Key Points
-
Tumours aberrantly express various glycans. Glycans regulate many different aspects of tumour progression, including proliferation, invasion, angiogenesis and metastasis.
-
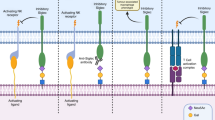
The proliferation of tumour cells is potentiated by the ability of glycoproteins and glycosphingolipids to directly activate growth-factor receptor tyrosine kinases and by the ability of proteoglycans to function as co-receptors for soluble tumour growth factors.
-
The overexpression of specific glycosyltransferases by tumour cells promotes the formation of tumour glycans that facilitate invasion.
-
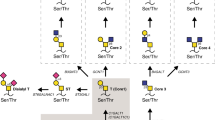
Carcinomas commonly overexpress O-linked glycans in the form of cell-surface and secreted mucins that present ligands for adhesion receptors, such as the selectins, which promote the ability of tumour cells to interact with host platelets, leukocytes and endothelial cells. These interactions facilitate haematogenous metastasis of tumour cells.
-
Glycosphingolipids, in the form of gangliosides, are overexpressed by a range of tumours, and their shedding into the bloodstream might impair host immunity to some tumours.
-
During tumour proliferation and invasion, heparan-sulphate proteoglycans (HSPGs) that are present on the surface of tumour cells function as co-receptors to stabilize growth-factor receptor signalling complexes. Secreted HSPGs that are present in the extracellular matrix store growth factors that can be mobilized by the action of tumour heparanases. A similar mechanism that involves endothelial-associated HSPGs and endothelial growth factors facilitates vascular sprouting during tumour angiogenesis.
-
Some glycans can be measured in the bloodstream, and their use as markers of disease burden can be used to screen for specific cancers as well as track response to therapy.
-
Experiments in which glycan function is genetically altered in cell-culture systems or mouse tumour models validate their potential as targets for anticancer therapy.
-
A few glycan-based targeting strategies are currently being tested in clinical trials. As we learn more about the roles of glycans in tumour progression, new targets will continue to emerge for drug design.
Abstract
A growing body of evidence supports crucial roles for glycans at various pathophysiological steps of tumour progression. Glycans regulate tumour proliferation, invasion, haematogenous metastasis and angiogenesis, and increased understanding of these roles sets the stage for developing pharmaceutical agents that target these molecules. Such novel agents might be used alone or in combination with operative and/or chemoradiation strategies for treating cancer.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Wells, L., Vosseller, K. & Hart, G. W. Glycosylation of nucleocytoplasmic proteins: signal transduction and O-GlcNAc. Science 291, 2376–2378 (2001).
Marth, J. Glycosylation changes in ontogeny and cell activation. in Essentials of Glycobiology (eds Varki, A. et al.) 515–536 (Cold Spring Harbor Laboratory Press, New York, 1999).
Varki, A. in Essentials of Glycobiology (eds Varki, A. et al.) 537–549 (Cold Spring Harbor Laboratory Press, New York, 1999).
Feizi, T. Carbohydrate antigens in human cancer. Cancer Surv. 4, 245–269 (1985).
Raedler, A. & Schreiber, S. Analysis of differentiation and transformation of cells by lectins. Crit. Rev. Clin. Lab. Sci. 26, 153–193 (1988).
Weis, W. I. & Drickamer, K. Structural basis of lectin-carbohydrate recognition. Annu. Rev. Biochem. 65, 441–473 (1996). Describes the structural basis for sugar recognition and adhesion by lectins expressed in bacteria, animals and plants.
Bogenrieder, T. & Herlyn, M. Axis of evil: molecular mechanisms of cancer metastasis. Oncogene 22, 6524–6536 (2003).
Hakomori, S. Tumor malignancy defined by aberrant glycosylation and sphingo(glyco)lipid metabolism. Cancer Res. 56, 5309–5318 (1996).
Iozzo, R. V. & San Antonio, J. D. Heparan sulfate proteoglycans: heavy hitters in the angiogenesis arena. J. Clin. Invest. 108, 349–355 (2001).
Liu, D., Shriver, Z., Qi, Y., Venkataraman, G. & Sasisekharan, R. Dynamic regulation of tumor growth and metastasis by heparan sulfate glycosaminoglycans. Semin. Thromb. Hemost. 28, 67–78 (2002).
Maeder, T. Sweet medicines. Sci. Am. 287, 40–47 (2002).
Hollingsworth, M. A. & Swanson, B. J. Mucins in cancer: protection and control of the cell surface. Nature Rev. Cancer 4, 45–60 (2004).
Toole, B. P. Hyaluronan: from extracellular glue to pericellular cue. Nature Rev. Cancer 4, 528–539 (2004).
Felsher, D. W. Reversibility of oncogene-induced cancer. Curr. Opin. Genet. Dev. 14, 37–42 (2004).
Marth, J. in Essentials of Glycobiology (eds Varki, A. et al.) 85–100 (Cold Spring Harbor Laboratory Press, New York, 1999).
Girnita, L. et al. Inhibition of N-linked glycosylation down-regulates insulin-like growth factor-1 receptor at the cell surface and kills Ewing's sarcoma cells: therapeutic implications. Anticancer Drug Des. 15, 67–72 (2000).
