab. 21, 81–94 (2015).
Hua, X. et al. SREBP-2, a second basic-helix-loop-helix-leucine zipper protein that stimulates transcription by binding to a sterol regulatory element. Proc. Natl Acad. Sci. USA 90, 11603–11607 (1993). Brown and Goldstein follow up their Nobel Prize-winning work by identifying SREBP2.
Yokoyama, C. et al. SREBP-1, a basic-helix-loop-helix-leucine zipper protein that controls transcription of the low density lipoprotein receptor gene. Cell 75, 187–197 (1993).
Amemiya-Kudo, M. et al. Transcriptional activities of nuclear SREBP-1a, -1c, and -2 to different target promoters of lipogenic and cholesterogenic genes. J. Lipid Res. 43, 1220–1235 (2002).
Shimomura, I., Shimano, H., Horton, J. D., Goldstein, J. L. & Brown, M. S. Differential expression of exons 1a and 1c in mRNAs for sterol regulatory element binding protein-1 in human and mouse organs and cultured cells. J. Clin. Invest. 99, 838–845 (1997).
Seo, Y. K. et al. Genome-wide analysis of SREBP-1 binding in mouse liver chromatin reveals a preference for promoter proximal binding to a new motif. Proc. Natl Acad. Sci. USA 106, 13765–13769 (2009).
Seo, Y. K. et al. Genome-wide localization of SREBP-2 in hepatic chromatin predicts a role in autophagy. Cell Metab. 13, 367–375 (2011). This study was the first to map the chromatin binding of SREBP2 genome-wide.
Fruman, D. A. & Rommel, C. PI3K and cancer: lessons, challenges and opportunities. Nat. Rev. Drug Discov. 13, 140–156 (2014).
Engelman, J. A. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat. Rev. Cancer 9, 550–562 (2009).
Song, M. S., Salmena, L. & Pandolfi, P. P. The functions and regulation of the PTEN tumour suppressor. Nat. Rev. Mol. Cell. Biol. 13, 283–296 (2012).
Demoulin, J. B. et al. Platelet-derived growth factor stimulates membrane lipid synthesis through activation of phosphatidylinositol 3-kinase and sterol regulatory element-binding proteins. J. Biol. Chem. 279, 35392–35402 (2004).
Zhou, R. H. et al. Vascular endothelial growth factor activation of sterol regulatory element binding protein: a potential role in angiogenesis. Circ. Res. 95, 471–478 (2004).
Fleischmann, M. & Iynedjian, P. B. Regulation of sterol regulatory-element binding protein 1 gene expression in liver: role of insulin and protein kinase B/cAkt. Biochem. J. 349, 13–17 (2000).
Luu, W., Sharpe, L. J., Stevenson, J. & Brown, A. J. Akt acutely activates the cholesterogenic transcription factor SREBP-2. Biochim. Biophys. Acta 1823, 458–464 (2012).
Porstmann, T. et al. PKB/Akt induces transcription of enzymes involved in cholesterol and fatty acid biosynthesis via activation of SREBP. Oncogene 24, 6465–6481 (2005).
Suzuki, R. et al. Diabetes and insulin in regulation of brain cholesterol metabolism. Cell Metab. 12, 567–579 (2010).
Jeon, T. I. & Osborne, T. F. SREBPs: metabolic integrators in physiology and metabolism. Trends Endocrinol. Metab. 23, 65–72 (2012).
Ricoult, S. J., Yecies, J. L., Ben-Sahra, I. & Manning, B. D. Oncogenic PI3K and K-Ras stimulate de novo lipid synthesis through mTORC1 and SREBP. Oncogene 35, 1250–1260 (2015).
Sundqvist, A. et al. Control of lipid metabolism by phosphorylation-dependent degradation of the SREBP family of transcription factors by SCF(Fbw7). Cell Metab. 1, 379–391 (2005).
