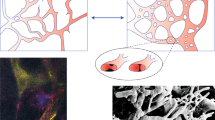
Abstract
Networks of blood vessels in normal and tumour tissues have heterogeneous structures, with widely varying blood flow pathway lengths. To achieve efficient blood flow distribution, mechanisms for the structural adaptation of vessel diameters must be able to inhibit the formation of functional shunts (whereby short pathways become enlarged and flow bypasses long pathways). Such adaptation requires information about tissue metabolic status to be communicated upstream to feeding vessels, through conducted responses. We propose that impaired vascular communication in tumour microvascular networks, leading to functional shunting, is a primary cause of dysfunctional microcirculation and local hypoxia in cancer. We suggest that anti-angiogenic treatment of tumours may restore vascular communication and thereby improve or normalize flow distribution in tumour vasculature.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
References
Duling, B. R. & Damon, D. H. An examination of the measurement of flow heterogeneity in striated muscle. Circ. Res. 60, 1–13 (1987).
Decking, U. K. Spatial heterogeneity in the heart: recent insights and open questions. News Physiol. Sci. 17, 246–250 (2002).
Pries, A. R., Secomb, T. W. & Gaehtgens, P. Structure and hemodynamics of microvascular networks: heterogeneity and correlations. Am. J. Physiol. 269, H1713–H1722 (1995).
Pries, A. R. & Secomb, T. W. Origins of heterogeneity in tissue perfusion and metabolism. Cardiovasc. Res. 81, 328–335 (2009).
Carmeliet, P. Angiogenesis in life, disease and medicine. Nature 438, 932–936 (2005).
Gewirtz, H., Tawakol, A. & Bacharach, S. L. Heterogeneity of myocardial blood flow and metabolism: review of physiologic principles and implications for radionuclide imaging of the heart. J. Nucl. Cardiol. 9, 534–541 (2002).
Bassingthwaighte, J. B., King, R. B. & Roger, S. A. Fractal nature of regional myocardial blood flow heterogeneity. Circ. Res. 65, 578–590 (1989).
Jain, R. K. Lessons from multidisciplinary translational trials on anti-angiogenic therapy of cancer. Nature Rev. Cancer 8, 309–316 (2008).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Horsman, M. R. & Siemann, D. W. Pathophysiologic effects of vascular-targeting agents and the implications for combination with conventional therapies. Cancer Res. 66, 11520–11539 (2006).
Bergers, G. & Hanahan, D. Modes of resistance to anti-angiogenic therapy. Nature Rev. Cancer 8, 592–603 (2008).
Martinez-Lemus, L. A., Hill, M. A. & Meininger, G. A. The plastic nature of the vascular wall: a continuum of remodeling events contributing to control of arteriolar diameter and structure. Physiology (Bethesda) 24, 45–57 (2009).
Pries, A. R., Reglin, B. & Secomb, T. W. Remodeling of blood vessels: responses of diameter and wall thickness to hemodynamic and metabolic stimuli. Hypertension 46, 726–731 (2005).
Zakrzewicz, A., Secomb, T. W. & Pries, A. R. Angioadaptation: keeping the vascular system in shape. News Physiol. Sci. 17, 197–201 (2002).
Dzau, V. J. & Gibbons, G. H. Vascular remodeling: mechanisms and implications. J. Cardiovasc. Pharmacol. 21, S1–S5 (1993).
Pries, A. R., Reglin, B. & Secomb, T. W. Structural adaptation of microvascular networks: functional roles of adaptive responses. Am. J. Physiol. 281, H1015–H1025 (2001).
Kamiya, A., Ando, J., Shibata, M. & Masuda, H. Roles of fluid shear stress in physiological regulation of vascular structure and function. Biorheology 25, 271–278 (1988).
Langille, B. L. & O'Donnell, F. Reductions in arterial diameter produced by chronic decreases in blood flow are endothelium-dependent. Science 231, 405–407 (1986).
Langille, B. L. Arterial remodeling: relation to hemodynamics. Can. J. Physiol. Pharmacol. 74, 834–841 (1996).
Rodbard, S. Vascular caliber. Cardiology 60, 4–49 (1975).
Pries, A. R., Secomb, T. W. & Gaehtgens, P. Structural adaptation and stability of microvascular networks: theory and simulations. Am. J. Physiol. 275, H349–H360 (1998).