Bharathan, S., Moriarty, J., Moody, C. E. & Sherblom, A. P. Effect of tunicamycin on sialomucin and natural killer susceptibility of rat mammary tumor ascites cells. Cancer Res. 50, 5250–5256 (1990).
Komatsu, M., Jepson, S., Arango, M. E., Carothers Carraway, C. A. & Carraway, K. L. Muc4/sialomucin complex, an intramembrane modulator of ErbB2/HER2/Neu, potentiates primary tumor growth and suppresses apoptosis in a xenotransplanted tumor. Oncogene 20, 461–470 (2001). Demonstrates how genetic upregulation of MUC4 in tumour A375 melanoma cells inhibits apoptosis of tumour cells and promotes tumour growth in vivo . MUC4 modulates phosphorylation of the receptor tyrosine kinase in the presence and absence of the major EGF receptor ligand, heregulin.
Hakomori, S. Bifunctional role of glycosphingolipids. Modulators for transmembrane signaling and mediators for cellular interactions. J. Biol. Chem. 265, 18713–18716 (1990).
Nagy, P. et al. Lipid rafts and the local density of ErbB proteins influence the biological role of homo- and heteroassociations of ErbB2. J. Cell Sci. 115, 4251–4262 (2002).
Esko, J. D. in Essentials of Glycobiology (eds Varki, A. et al.) 145–159 (Cold Spring Harbor Laboratory Press, New York, 1999).
Esko, J. D. & Lindahl, U. Molecular diversity of heparan sulfate. J. Clin. Invest. 108, 169–173 (2001).
Bernfield, M. et al. Functions of cell surface heparan sulfate proteoglycans. Annu. Rev. Biochem. 68, 729–777 (1999).
Kleeff, J. et al. The cell-surface heparan sulfate proteoglycan glypican-1 regulates growth factor action in pancreatic carcinoma cells and is overexpressed in human pancreatic cancer. J. Clin. Invest. 102, 1662–1673 (1998).
Lai, J. et al. Loss of HSulf-1 up-regulates heparin-binding growth factor signaling in cancer. J. Biol. Chem. 278, 23107–23117 (2003). Demonstration of the importance of HSPGs in tumour proliferation. The cell-surface-associated enzyme SULF1 diminishes HSPG sulphation. Downregulation of SULF1 by tumours increases growth-factor signalling and resistance to apoptosis, representing a novel mechanism by which cancer cells can enhance growth.
DeBaun, M. R., Ess, J. & Saunders, S. Simpson Golabi Behmel syndrome: progress toward understanding the molecular basis for overgrowth, malformation, and cancer predisposition. Mol. Genet. Metab. 72, 279–286 (2001).
Filmus, J. Glypicans in growth control and cancer. Glycobiology 11, 19R–23R (2001).
Turley, E. A., Noble, P. W. & Bourguignon, L. Y. Signaling properties of hyaluronan receptors. J. Biol. Chem. 277, 4589–4592 (2002).
Simpson, M. A., Wilson, C. M. & McCarthy, J. B. Inhibition of prostate tumor cell hyaluronan synthesis impairs subcutaneous growth and vascularization in immunocompromised mice. Am. J. Pathol. 161, 849–857 (2002).
Misra, S., Ghatak, S., Zoltan-Jones, A. & Toole, B. P. Regulation of multidrug resistance in cancer cells by hyaluronan. J. Biol. Chem. 278, 25285–25288 (2003).
Chou, T. Y. & Hart, G. W. O-linked N-acetylglucosamine and cancer: messages from the glycosylation of c-Myc. Adv. Exp. Med. Biol. 491, 413–418 (2001). Discusses the importance of modification by O -GlcNAc of key cellular oncogenes or tumour-suppressor proteins. O -GlcNAc transferase glycosylates tumour-suppressor proteins at amino-acid residues that either promote oncogene activity or inhibit tumour-suppressor functions, highlighting the importance of O -GlcNAc in tumorigenesis and tumour proliferation.
Noujaim, A. A., Schultes, B. C., Baum, R. P. & Madiyalakan, R. Induction of CA125-specific B and T cell responses in patients injected with MAb-B43.13 — evidence for antibody-mediated antigen-processing and presentation of CA125 in vivo. Cancer Biother. Radiopharm. 16, 187–203 (2001).
Musselli, C. et al. Reevaluation of the cellular immune response in breast cancer patients vaccinated with MUC1. Int. J. Cancer 97, 660–667 (2002).
Ramanathan, R. K. et al. Phase I study of a MUC1 vaccine composed of different doses of MUC1 peptide with SB-AS2 adjuvant in resected and locally advanced pancreatic cancer. Cancer Immunol. Immunother. 54, 254–264 (2005).
Bestagno, M., Occhino, M., Corrias, M. V., Burrone, O. & Pistoia, V. Recombinant antibodies in the immunotherapy of neuroblastoma: perspectives of new developments. Cancer Lett. 197, 193–198 (2003).
Carr, A. et al. Immunotherapy of advanced breast cancer with a heterophilic ganglioside (NeuGcGM3) cancer vaccine. J. Clin. Oncol. 21, 1015–1021 (2003).
Thomas, T. & Thomas, T. J. Polyamine metabolism and cancer. J. Cell Mol. Med. 7, 113–126 (2003).
Belting, M. et al. Tumor attenuation by combined heparan sulfate and polyamine depletion. Proc. Natl Acad. Sci. USA 99, 371–376 (2002).