Yellaturu, C. R., Deng, X., Park, E. A., Raghow, R. & Elam, M. B. Insulin enhances the biogenesis of nuclear sterol regulatory element-binding protein (SREBP)-1c by posttranscriptional down-regulation of Insig-2A and its dissociation from SREBP cleavage-activating protein (SCAP). SREBP-1c complex. J. Biol. Chem. 284, 31726–31734 (2009).
Hegarty, B. D. et al. Distinct roles of insulin and liver X receptor in the induction and cleavage of sterol regulatory element-binding protein-1c. Proc. Natl Acad. Sci. USA 102, 791–796 (2005).
Yecies, J. L. et al. Akt stimulates hepatic SREBP1c and lipogenesis through parallel mTORC1-dependent and independent pathways. Cell Metab. 14, 21–32 (2011).
Du, X., Kristiana, I., Wong, J. & Brown, A. J. Involvement of Akt in ER-to-Golgi transport of SCAP/SREBP: a link between a key cell proliferative pathway and membrane synthesis. Mol. Biol. Cell 17, 2735–2745 (2006).
Yellaturu, C. R. et al. Insulin enhances post-translational processing of nascent SREBP-1c by promoting its phosphorylation and association with COPII vesicles. J. Biol. Chem. 284, 7518–7532 (2009).
Yamauchi, Y., Furukawa, K. & Hamamura, K. Positive feedback loop between PI3K-Akt-mTORC1 signaling and the lipogenic pathway boosts Akt signaling: induction of the lipogenic pathway by a melanoma antigen. Cancer Res. 71, 4989–4997 (2011).
Calvisi, D. F. et al. Increased lipogenesis, induced by AKT-mTORC1-RPS6 signaling, promotes development of human hepatocellular carcinoma. Gastroenterology 140, 1071–1083 (2011).
DeBerardinis, R. J., Lum, J. J., Hatzivassiliou, G. & Thompson, C. B. The biology of cancer: metabolic reprogramming fuels cell growth and proliferation. Cell Metab. 7, 11–20 (2008).
Kusama, T. et al. 3-Hydroxy-3-methylglutaryl-coenzyme a reductase inhibitors reduce human pancreatic cancer cell invasion and metastasis. Gastroenterology 122, 308–317 (2002).
Asslan, R. et al. Epidermal growth factor stimulates 3-hydroxy-3-methylglutaryl-coenzyme A reductase expression via the ErbB-2 pathway in human breast adenocarcinoma cells. Biochem. Biophys. Res. Commun. 260, 699–706 (1999).
Shimobayashi, M. & Hall, M. N. Making new contacts: the mTOR network in metabolism and signalling crosstalk. Nat. Rev. Mol. Cell. Biol. 15, 155–162 (2014).
Chung, J., Kuo, C. J., Crabtree, G. R. & Blenis, J. Rapamycin-FKBP specifically blocks growth-dependent activation of and signaling by the 70 kd S6 protein kinases. Cell 69, 1227–1236 (1992).
Kuo, C. J. et al. Rapamycin selectively inhibits interleukin-2 activation of p70 S6 kinase. Nature 358, 70–73 (1992).
Von Manteuffel, S. R., Gingras, A. C., Ming, X. F., Sonenberg, N. & Thomas, G. 4E-BP1 phosphorylation is mediated by the FRAP-p70s6k pathway and is independent of mitogen-activated protein kinase. Proc. Natl Acad. Sci. USA 93, 4076–4080 (1996).
Porstmann, T. et al. SREBP activity is regulated by mTORC1 and contributes to Akt-dependent cell growth. Cell Metab. 8, 224–236 (2008). This study shows that the activation of SREBPs through AKT–mTORC1 is required for cell growth.
Li, S., Brown, M. S. & Goldstein, J. L. Bifurcation of insulin signaling pathway in rat liver: mTORC1 required for stimulation of lipogenesis, but not inhibition of gluconeogenesis. Proc. Natl Acad. Sci. USA 107, 3441–3446 (2010). This study offers an explanation for the paradox of insulin resistance, in which insulin fails to suppress glucose production but continues to promote lipid synthesis.
Duvel, K. et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell. 39, 171–183 (2010).