Ellis, C. G., Bateman, R. M., Sharpe, M. D., Sibbald, W. J. & Gill, R. Effect of a maldistribution of microvascular blood flow on capillary O2 extraction in sepsis. Am. J. Physiol. Heart Circ. Physiol. 282, H156–H164 (2002).
Pries, A. R., Reglin, B. & Secomb, T. W. Structural response of microcirculatory networks to changes in demand: information transfer by shear stress. Am. J. Physiol. 284, H2204–H2212 (2003).
Berne, R. M., Knabb, R. M., Ely, S. W. & Rubio, R. Adenosine in the local regulation of blood flow: a brief overview. Fed. Proc. 42, 3136–3142 (1983).
Arciero, J. C., Carlson, B. E. & Secomb, T. W. Theoretical model of metabolic blood flow regulation: roles of ATP release by red blood cells and conducted responses. Am. J. Physiol. Heart Circ. Physiol. 295, H1562–H1571 (2008).
Ellsworth, M. L. Red blood cell-derived ATP as a regulator of skeletal muscle perfusion. Med. Sci. Sports Exerc. 36, 35–41 (2004).
Cosby, K. et al. Nitrite reduction to nitric oxide by deoxyhemoglobin vasodilates the human circulation. Nature Med. 9, 1498–1505 (2003).
Stamler, J. S. et al. Blood flow regulation by S-nitrosohemoglobin in the physiological oxygen gradient. Science 276, 2034–2037 (1997).
Bakker, E. N. et al. Small artery remodeling depends on tissue-type transglutaminase. Circ. Res. 96, 119–126 (2005).
Bakker, E. N. et al. Inward remodeling follows chronic vasoconstriction in isolated resistance arteries. J. Vasc. Res. 39, 12–20 (2002).
Segal, S. S. & Duling, B. R. Flow control among microvessels coordinated by intercellular conduction. Science 234, 868–870 (1986).
Figueroa, X. F. & Duling, B. R. Gap junctions in the control of vascular function. Antioxid. Redox Signal. 11, 251–266 (2009).
de Wit, C., Wolfle, S. E. & Hopfl, B. Connexin-dependent communication within the vascular wall: contribution to the control of arteriolar diameter. Adv. Cardiol. 42, 268–283 (2006).
Brisset, A. C., Isakson, B. E. & Kwak, B. R. Connexins in vascular physiology and pathology. Antioxid. Redox Signal. 11, 267–282 (2009).
Little, T. L., Beyer, E. C. & Duling, B. R. Connexin 43 and connexin 40 gap junctional proteins are present in arteriolar smooth muscle and endothelium in vivo. Am. J. Physiol. 268, H729–H739 (1995).
Johnstone, S., Isakson, B. & Locke, D. Biological and biophysical properties of vascular connexin channels. Int. Rev. Cell Mol. Biol. 278, 69–118 (2009).
Wolfle, S. E. et al. Connexin45 cannot replace the function of connexin40 in conducting endothelium-dependent dilations along arterioles. Circ. Res. 101, 1292–1299 (2007).
de Wit, C., Roos, F., Bolz, S. S. & Pohl, U. Lack of vascular connexin 40 is associated with hypertension and irregular arteriolar vasomotion. Physiol. Genomics 13, 169–177 (2003).
Figueroa, X. F. et al. Central role of connexin40 in the propagation of electrically activated vasodilation in mouse cremasteric arterioles in vivo. Circ. Res. 92, 793–800 (2003).
Liao, Y., Day, K. H., Damon, D. N. & Duling, B. R. Endothelial cell-specific knockout of connexin 43 causes hypotension and bradycardia in mice. Proc. Natl Acad. Sci. USA 98, 9989–9994 (2001).
de Wit, C. Different pathways with distinct properties conduct dilations in the microcirculation in vivo. Cardiovasc. Res. 85, 604–613 (2010).
Heyman, N. S., Kurjiaka, D. T., Ek Vitorin, J. F. & Burt, J. M. Regulation of gap junctional charge selectivity in cells coexpressing connexin 40 and connexin 43. Am. J. Physiol. Heart Circ. Physiol. 297, H450–H459 (2009).