Zeng, C., Toole, B. P., Kinney, S. D., Kuo, J. W. & Stamenkovic, I. Inhibition of tumor growth in vivo by hyaluronan oligomers. Int. J. Cancer 77, 396–401 (1998). Disruption of the interaction between host tissue stromal hyaluronan and the hyaluronan receptor CD44 on tumour cells can be achieved by treatment with hyaluronan oligomers. This study illustrates how infusion with such oligomers can block growth of experimental melanomas in vivo.
Dennis, J. W., Pawling, J., Cheung, P., Partridge, E. & Demetriou, M. UDP-N-acetylglucosamine:α-6-D-mannoside β1, 6 N-acetylglucosaminyltransferase V (Mgat5) deficient mice. Biochim. Biophys. Acta. 1573, 414–422 (2002).
Yoshimura, M., Ihara, Y., Matsuzawa, Y. & Taniguchi, N. Aberrant glycosylation of E-cadherin enhances cell–cell binding to suppress metastasis. J. Biol. Chem. 271, 13811–13815 (1996).
Hirohashi, S. & Kanai, Y. Cell adhesion system and human cancer morphogenesis. Cancer Sci. 94, 575–581 (2003).
Seidenfaden, R., Krauter, A., Schertzinger, F., Gerardy-Schahn, R. & Hildebrandt, H. Polysialic acid directs tumor cell growth by controlling heterophilic neural cell adhesion molecule interactions. Mol. Cell. Biol. 23, 5908–5918 (2003). This study examines the role of polysialic acid on neural cell adhesion molecule (NCAM) in neuroblastoma cells, and demonstrates the ability of polysialic acid to control tumour-cell growth and differentiation by interfering with NCAM signalling at cell–cell contacts.
Angata, K. & Fukuda, M. Polysialyltransferases: major players in polysialic acid synthesis on the neural cell adhesion molecule. Biochimie 85, 195–206 (2003).
Lin, S., Kemmner, W., Grigull, S. & Schlag, P. M. Cell surface α 2, 6 sialylation affects adhesion of breast carcinoma cells. Exp. Cell Res. 276, 101–110 (2002).
Ju, T. & Cummings, R. D. A unique molecular chaperone Cosmc required for activity of the mammalian core 1b 3-galactosyltransferase. Proc. Natl Acad. Sci. USA 99, 16613–16618 (2002).
Julien, S. et al. Expression of sialyl-Tn antigen in breast cancer cells transfected with the human CMP-Neu5Ac: GalNAc α2, 6-sialyltransferase (ST6GalNac I) cDNA. Glycoconj. J. 18, 883–893 (2001).
Guo, H. B., Lee, I., Kamar, M., Akiyama, S. K. & Pierce, M. Aberrant N-glycosylation of β1 integrin causes reduced α5β1 integrin clustering and stimulates cell migration. Cancer Res. 62, 6837–6845 (2002).
Wozniak, M. A., Modzelewska, K., Kwong, L. & Keely, P. J. Focal adhesion regulation of cell behavior. Biochim. Biophys. Acta 1692, 103–119 (2004).
Tumova, S., Woods, A. & Couchman, J. R. Heparan sulfate proteoglycans on the cell surface: versatile coordinators of cellular functions. Int. J. Biochem. Cell Biol. 32, 269–288 (2000).
Woods, A., McCarthy, J. B., Furcht, L. T. & Couchman, J. R. A synthetic peptide from the COOH-terminal heparin-binding domain of fibronectin promotes focal adhesion formation. Mol. Biol. Cell 4, 605–613 (1993).
Saoncella, S. et al. Syndecan-4 signals cooperatively with integrins in a Rho-dependent manner in the assembly of focal adhesions and actin stress fibers. Proc. Natl Acad. Sci. USA 96, 2805–2810 (1999).
Kusano, Y., Yoshitomi, Y., Munesue, S., Okayama, M. & Oguri, K. Cooperation of syndecan-2 and syndecan-4 among cell surface heparan sulfate proteoglycans in the actin cytoskeletal organization of Lewis lung carcinoma cells. J. Biochem. (Tokyo) 135, 129–137 (2004).
Culp, L. A. et al. Heparan sulfate proteoglycans of Ras-transformed 3T3 or neuroblastoma cells. Differing functions in adhesion on fibronectin. Ann. NY Acad. Sci. 556, 194–216 (1989).
Vlodavsky, I. et al. Inhibition of tumor metastasis by heparanase inhibiting species of heparin. Invasion Metastasis 14, 290–302 (1994).
Vlodavsky, I. & Friedmann, Y. Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J. Clin. Invest. 108, 341–347 (2001). Reviews the many roles of heparanase in the tumour environment, and its importance in tumour progression.
Kato, M., Saunders, S., Nguyen, H. & Bernfield, M. Loss of cell surface syndecan-1 causes epithelia to transform into anchorage-independent mesenchyme-like cells. Mol. Biol. Cell 6, 559–576 (1995).
Beauvais, D. M. & Rapraeger, A. C. Syndecans in tumor cell adhesion and signaling. Reprod. Biol. Endocrinol. 2, 3 (2004).
Beauvais, D. M., Burbach, B. J. & Rapraeger, A. C. The syndecan-1 ectodomain regulates αvβ3 integrin activity in human mammary carcinoma cells. J. Cell Biol. 167, 171–181 (2004).