Wang, B. T. et al. The mammalian target of rapamycin regulates cholesterol biosynthetic gene expression and exhibits a rapamycin-resistant transcriptional profile. Proc. Natl Acad. Sci. USA 108, 15201–15206 (2011).
Thoreen, C. C. et al. An ATP-competitive mammalian target of rapamycin inhibitor reveals rapamycin-resistant functions of mTORC1. J. Biol. Chem. 284, 8023–8032 (2009).
Liu, Q. et al. Development of ATP-competitive mTOR inhibitors. Methods Mol. Biol. 821, 447–460 (2012).
Peterson, T. R. et al. mTOR complex 1 regulates lipin 1 localization to control the SREBP pathway. Cell 146, 408–420 (2011). New mTOR inhibitors enabled this work to identify a target of mTOR that regulates SREBP activity.
Zhang, P., Verity, M. A. & Reue, K. Lipin-1 regulates autophagy clearance and intersects with statin drug effects in skeletal muscle. Cell Metab. 20, 267–279 (2014).
Shao, W. & Espenshade, P. J. Expanding roles for SREBP in metabolism. Cell Metab. 16, 414–419 (2012).
Griffiths, B. et al. Sterol regulatory element binding protein-dependent regulation of lipid synthesis supports cell survival and tumor growth. Cancer Metab. 1, 3 (2013). This is the first study to show that ablation of SREBPs affects both lipid and protein biosynthesis.
Hardie, D. G. & Alessi, D. R. LKB1 and AMPK and the cancer-metabolism link – ten years after. BMC Biol. 11, 36 (2013).
Beg, Z. H., Allmann, D. W. & Gibson, D. M. Modulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase activity with cAMP and wth protein fractions of rat liver cytosol. Biochem. Biophys. Res. Commun. 54, 1362–1369 (1973).
Beg, Z. H., Stonik, J. A. & Brewer, H. B. Jr. 3-Hydroxy-3-methylglutaryl coenzyme A reductase: regulation of enzymatic activity by phosphorylation and dephosphorylation. Proc. Natl Acad. Sci. USA 75, 3678–3682 (1978).
Clarke, P. R. & Hardie, D. G. Regulation of HMG-CoA reductase: identification of the site phosphorylated by the AMP-activated protein kinase in vitro and in intact rat liver. EMBO J. 9, 2439–2446 (1990).
Sato, R., Goldstein, J. L. & Brown, M. S. Replacement of serine-871 of hamster 3-hydroxy-3-methylglutaryl-CoA reductase prevents phosphorylation by AMP-activated kinase and blocks inhibition of sterol synthesis induced by ATP depletion. Proc. Natl Acad. Sci. USA 90, 9261–9265 (1993).
Li, Y. et al. AMPK phosphorylates and inhibits SREBP activity to attenuate hepatic steatosis and atherosclerosis in diet-induced insulin-resistant mice. Cell Metab. 13, 376–388 (2011).
Collins, S. P., Reoma, J. L., Gamm, D. M. & Uhler, M. D. LKB1, a novel serine/threonine protein kinase and potential tumour suppressor, is phosphorylated by cAMP-dependent protein kinase (PKA) and prenylated in vivo. Biochem. J. 345, 673–680 (2000).
Houde, V. P. et al. Investigation of LKB1 Ser431 phosphorylation and Cys433 farnesylation using mouse knockin analysis reveals an unexpected role of prenylation in regulating AMPK activity. Biochem. J. 458, 41–56 (2014).
Vogelstein, B., Lane, D. & Levine, A. J. Surfing the p53 network. Nature 408, 307–310 (2000).
Petitjean, A., Achatz, M. I., Borresen-Dale, A. L., Hainaut, P. & Olivier, M. TP53 mutations in human cancers: functional selection and impact on cancer prognosis and outcomes. Oncogene 26, 2157–2165 (2007).
Freed-Pastor, W. A. et al. Mutant p53 disrupts mammary tissue architecture via the mevalonate pathway. Cell 148, 244–258 (2012). This study was the first to demonstrate that specific gain-of-function p53 mutants activate the MVA pathway in cancer cells.