Bukauskas, F. F., Angele, A. B., Verselis, V. K. & Bennett, M. V. Coupling asymmetry of heterotypic connexin 45/ connexin 43-EGFP gap junctions: properties of fast and slow gating mechanisms. Proc. Natl Acad. Sci. USA 99, 7113–7118 (2002).
Cottrell, G. T. & Burt, J. M. Heterotypic gap junction channel formation between heteromeric and homomeric Cx40 and Cx43 connexons. Am. J. Physiol. Cell Physiol. 281, C1559–C1567 (2001).
Cottrell, G. T., Wu, Y. & Burt, J. M. Cx40 and Cx43 expression ratio influences heteromeric/ heterotypic gap junction channel properties. Am. J. Physiol. Cell Physiol. 282, C1469–C1482 (2002).
Cottrell, G. T. & Burt, J. M. Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim. Biophys. Acta 1711, 126–141 (2005).
Rackauskas, M. et al. Gating properties of heterotypic gap junction channels formed of connexins 40, 43, and 45. Biophys. J. 92, 1952–1965 (2007).
Ebong, E. E., Kim, S. & DePaola, N. Flow regulates intercellular communication in HAEC by assembling functional Cx40 and Cx37 gap junctional channels. Am. J. Physiol. Heart Circ. Physiol. 290, H2015–H2023 (2006).
Theis, M. et al. Endothelium-specific replacement of the connexin43 coding region by a lacZ reporter gene. Genesis 29, 1–13 (2001).
Gabriels, J. E. & Paul, D. L. Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ. Res. 83, 636–643 (1998).
Pries, A. R. et al. Structural adaptation and heterogeneity of normal and tumor microvascular networks. PLoS Comput. Biol. 5, e1000394 (2009).
Ince, C. The microcirculation is the motor of sepsis. Crit. Care 9 (Suppl. 4) S13–S19 (2005).
Tyml, K., Li, F. & Wilson, J. X. Delayed ascorbate bolus protects against maldistribution of microvascular blood flow in septic rat skeletal muscle. Crit. Care Med. 33, 1823–1828 (2005).
Lauterbach, M. et al. Shunting of the microcirculation after mesenteric ischemia and reperfusion is a function of ischemia time and increases mortality. Microcirculation 13, 411–422 (2006).
Kruger, O. et al. Defective vascular development in connexin 45-deficient mice. Development 127, 4179–4193 (2000).
Simon, A. M. & McWhorter, A. R. Vascular abnormalities in mice lacking the endothelial gap junction proteins connexin37 and connexin40. Dev. Biol. 251, 206–220 (2002).
Simon, A. M. & McWhorter, A. R. Role of connexin37 and connexin40 in vascular development. Cell Commun. Adhes. 10, 379–385 (2003).
Limaye, N., Boon, L. M. & Vikkula, M. From germline towards somatic mutations in the pathophysiology of vascular anomalies. Hum. Mol. Genet. 18, R65–R74 (2009).
Brouillard, P. & Vikkula, M. Genetic causes of vascular malformations. Hum. Mol. Genet. 16, R140–R149 (2007).
Tille, J. C. & Pepper, M. S. Hereditary vascular anomalies: new insights into their pathogenesis. Arterioscler. Thromb. Vasc. Biol. 24, 1578–1590 (2004).
Buschmann, I. et al. Pulsatile shear and Gja5 modulate arterial identity and remodeling events during flow-driven arteriogenesis. Development 137, 2187–2196 (2010).
Elzarrad, M. K. et al. Connexin-43 upregulation in micrometastases and tumor vasculature and its role in tumor cell attachment to pulmonary endothelium. BMC Med. 6, 20 (2008).
van Beijnum, J. R. et al. Gene expression of tumor angiogenesis dissected: specific targeting of colon cancer angiogenic vasculature. Blood 108, 2339–2348 (2006).
Seaman, S. et al. Genes that distinguish physiological and pathological angiogenesis. Cancer Cell 11, 539–554 (2007).
Nebert, D. W. & Mason, H. S. An electron spin resonance study of neoplasms. Cancer Res. 23, 833–840 (1963).
Chen, C. N., Hsieh, F. J., Cheng, Y. M., Chang, K. J. & Lee, P. H. Expression of inducible nitric oxide synthase and cyclooxygenase-2 in angiogenesis and clinical outcome of human gastric cancer. J. Surg. Oncol. 94, 226–233 (2006).