Sanderson, R. D. Heparan sulfate proteoglycans in invasion and metastasis. Semin. Cell Dev. Biol. 12, 89–98 (2001).
Iida, J., Meijne, A. M., Knutson, J. R., Furcht, L. T. & McCarthy, J. B. Cell surface chondroitin sulfate proteoglycans in tumor cell adhesion, motility and invasion. Semin. Cancer Biol. 7, 155–162 (1996).
Nutt, C. L., Zerillo, C. A., Kelly, G. M. & Hockfield, S. Brain enriched hyaluronan binding (BEHAB)/brevican increases aggressiveness of CNS-1 gliomas in Lewis rats. Cancer Res. 61, 7056–7059 (2001).
Faassen, A. E. et al. A cell surface chondroitin sulfate proteoglycan, immunologically related to CD44, is involved in type I collagen-mediated melanoma cell motility and invasion. J. Cell Biol. 116, 521–531 (1992).
Gunthert, U. et al. A new variant of glycoprotein CD44 confers metastatic potential to rat carcinoma cells. Cell 65, 13–24 (1991).
Aruffo, A., Stamenkovic, I., Melnick, M., Underhill, C. B. & Seed, B. CD44 is the principal cell surface receptor for hyaluronate. Cell 61, 1303–1313 (1990).
Jalkanen, S. & Jalkanen, M. Lymphocyte CD44 binds the COOH-terminal heparin-binding domain of fibronectin. J. Cell Biol. 116, 817–825 (1992).
Wolff, E. A. et al. Generation of artificial proteoglycans containing glycosaminoglycan-modified CD44. Demonstration of the interaction between rantes and chondroitin sulfate. J. Biol. Chem. 274, 2518–2524 (1999).
Vlodavsky, I. et al. Mammalian heparanase: gene cloning, expression and function in tumor progression and metastasis. Nature Med. 5, 793–802 (1999).
Goldshmidt, O. et al. Cell surface expression and secretion of heparanase markedly promote tumor angiogenesis and metastasis. Proc. Natl Acad. Sci. USA 99, 10031–10036 (2002).
Vlodavsky, I. et al. Molecular properties and involvement of heparanase in cancer progression and normal development. Biochimie 83, 831–839 (2001).
Jiang, X. & Couchman, J. R. Perlecan and tumor angiogenesis. J. Histochem. Cytochem. 51, 1393–1410 (2003).
Adatia, R. et al. Suppression of invasive behavior of melanoma cells by stable expression of anti-sense perlecan cDNA. Ann. Oncol. 8, 1257–1261 (1997).
Zcharia, E. et al. Molecular properties and involvement of heparanase in cancer progression and mammary gland morphogenesis. J. Mammary Gland Biol. Neoplasia 6, 311–322 (2001).
Granovsky, M. et al. Suppression of tumor growth and metastasis in Mgat5-deficient mice. Nature Med. 6, 306–312 (2000). Demonstrates the importance of N -acetylglucosaminyltransferase V (GnTV) in tumour progression by examining mammary tumour growth and metastasis in mice bearing a deletion in the enzyme.
Goss, P. E., Baptiste, J., Fernandes, B., Baker, M. & Dennis, J. W. A phase I study of swainsonine in patients with advanced malignancies. Cancer Res. 54, 1450–1457 (1994).
Schachter, H. The joys of HexNAc. The synthesis and function of N- and O-glycan branches. Glycoconj. J. 17, 465–483 (2000).
Horstkorte, R. et al. Selective inhibition of polysialyltransferase ST8SiaII by unnatural sialic acids. Exp. Cell Res. 298, 268–274 (2004).
Kakkar, A. K. An expanding role for antithrombotic therapy in cancer patients. Cancer Treat. Rev. 29 (Suppl. 2), 23–26 (2003).
Bumol, T. F. & Reisfeld, R. A. Unique glycoprotein-proteoglycan complex defined by monoclonal antibody on human melanoma cells. Proc. Natl Acad. Sci. USA 79, 1245–1249 (1982).
Olsen, E. B., Trier, K., Eldov, K. & Ammitzboll, T. Glycosaminoglycans in human breast cancer. Acta Obstet. Gynecol. Scand. 67, 539–542 (1988).
Lee, C. M. et al. Novel chondroitin sulfate-binding cationic liposomes loaded with cisplatin efficiently suppress the local growth and liver metastasis of tumor cells in vivo. Cancer Res. 62, 4282–4288 (2002).
Folkman, J. in The Molecular Basis of Cancer (eds Mendelsohn, J., Howley P. M., Israel M. A. & Liotta L. A.) 206–232 (W. B. Saunders, Philadelphia, 1995).
Marcum, J. A. & Rosenberg, R. D. Heparinlike molecules with anticoagulant activity are synthesized by cultured endothelial cells. Biochem. Biophys. Res. Commun. 126, 365–372 (1985).
Zhou, Z. et al. Impaired angiogenesis, delayed wound healing and retarded tumor growth in perlecan heparan sulfate-deficient mice. Cancer Res. 64, 4699–4702 (2004).
Sharma, B. et al. Antisense targeting of perlecan blocks tumor growth and angiogenesis in vivo. J. Clin. Invest. 102, 1599–1608 (1998).