Assaily, W. et al. ROS-mediated p53 induction of Lpin1 regulates fatty acid oxidation in response to nutritional stress. Mol. Cell. 44, 491–501 (2011).
Cancer Genome Atlas Research Network. Integrated genomic analyses of ovarian carcinoma. Nature 474, 609–615 (2011).
Cancer Genome Atlas Research Network. The molecular taxonomy of primary prostate cancer. Cell 163, 1011–1025 (2015).
Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature 489, 519–525 (2012).
Shamma, A. et al. Rb regulates DNA damage response and cellular senescence through E2F-dependent suppression of N-ras isoprenylation. Cancer Cell 15, 255–269 (2009).
Dang, C. V. MYC on the path to cancer. Cell 149, 22–35 (2012).
Tu, W. B. et al. Myc and its interactors take shape. Biochim. Biophys. Acta 1849, 469–483 (2015).
Gao, P. et al. c-Myc suppression of miR-23a/b enhances mitochondrial glutaminase expression and glutamine metabolism. Nature 458, 762–765 (2009).
Meyer, N. & Penn, L. Z. Reflecting on 25 years with MYC. Nat. Rev. Cancer 8, 976–990 (2008).
Wu, Y. et al. Srebp-1 interacts with c-Myc to enhance somatic cell reprogramming. Stem Cells 34, 83–92 (2015).
The ENCODE Project Consortium. An integrated encyclopedia of DNA elements in the human genome. Nature 489, 57–74 (2012).
Dingar, D. et al. BioID identifies novel c-MYC interacting partners in cultured cells and xenograft tumors. J. Proteom. 118, 95–111 (2015).
Cao, Z. et al. MYC phosphorylation, activation, and tumorigenic potential in hepatocellular carcinoma are regulated by HMG-CoA reductase. Cancer Res. 71, 2286–2297 (2011).
Hofmann, J. W. et al. Reduced expression of MYC increases longevity and enhances healthspan. Cell 160, 477–488 (2015).
Sorrentino, G. et al. Metabolic control of YAP and TAZ by the mevalonate pathway. Nat. Cell Biol. 16, 357–366 (2014). This study provides compelling evidence of the importance of MVA-derived metabolites in cancer.
Zanconato, F., Cordenonsi, M. & Piccolo, S. YAP/TAZ at the roots of cancer. Cancer Cell 29, 783–803 (2016).
Moroishi, T., Hansen, C. G. & Guan, K. L. The emerging roles of YAP and TAZ in cancer. Nat. Rev. Cancer 15, 73–79 (2015).
Wang, Z. et al. Interplay of mevalonate and Hippo pathways regulates RHAMM transcription via YAP to modulate breast cancer cell motility. Proc. Natl Acad. Sci. USA 111, E89–E98 (2014).
Mi, W. et al. Geranylgeranylation signals to the Hippo pathway for breast cancer cell proliferation and migration. Oncogene 34, 3095–3106 (2015).
Aylon, Y. et al. The LATS2 tumor suppressor inhibits SREBP and suppresses hepatic cholesterol accumulation. Genes Dev. 30, 786–797 (2016).
Riobo, N. A. Cholesterol and its derivatives in Sonic Hedgehog signaling and cancer. Curr. Opin. Pharmacol. 12, 736–741 (2012).
Eaton, S. Multiple roles for lipids in the Hedgehog signalling pathway. Nat. Rev. Mol. Cell. Biol. 9, 437–445 (2008).
Corcoran, R. B. & Scott, M. P. Oxysterols stimulate Sonic hedgehog signal transduction and proliferation of medulloblastoma cells. Proc. Natl Acad. Sci. USA 103, 8408–8413 (2006).
Nguyen, V. T. et al. Differential epigenetic reprogramming in response to specific endocrine therapies promotes cholesterol biosynthesis and cellular invasion. Nat. Commun. 6, 10044 (2015).