Solan, J. L. et al. Phosphorylation at S365 is a gatekeeper event that changes the structure of Cx43 and prevents down-regulation by PKC. J. Cell Biol. 179, 1301–1309 (2007).
Upham, B. L., Kang, K. S., Cho, H. Y. & Trosko, J. E. Hydrogen peroxide inhibits gap junctional intercellular communication in glutathione sufficient but not glutathione deficient cells. Carcinogenesis 18, 37–42 (1997).
Suarez, S. & Ballmer-Hofer, K. VEGF transiently disrupts gap junctional communication in endothelial cells. J. Cell Sci. 114, 1229–1235 (2001).
Fukumura, D. & Jain, R. K. Tumor microvasculature and microenvironment: targets for anti-angiogenesis and normalization. Microvasc. Res. 74, 72–84 (2007).
Jain, R. K., Finn, A. V., Kolodgie, F. D., Gold, H. K. & Virmani, R. Antiangiogenic therapy for normalization of atherosclerotic plaque vasculature: a potential strategy for plaque stabilization. Nature Clin. Pract. Cardiovasc. Med. 4, 491–502 (2007).
Zhong, H. & Bowen, J. P. Antiangiogenesis drug design: multiple pathways targeting tumor vasculature. Curr. Med. Chem. 13, 849–862 (2006).
Acknowledgements
This work was supported by the US National Institutes of Health (NIH) grants CA040355 and HL034555. The authors thank B. Reglin for stimulating discussions and for help with figures 4–6.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Glossary
- Conducted response
-
A signal that is propagated along the wall of a blood vessel through the gap junctions that connect adjacent endothelial and/or smooth muscle cells. In a conducted vasomotor response, a stimulus applied at one point on a vessel elicits a contraction or dilation at another point. Some studies show that conducted responses can propagate for several millimetres without appreciable decay, implying that they regenerate themselves in a manner analogous to that used by nerve impulses.
- Distal
-
Further from the heart. Capillaries are the most distal vessels.
- Gap junction
-
A molecular structure that forms a passage connecting the cytoplasm of two adjacent cells and selectively allows solutes to pass between the cells. A gap junction consists of two linked hemichannels, called connexons, each embedded in the membrane of one of the cells. Each hemichannel consists of six connexins, which are proteins with multiple membrane-spanning domains.
- Proximal
-
Closer to the heart — that is, upstream in arterial vessels and downstream in venous vessels.
- Structural adaptation
-
Also known as vascular remodelling. Long-term changes in the structure of blood vessels (such as their diameter and wall mass) that occur during growth, in response to changing functional needs and in various disease states.
- Wall shear stress
-
The mechanical tangential force per unit area that is exerted on the walls of blood vessels by flowing blood as a consequence of the blood viscosity. It is directed parallel to the surface of the wall in the direction of flow and is transmitted to the endothelial cells, which can respond to changes in wall shear stress. In a cylindrical microvessel, wall shear stress is given by the formula τ = (D/4) × (ΔP/L), where ΔP is the change in pressure, L is the length of the vessel, and D is the diameter of the vessel.
Rights and permissions
About this article
Cite this article
Pries, A., Höpfner, M., le Noble, F. et al. The shunt problem: control of functional shunting in normal and tumour vasculature. Nat Rev Cancer 10, 587–593 (2010). https://doi.org/10.1038/nrc2895
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc2895
This article is cited by
-
The diagnostic performance of ultrafast MRI to differentiate benign from malignant breast lesions: a systematic review and meta-analysis
European Radiology (2024)
-
Prediction of pseudoprogression in post-treatment glioblastoma using dynamic susceptibility contrast-derived oxygenation and microvascular transit time heterogeneity measures
European Radiology (2023)
-
Fibrin-based factor delivery for therapeutic angiogenesis: friend or foe?
Cell and Tissue Research (2022)
-
Vessel size and perfusion-derived vascular habitat refines prediction of treatment failure to bevacizumab in recurrent glioblastomas: validation in a prospective cohort
European Radiology (2022)
-
Contrast enhancing pattern on pre-treatment MRI predicts response to anti-angiogenic treatment in recurrent glioblastoma: comparison of bevacizumab and temozolomide treatment
Journal of Neuro-Oncology (2022)