Folkman, J. & Shing, Y. Control of angiogenesis by heparin and other sulfated polysaccharides. Adv. Exp. Med. Biol. 313, 355–364 (1992).
Zcharia, E. et al. Transgenic expression of mammalian heparanase uncovers physiological functions of heparan sulfate in tissue morphogenesis, vascularization, and feeding behavior. FASEB J. 18, 252–263 (2004).
Boehm, T., Folkman, J., Browder, T. & O'Reilly, M. S. Antiangiogenic therapy of experimental cancer does not induce acquired drug resistance. Nature 390, 404–407 (1997).
Lapierre, F. et al. Chemical modifications of heparin that diminish its anticoagulant but preserve its heparanase-inhibitory, angiostatic, anti-tumor and anti-metastatic properties. Glycobiology 6, 355–366 (1996).
Parish, C. R., Freeman, C., Brown, K. J., Francis, D. J. & Cowden, W. B. Identification of sulfated oligosaccharide-based inhibitors of tumor growth and metastasis using novel in vitro assays for angiogenesis and heparanase activity. Cancer Res. 59, 3433–3441 (1999).
Presta, M. et al. Heparin derivatives as angiogenesis inhibitors. Curr. Pharm. Des. 9, 553–566 (2003).
Liekens, S. et al. Modulation of fibroblast growth factor-2 receptor binding, signaling, and mitogenic activity by heparin-mimicking polysulfonated compounds. Mol. Pharmacol. 56, 204–213 (1999).
Jemth, P. et al. Biosynthetic oligosaccharide libraries for identification of protein-binding heparan sulfate motifs. Exploring the structural diversity by screening for fibroblast growth factor (FGF)1 and FGF2 binding. J. Biol. Chem. 277, 30567–30573 (2002).
Pisano, C. et al. Undersulfated, low-molecular-weight glycol-split heparin as an antiangiogenic VEGF antagonist. Glycobiology 15, 1C–6C (2005).
Hwang, R. & Varner, J. The role of integrins in tumor angiogenesis. Hematol. Oncol. Clin. North Am. 18, 991–1006, vii (2004).
Nguyen, M., Folkman, J. & Bischoff, J. 1-Deoxymannojirimycin inhibits capillary tube formation in vitro. Analysis of N-linked oligosaccharides in bovine capillary endothelial cells. J. Biol. Chem. 267, 26157–26165 (1992).
Krause, T. & Turner, G. A. Are selectins involved in metastasis? Clin. Exp. Metastasis 17, 183–192 (1999).
Borsig, L. et al. Pictures in molecular medicine: three-dimensional visualization of intravascular tumor cells in mice. Trends Mol. Med. 7, 377 (2001).
Cummings, R. D. Selectins. in Essentials of Glycobiology (eds Varki, A. et al.) 391–415 (Cold Spring Harbor Laboratory Press, New York, 1999).
Kim, Y. J., Borsig, L., Varki, N. M. & Varki, A. P-selectin deficiency attenuates tumor growth and metastasis. Proc. Natl Acad. Sci. USA 95, 9325–9330 (1998). Describes the observation that P-selectin is important for the formation of tumour-platelet emboli during metastasis, and facilitates haematogenous dissemination of mucin-producing carcinoma cells.
Borsig, L., Wong, R., Hynes, R. O., Varki, N. M. & Varki, A. Synergistic effects of L- and P-selectin in facilitating tumor metastasis can involve non-mucin ligands and implicate leukocytes as enhancers of metastasis. Proc. Natl Acad. Sci. USA 99, 2193–2198 (2002).
Varki, A. Selectin ligands: will the real ones please stand up? J. Clin. Invest. 100, S31–S35 (1997).
Nakamori, S. et al. Involvement of carbohydrate antigen sialyl Lewis(x) in colorectal cancer metastasis. Dis. Colon Rectum. 40, 420–431 (1997).
Baldus, S. E. et al. Histopathological subtypes and prognosis of gastric cancer are correlated with the expression of mucin-associated sialylated antigens: Sialosyl-Lewis(a), Sialosyl-Lewis(x) and sialosyl-Tn. Tumour Biol. 19, 445–453 (1998).
Nakamori, S. et al. Molecular mechanism involved in increased expression of sialyl Lewis antigens in ductal carcinoma of the pancreas. J. Exp. Clin. Cancer Res. 18, 425–432 (1999).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Almofti, A. et al. The clinicopathological significance of the expression of CXCR4 protein in oral squamous cell carcinoma. Int. J. Oncol. 25, 65–71 (2004).
Crocker, P. R. Siglecs: sialic-acid-binding immunoglobulin-like lectins in cell-cell interactions and signalling. Curr. Opin. Struct. Biol. 12, 609–615 (2002).
Brinkman-Van der Linden, E. C. & Varki, A. New aspects of siglec binding specificities, including the significance of fucosylation and of the sialyl-Tn epitope. Sialic acid-binding immunoglobulin superfamily lectins. J. Biol. Chem. 275, 8625–8632 (2000).
Liu, F. T. & Rabinovich, G. A. Galectins as modulators of tumour progression. Nature Rev. Cancer 5, 29–41 (2005).