Locke, J. A. et al. Androgen levels increase by intratumoral de novo steroidogenesis during progression of castration-resistant prostate cancer. Cancer Res. 68, 6407–6415 (2008).
Ettinger, S. L. et al. Dysregulation of sterol response element-binding proteins and downstream effectors in prostate cancer during progression to androgen independence. Cancer Res. 64, 2212–2221 (2004).
Huang, W. C., Li, X., Liu, J., Lin, J. & Chung, L. W. Activation of androgen receptor, lipogenesis, and oxidative stress converged by SREBP-1 is responsible for regulating growth and progression of prostate cancer cells. Mol. Cancer Res. 10, 133–142 (2012).
Goldstein, J. L. & Brown, M. S. Regulation of the mevalonate pathway. Nature 343, 425–430 (1990).
Ahern, T. P. et al. Statin prescriptions and breast cancer recurrence risk: a Danish nationwide prospective cohort study. J. Natl Cancer Inst. 103, 1461–1468 (2011).
Fortuny, J. et al. Use of analgesics and nonsteroidal anti-inflammatory drugs, genetic predisposition, and bladder cancer risk in Spain. Cancer Epidemiol. Biomarkers Prev. 15, 1696–1702 (2006).
Nielsen, S. F., Nordestgaard, B. G. & Bojesen, S. E. Statin use and reduced cancer-related mortality. N. Engl. J. Med. 367, 1792–1802 (2012). An important study showing reduced deaths from cancer in statin users.
Freedland, S. J. et al. Statin use and risk of prostate cancer and high-grade prostate cancer: results from the REDUCE study. Prostate Cancer Prostat. Dis. 16, 254–259 (2013).
Kuoppala, J., Lamminpaa, A. & Pukkala, E. Statins and cancer: a systematic review and meta-analysis. Eur. J. Cancer 44, 2122–2132 (2008).
Chae, Y. K. et al. Reduced risk of breast cancer recurrence in patients using ACE inhibitors, ARBs, and/or statins. Cancer Invest. 29, 585–593 (2011).
Boudreau, D. M. et al. Comparative safety of cardiovascular medication use and breast cancer outcomes among women with early stage breast cancer. Breast Cancer Res. Treat. 144, 405–416 (2014).
Kwan, M. L., Habel, L. A., Flick, E. D., Quesenberry, C. P. & Caan, B. Post-diagnosis statin use and breast cancer recurrence in a prospective cohort study of early stage breast cancer survivors. Breast Cancer Res. Treat. 109, 573–579 (2008).
Chen, C., Lin, J., Smolarek, T. & Tremaine, L. P-Glycoprotein has differential effects on the disposition of statin acid and lactone forms in mdr1a/b knockout and wild-type mice. Drug Metab. Dispos. 35, 1725–1729 (2007).
Moon, H., Hill, M. M., Roberts, M. J., Gardiner, R. A. & Brown, A. J. Statins: protectors or pretenders in prostate cancer? Trends Endocrinol. Metab. 25, 188–196 (2014).
Wong, W. W., Dimitroulakos, J., Minden, M. D. & Penn, L. Z. HMG-CoA reductase inhibitors and the malignant cell: the statin family of drugs as triggers of tumor-specific apoptosis. Leukemia 16, 508–519 (2002).
Wong, W. W. et al. Cerivastatin triggers tumor-specific apoptosis with higher efficacy than lovastatin. Clin. Cancer Res. 7, 2067–2075 (2001).
Dimitroulakos, J. et al. Lovastatin induces a pronounced differentiation response in acute myeloid leukemias. Leuk. Lymphoma 40, 167–178 (2000).
Martirosyan, A., Clendening, J. W., Goard, C. A. & Penn, L. Z. Lovastatin induces apoptosis of ovarian cancer cells and synergizes with doxorubicin: potential therapeutic relevance. BMC Cancer 10, 103 (2010).
Mas, E. & Mori, T. A. Coenzyme Q(10) and statin myalgia: what is the evidence? Curr. Atheroscler. Rep. 12, 407–413 (2010).