Pearlstein, E., Salk, P. L., Yogeeswaran, G. & Karpatkin, S. Correlation between spontaneous metastatic potential, platelet-aggregating activity of cell surface extracts, and cell surface sialylation in 10 metastatic-variant derivatives of a rat renal sarcoma cell line. Proc. Natl Acad. Sci. USA 77, 4336–4339 (1980).
Fuster, M. M., Brown, J. R., Wang, L. & Esko, J. D. A disaccharide precursor of sialyl Lewis X inhibits metastatic potential of tumor cells. Cancer Res. 63, 2775–2781 (2003).
Al-Mehdi, A. B. et al. Intravascular origin of metastasis from the proliferation of endothelium-attached tumor cells: a new model for metastasis. Nature Med. 6, 100–102 (2000).
Winn, R. K., Liggitt, D., Vedder, N. B., Paulson, J. C. & Harlan, J. M. Anti-P-selectin monoclonal antibody attenuates reperfusion injury to the rabbit ear. J. Clin. Invest. 92, 2042–2047 (1993).
Fukuda, M. N. et al. A peptide mimic of E-selectin ligand inhibits sialyl Lewis X-dependent lung colonization of tumor cells. Cancer Res. 60, 450–456 (2000).
Kaila, N. & Thomas, B. E. t. Design and synthesis of sialyl Lewis(x) mimics as E- and P-selectin inhibitors. Med. Res. Rev. 22, 566–601 (2002).
Leppanen, A. et al. A novel glycosulfopeptide binds to P-selectin and inhibits leukocyte adhesion to P-selectin. J. Biol. Chem. 274, 24838–24848 (1999).
Varki, N. M. & Varki, A. Heparin inhibition of selectin-mediated interactions during the haematogenous phase of carcinoma metastasis: rationale for clinical studies in humans. Semin. Thromb. Hemost. 28, 53–66 (2002).
Orner, B. P., Derda, R., Lewis, R. L., Thomson, J. A. & Kiessling, L. L. Arrays for the combinatorial exploration of cell adhesion. J. Am. Chem. Soc. 126, 10808–10809 (2004).
Brown, J. R., Fuster, M. M., Whisenant, T. & Esko, J. D. Expression patterns of α 2, 3-sialyltransferases and α 1, 3-fucosyltransferases determine the mode of sialyl Lewis X inhibition by disaccharide decoys. J. Biol. Chem. 278, 23352–23359 (2003).
Sarkar, A. K., Rostand, K. S., Jain, R. K., Matta, K. L. & Esko, J. D. Fucosylation of disaccharide precursors of sialyl LewisX inhibit selectin-mediated cell adhesion. J. Biol. Chem. 272, 25608–25616 (1997). Demonstrates the ability of disaccharide decoys of glycosylation to alter expression of the glycan selectin ligand SLeX on the surface of lymphoma cells. This is a novel method to reduce the levels of selectin ligands on a range of carcinomas, thereby altering the metastatic potential of tumour cells.
Qian, F., Hanahan, D. & Weissman, I. L. L-selectin can facilitate metastasis to lymph nodes in a transgenic mouse model of carcinogenesis. Proc. Natl Acad. Sci. USA 98, 3976–3981 (2001).
Renkonen, J., Paavonen, T. & Renkonen, R. Endothelial and epithelial expression of sialyl Lewis(x) and sialyl Lewis(a) in lesions of breast carcinoma. Int. J. Cancer 74, 296–300 (1997).
Glithero, A. et al. Crystal structures of two H-2Db/glycopeptide complexes suggest a molecular basis for CTL cross-reactivity. Immunity 10, 63–74 (1999).
Ibrahim, N. K. & Murray, J. L. Clinical Development of the STn–KLH Vaccine (Theratope(R)). Clin. Breast Cancer 3 (Suppl. 4), 139–143 (2003). The authors describe the development of a unique tumour vaccine by conjugating a synthetic STn epitope to a high-molecular-weight carrier, KLH. The STn–KLH vaccine generates a humoral and cellular immune response, and its safety was confirmed in the ongoing Phase III trial described in the article.
Holmberg, L. A., Oparin, D. V., Gooley, T. & Sandmaier, B. M. The role of cancer vaccines following autologous stem cell rescue in breast and ovarian cancer patients: experience with the sTn–KLH vaccine (Theratope(R)). Clin. Breast Cancer 3 (Suppl. 4), 144–151 (2003).
de Kleijn, E. M. & Punt, C. J. Biological therapy of colorectal cancer. Eur. J. Cancer 38, 1016–1022 (2002).
Monzavi-Karbassi, B., Cunto-Amesty, G., Luo, P. & Kieber-Emmons, T. Use of surrogate antigens as vaccines against cancer. Hybrid Hybridomics 21, 103–109 (2002).
Lemieux, G. A. & Bertozzi, C. R. Modulating cell surface immunoreactivity by metabolic induction of unnatural carbohydrate antigens. Chem. Biol. 8, 265–275 (2001). Sialic acid is often expressed by tumour-associated oligosaccharide antigens; the authors explored whether intercepting the sialic acid biosynthetic pathway with unnatural precursors might modulate the immunogenicity of targeted tumour cells. Antibodies generated against the unnatural sialic acid directed complement-mediated lysis of tumour cells.
Wolfl, M., Batten, W. Y., Posovszky, C., Bernhard, H. & Berthold, F. Gangliosides inhibit the development from monocytes to dendritic cells. Clin. Exp. Immunol. 130, 441–448 (2002).