Harper, C. R. & Jacobson, T. A. Evidence-based management of statin myopathy. Curr. Atheroscler. Rep. 12, 322–330 (2010).
Bjarnadottir, O. et al. Targeting HMG-CoA reductase with statins in a window-of-opportunity breast cancer trial. Breast Cancer Res. Treat. 138, 499–508 (2013).
Garwood, E. R. et al. Fluvastatin reduces proliferation and increases apoptosis in women with high grade breast cancer. Breast Cancer Res. Treat. 119, 137–144 (2010). The first window-of-opportunity, pre-operative trial to demonstrate that fluvastatin can reduce proliferation and increase the apoptosis of tumour cells in women with early stage, high-grade breast cancer.
Graf, H. et al. Chemoembolization combined with pravastatin improves survival in patients with hepatocellular carcinoma. Digestion 78, 34–38 (2008).
Kornblau, S. M. et al. Blockade of adaptive defensive changes in cholesterol uptake and synthesis in AML by the addition of pravastatin to idarubicin + high-dose Ara-C: a phase 1 study. Blood 109, 2999–3006 (2007).
Hus, M. et al. Thalidomide, dexamethasone and lovastatin with autologous stem cell transplantation as a salvage immunomodulatory therapy in patients with relapsed and refractory multiple myeloma. Ann. Hematol. 90, 1161–1166 (2011).
Sondergaard, T. E. et al. A phase II clinical trial does not show that high dose simvastatin has beneficial effect on markers of bone tunrover in multiple myeloma. Hematol. Oncol. 27, 17–22 (2009).
Shachaf, C. M. et al. Inhibition of HMGcoA reductase by atorvastatin prevents and reverses MYC-induced lymphomagenesis. Blood 110, 2674–2684 (2007).
Hamilton, R. J. et al. Statin medication use and the risk of biochemical recurrence after radical prostatectomy: results from the Shared Equal Access Regional Cancer Hospital (SEARCH) Database. Cancer 116, 3389–3398 (2010).
Harshman, L. C. et al. Statin use at the time of initiation of androgen deprivation therapy and time to progression in patients with hormone-sensitive prostate cancer. JAMA Oncol. 1, 495–504 (2015).
Demierre, M. F., Higgins, P. D., Gruber, S. B., Hawk, E. & Lippman, S. M. Statins and cancer prevention. Nat. Rev. Cancer 5, 930–942 (2005).
Ho, Y. K., Smith, R. G., Brown, M. S. & Goldstein, J. L. Low-density lipoprotein (LDL) receptor activity in human acute myelogenous leukemia cells. Blood 52, 1099–1114 (1978).
Yue, S. et al. Cholesteryl ester accumulation induced by PTEN loss and PI3K/AKT activation underlies human prostate cancer aggressiveness. Cell Metab. 19, 393–406 (2014).
Guillaumond, F. et al. Cholesterol uptake disruption, in association with chemotherapy, is a promising combined metabolic therapy for pancreatic adenocarcinoma. Proc. Natl Acad. Sci. USA 112, 2473–2478 (2015).
Hirsch, H. A. et al. A transcriptional signature and common gene networks link cancer with lipid metabolism and diverse human diseases. Cancer Cell 17, 348–361 (2010).
Pandyra, A. A. et al. Genome-wide RNAi analysis reveals that simultaneous inhibition of specific mevalonate pathway genes potentiates tumor cell death. Oncotarget. 6, 26909–26921 (2015).
Nazarian, R. et al. Melanomas acquire resistance to B-RAF(V600E) inhibition by RTK or N-RAS upregulation. Nature 468, 973–977 (2010).
Spagnolo, F., Ghiorzo, P. & Queirolo, P. Overcoming resistance to BRAF inhibition in BRAF-mutated metastatic melanoma. Oncotarget 5, 10206–10221 (2014).
Tuerdi, G. et al. Synergistic effect of combined treatment with gamma-tocotrienol and statin on human malignant mesothelioma cells. Cancer Lett. 339, 116–127 (2013).
Krycer, J. R., Phan, L. & Brown, A. J. A key regulator of cholesterol homoeostasis, SREBP-2, can be targeted in prostate cancer cells with natural products. Biochem. J. 446, 191–201 (2012). This study highlights the potential of inhibiting SREBP2 as an anticancer therapeutic.
Kamisuki, S. et al. A small molecule that blocks fat synthesis by inhibiting the activation of SREBP. Chem. Biol. 16, 882–892 (2009).
Li, X., Chen, Y. T., Hu, P. & Huang, W. C. Fatostatin displays high antitumor activity in prostate cancer by blocking SREBP-regulated metabolic pathways and androgen receptor signaling. Mol. Cancer Ther. 13, 855–866 (2014).
Li, X., Wu, J. B., Chung, L. W. & Huang, W. C. Anti-cancer efficacy of SREBP inhibitor, alone or in combination with docetaxel, in prostate cancer harboring p53 mutations. Oncotarget 6, 41018–41032 (2015).
Goldstein, J. L. & Brown, M. S. A century of cholesterol and coronaries: from plaques to genes to statins. Cell 161, 161–172 (2015). A retrospective of the work that uncovered and helped us to understand the role of cholesterol in disease.
Saad, F. et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J. Natl Cancer Inst. 94, 1458–1468 (2002).
Aft, R. et al. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 11, 421–428 (2010).
Morgan, G. J. et al. First-line treatment with zoledronic acid as compared with clodronic acid in multiple myeloma (MRC Myeloma IX): a randomised controlled trial. Lancet 376, 1989–1999 (2010).
Harousseau, J. L. et al. A randomized phase 3 study of tipifarnib compared with best supportive care, including hydroxyurea, in the treatment of newly diagnosed acute myeloid leukemia in patients 70 years or older. Blood 114, 1166–1173 (2009).
Sparano, J. A. et al. Phase II trial of tipifarnib plus neoadjuvant doxorubicin-cyclophosphamide in patients with clinical stage IIB-IIIC breast cancer. Clin. Cancer Res. 15, 2942–2948 (2009).
Tang, J. J. et al. Inhibition of SREBP by a small molecule, betulin, improves hyperlipidemia and insulin resistance and reduces atherosclerotic plaques. Cell Metab. 13, 44–56 (2011).
Guan, M. et al. Nelfinavir induces liposarcoma apoptosis through inhibition of regulated intramembrane proteolysis of SREBP-1 and ATF6. Clin. Cancer. Res. 17, 1796–1806 (2011).
Brunner, T. B. et al. Phase I trial of the human immunodeficiency virus protease inhibitor nelfinavir and chemoradiation for locally advanced pancreatic cancer. J. Clin. Oncol. 26, 2699–2706 (2008).
Rengan, R. et al. A phase I trial of the HIV protease inhibitor nelfinavir with concurrent chemoradiotherapy for unresectable stage IIIA/IIIB non-small cell lung cancer: a report of toxicities and clinical response. J. Thorac. Oncol. 7, 709–715 (2012).
Acknowledgements
The authors thank J. van Leeuwen and W. B. Tu for helping to prepare this Review. The authors also thank other current and former members of the Penn laboratory for their helpful comments, including A. Pandyra, E. Chamberlain, J. De Melo, D. Dingar, A. Hickman, M. Kalkat, C. Lourenco, D. Resetca and A. Tamachi. The authors also acknowledge the many important contributions by their colleagues that could not be cited here owing to space and reference constraints. The funding agencies that enable the authors' research include the Ontario Institute for Cancer Research through funding provided by the Province of Ontario, the Canadian Institute for Health Research, Prostate Cancer Canada, the Department of Defense Breast Cancer Research Program, the Princess Margaret Cancer Foundation Hold'em for Life Prostate Cancer Research Fund, and the Terry Fox Foundation Canada. L.Z.P. holds the Canada Research Chair in Molecular Oncology.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Acetyl-CoA
-
An essential metabolite that is used to drive many cellular processes, including the tricarboxylic acid (TCA) cycle, fatty acid and sterol biosynthesis, and acetylation of histones.