Caldwell, S. et al. Mechanisms of ganglioside inhibition of APC function. J. Immunol. 171, 1676–1683 (2003).
Freeze, H. H., Sampath, D. & Varki, A. α- and β-xylosides alter glycolipid synthesis in human melanoma and Chinese hamster ovary cells. J. Biol. Chem. 268, 1618–1627 (1993).
Weiss, M., Hettmer, S., Smith, P. & Ladisch, S. Inhibition of melanoma tumor growth by a novel inhibitor of glucosylceramide synthase. Cancer Res. 63, 3654–3658 (2003).
Ragupathi, G. et al. Consistent antibody response against ganglioside GD2 induced in patients with melanoma by a GD2 lactone-keyhole limpet hemocyanin conjugate vaccine plus immunological adjuvant QS-21. Clin. Cancer Res. 9, 5214–5220 (2003).
Slovin, S. F. et al. Carbohydrate vaccines in cancer: immunogenicity of a fully synthetic globo H hexasaccharide conjugate in man. Proc. Natl Acad. Sci. USA 96, 5710–5715 (1999).
Pagnan, G. et al. Delivery of c-myb antisense oligodeoxynucleotides to human neuroblastoma cells via disialoganglioside GD(2)-targeted immunoliposomes: antitumor effects. J. Natl Cancer Inst. 92, 253–261 (2000).
Nakagoe, T. et al. Difference in prognostic value between sialyl Lewis(a) and sialyl Lewis(x) antigen levels in the preoperative serum of gastric cancer patients. J. Clin. Gastroenterol. 34, 408–415 (2002).
Nakagoe, T. et al. Preoperative serum level of CA19–9 predicts recurrence after curative surgery in node-negative colorectal cancer patients. Hepatogastroenterology 50, 696–699 (2003).
Satoh, H. et al. Predictive value of preoperative serum sialyl Lewis X-i antigen levels in non-small cell lung cancer. Anticancer Res. 18, 2865–2868 (1998).
Seelenmeyer, C., Wegehingel, S., Lechner, J. & Nickel, W. The cancer antigen CA125 represents a novel counter receptor for galectin-1. J. Cell Sci. 116, 1305–1318 (2003).
Hayashi, N. et al. Association between expression levels of CA 19–9 and N-acetylglucosamine-β;1, 3-galactosyltransferase 5 gene in human pancreatic cancer tissue. Pathobiology 71, 26–34 (2004).
Schroeder, J. A., Adriance, M. C., Thompson, M. C., Camenisch, T. D. & Gendler, S. J. MUC1 alters β-catenin-dependent tumor formation and promotes cellular invasion. Oncogene 22, 1324–1332 (2003).
Luchansky, S. J., Goon, S. & Bertozzi, C. R. Expanding the diversity of unnatural cell-surface sialic acids. Chembiochem. 5, 371–374 (2004).
Saxon, E. & Bertozzi, C. R. Cell surface engineering by a modified Staudinger reaction. Science 287, 2007–2010 (2000).
Dwek, R. A., Butters, T. D., Platt, F. M. & Zitzmann, N. Targeting glycosylation as a therapeutic approach. Nature Rev. Drug Discov. 1, 65–75 (2002).
Brown, J. R., Fuster, M. M. & Esko, J. D. in Carbohydrate Based Drug Discovery, Vol. 2 (ed. Wong, C.-H.) 883–898 (Wiley VCH, Weinheim, 2003).
Fritz, T. A., Lugemwa, F. N., Sarkar, A. K. & Esko, J. D. Biosynthesis of heparan sulfate on β-D-xylosides depends on aglycone structure. J. Biol. Chem. 269, 300–307 (1994).
Dimitroff, C. J., Sharma, A. & Bernacki, R. J. Cancer metastasis: a search for therapeutic inhibition. Cancer Invest. 16, 279–290 (1998).
Prescher, J. A., Dube, D. H. & Bertozzi, C. R. Chemical remodelling of cell surfaces in living animals. Nature 430, 873–877 (2004).
Takenaka, Y., Fukumori, T. & Raz, A. Galectin-3 and metastasis. Glycoconj. J. 19, 543–549 (2004).
Kojima, N. & Hakomori, S. Cell adhesion, spreading, and motility of GM3-expressing cells based on glycolipid-glycolipid interaction. J. Biol. Chem. 266, 17552–17558 (1991).
Pili, R. et al. The α-glucosidase I inhibitor castanospermine alters endothelial cell glycosylation, prevents angiogenesis, and inhibits tumor growth. Cancer Res. 55, 2920–2926 (1995).
Kleeff, J. et al. Stable transfection of a glypican-1 antisense construct decreases tumorigenicity in PANC-1 pancreatic carcinoma cells. Pancreas 19, 281–288 (1999).
Miyamoto, S. et al. Heparin-binding EGF-like growth factor is a promising target for ovarian cancer therapy. Cancer Res. 64, 5720–5727 (2004).
Derksen, P. W. et al. Cell surface proteoglycan syndecan-1 mediates hepatocyte growth factor binding and promotes Met signaling in multiple myeloma. Blood 99, 1405–1410 (2002).