- SREBP cleavage-activating protein
-
(SCAP). Essential for sterol regulatory element-binding protein (SREBP) endoplasmic reticulum (ER)-to-Golgi translocation. SCAP contains a sterol-sensing domain and undergoes a conformational change when levels of ER membrane sterols are low. This change causes a dissociation of the SCAP–SREBP complex from insulin-induced genes (INSIGs).
- Insulin-induced genes
-
(INSIGs). INSIG1 and INSIG2 interact with SREBP cleavage-activating protein (SCAP) under sterol-rich conditions. They prevent sterol regulatory element-binding protein (SREBP) activation by retaining the SCAP–SREBP complex in the endoplasmic reticulum (ER). They also promote the sterol-regulated degradation of 3-hydroxy-3-methylglutaryl-CoA reductase (HMGCR).
- Site-1 protease and site-2 protease
-
(S1P and S2P). Two proteases that cleave the sterol regulatory element-binding proteins (SREBPs), in the Golgi. S1P cleaves at the luminal loop of the SREBPs, whereas S2P is a hydrophobic protein that cleaves the SREBPs at a transmembrane residue.
- Sterol regulatory elements
-
(SREs). Motifs found in the promoters of genes that are transcribed in response to sterol deprivation. SREs are necessary for the transcription of mevalonate (MVA) pathway genes by the sterol regulatory element-binding proteins (SREBPs).
- Lipid rafts
-
Membrane domains that contain high concentrations of cholesterol, saturated fatty acids and sphingolipids. They are tightly packed and form the liquid ordered phase of membranes. One key role is to enable protein complexes to be pre-organized for efficient signal transduction.
- γδ T cells
-
T cells with a T cell receptor that contains a γ- and a δ-chain instead of the more common α- and β-chains. They are known to recognize lipid antigens, are independent of major histocompatibility complex (MHC) class I presentation and are currently being investigated for their anticancer potential.
- Isoprenylation
-
The attachment of a hydrophobic farnesol or geranygeraniol to the carboxyl terminus of proteins that contain a CAAX motif, which anchors the proteins to lipid membranes. Geranylgeraniol can also be attached to non-CAAX motif-containing proteins.
- Quinone
-
A cyclic organic compound that contains two C=O groups. The quinone coenzyme Q is derived from the essential amino acid tyrosine.
- C-Cell adenoma
-
C-Cells (also known as parafollicular cells) are found in the thyroid and produce the hormone calcitonin. Tumours originating from the C-cells include medullary thyroid cancer, and mutations in the RET proto-oncogene are often found in patients.
- Aromatase inhibitors
-
Inhibitors of oestrogen production and a common treatment option for patients with oestrogen receptor-positive breast cancer.
- Ki67 index
-
The fraction of Ki67-positive tumour cells as determined using immunohistochemistry. The expression of Ki67 is associated with cell proliferation.
- Dipyridamole
-
A clinically approved drug used to prevent platelet aggregation.
Rights and permissions
About this article
Cite this article
Mullen, P., Yu, R., Longo, J. et al. The interplay between cell signalling and the mevalonate pathway in cancer. Nat Rev Cancer 16, 718–731 (2016). https://doi.org/10.1038/nrc.2016.76
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc.2016.76
This article is cited by
-
Shaping immune landscape of colorectal cancer by cholesterol metabolites
EMBO Molecular Medicine (2024)
-
Lipids as mediators of cancer progression and metastasis
Nature Cancer (2024)
-
Multiplex immunohistochemistry defines two cholesterol metabolism patterns predicting immunotherapeutic outcomes in gastric cancer
Journal of Translational Medicine (2023)
-
Sex differences in FASN protein concentrations in urinary exosomes related to serum triglycerides levels in healthy adults
Lipids in Health and Disease (2023)
-
Pyroptosis, ferroptosis, and autophagy cross-talk in glioblastoma opens up new avenues for glioblastoma treatment
Cell Communication and Signaling (2023)