Mercurio, A. M., Bachelder, R. E., Bates, R. C. & Chung, J. Autocrine signaling in carcinoma: VEGF and the α6β4 integrin. Semin. Cancer Biol. 14, 115–122 (2004).
Syrokou, A., Tzanakakis, G. N., Hjerpe, A. & Karamanos, N. K. Proteoglycans in human malignant mesothelioma. Stimulation of their synthesis induced by epidermal, insulin and platelet-derived growth factors involves receptors with tyrosine kinase activity. Biochimie 81, 733–744 (1999).
George, D. Targeting PDGF receptors in cancer—rationales and proof of concept clinical trials. Adv. Exp. Med. Biol. 532, 141–151 (2003).
Wang, L., Fuster, M. M., Sriranardo, P. & Esko, J. D. Endothelial deficiency of heparan sulphate impairs L-selectin and chemokine mediated neutrophil trafficking during inflammatory responses. Nature Immunol. (in the press).
Acknowledgements
We would like to thank A. Varki for many helpful comments. This work was supported by a Research Career Development Award from the US Department of Veteran's Affairs (to M.F.) and National Institutes of Health (to J.D.E.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
DATABASES
Entrez Gene
National Cancer Institute
FURTHER INFORMATION
Complex Carbohydrate Research Center
Consortium for Functional Glycomics
Glycobiology Research and Training Center
Glossary
- GLYCOCONJUGATE
-
A molecule in which one or more glycan units are covalently linked to a non-carbohydrate entity.
- N-LINKED GLYCANS
-
Glycans covalently linked to an asparagine residue of a polypeptide chain in the consensus sequence–Asn–X–Ser/Thr.
- GLYCOPROTEIN
-
A protein with one or more covalently bound glycans.
- O-LINKED GLYCANS
-
Glycans glycosidically linked to the hydroxyl group of the amino acids serine, threonine, tyrosine or hydroxylysine.
- MUCINS
-
Large glycoproteins with a high content of serine, threonine and proline residues, and numerous O-linked glycans, often occurring in clusters on the polypeptide. Tumour mucins are often decorated by unique small glycans such as Tn (O-linked GalNAc) or sialyl Tn antigens (sialic acid-capped O-linked GalNAc).
- PROTEOGLYCAN
-
A protein with one or more covalently attached glycosaminoglycan chains, such as heparan sulphate or chondroitin sulphate (and dermatan sulphate). In the tumour environment, a range of heparan-sulphate proteoglycans expressed by both tumour cells as well as endothelial cells affect growth-factor signalling.
- GLYCOCALYX
-
The cell-coat structure consisting of glycans and glycoconjugates surrounding animal cells. This is seen as an electron-dense layer by electron microscopy.
- LECTIN
-
A protein (other than an anti-carbohydrate antibody) that specifically recognizes and binds to glycans without catalysing a modification of the glycan.
- EGF-RECEPTOR FAMILY
-
Epidermal growth factor receptors have important roles in initiating the signalling that directs the behaviour of epithelial cells and tumours of epithelial origin. The four members of the family are also known as ERBB receptor tyrosine kinases (ERBB1–4), and share structural and functional similarities.
- GANGLIOSIDES
-
Anionic glycosphingolipids containing one or more residues of sialic acid.
- LIPID RAFTS
-
Microdomains in the plasma membrane that are enriched in sphingolipids, cholesterol and GPI-linked proteins. They function as signalling platforms through their ability to concentrate signalling proteins, resulting in increased output from receptors that require cross-activating interactions and increasing local concentrations of other downstream signalling components.
- SELECTINS
-
C-type calcium-dependent lectin expressed by cells in the vasculature and bloodstream. The three known selectins are L-selectin/CD62L (expressed by most leukocytes), E-selectin/CD62E (expressed by cytokine-activated endothelial cells) and P-selectin/CD62P (expressed by activated endothelial cells and platelets). Important ligands for the selectins include glycans containing sialyl Lewis X and sialyl Lewis A.
- METASTATIC SEEDING
-
The colonization of an organ or tissue by metastatic tumour cells.
- LEWIS TYPE BLOOD GROUP ANTIGENS
-
A structurally similar set of fucose-containing (α1-3-fucosylated) oligosaccharides found on normal epithelia and blood cells, a few of which (for example, the sialyl Lewis X or sialyl Lewis Y antigens) are overexpressed on the surface of certain epithelial tumour cells.
Rights and permissions
About this article
Cite this article
Fuster, M., Esko, J. The sweet and sour of cancer: glycans as novel therapeutic targets. Nat Rev Cancer 5, 526–542 (2005). https://doi.org/10.1038/nrc1649
Issue Date:
DOI: https://doi.org/10.1038/nrc1649
This article is cited by
-
Novel heavily fucosylated glycans as a promising therapeutic target in colorectal cancer
Journal of Translational Medicine (2023)
-
Targeting Tn-positive tumors with an afucosylated recombinant anti-Tn IgG
Scientific Reports (2023)
-
Inter- and intratumoral proteomics and glycosaminoglycan characterization of ALK rearranged lung adenocarcinoma tissues: a pilot study
Scientific Reports (2023)
-
Genetically encoded chemical crosslinking of carbohydrate
Nature Chemistry (2023)
-
The lectin DrfL inhibits cell migration, adhesion and triggers autophagy-dependent cell death in glioma cells
Glycoconjugate Journal (2023)