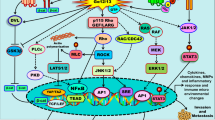
Key Points
-
Recent cancer genome deep sequencing efforts have revealed an unanticipated high frequency of mutations in G proteins and G-protein-coupled receptors (GPCRs) in most tumour types.
-
A striking 4.2% of all tumour sequences deposited to date show activating mutations in GNAS (a complex locus that encodes Gαs). Transforming mutations in GNAS have been well documented in human thyroid and pituitary tumours, and recent sequencing efforts have shown these mutations to be present in a wide variety of additional tumour types, including colon cancer, hepatocellular carcinoma, and parathyroid, ovarian, endometrial, biliary tract and pancreatic tumours.
-
Mutually exclusive activating mutations in GNAQ or GNA11 (encoding Gαq family members) occur in 5.6% of tumours, and they are present in ∼66% and ∼6% of melanomas arising in the eye and skin, respectively, where they can act as driver oncogenes.
-
Hotspot mutations in Gαs (R201 and Q227) as well as Gαq and Gα11 (R183 and Q209) disrupt the GTPase activity, thereby leading to constitutive activity and persistent signalling.
-
Nearly 20% of human cancers harbour mutations in GPCRs.
-
The most frequently mutated GPCRs include thyroid-stimulating hormone receptor (TSHR), Smoothened (SMO), glutamate metabotropic receptors (GRMs), members of the adhesion family of GPCRs and receptors for bioactive lipid mediators such as lysophosphatidic acid (LPA) and sphingosine-1-phosphate (S1P) that accumulate in the tumour microenvironment.
-
Many GPCR mutations are still uncharacterized with respect to their potential contribution to tumorigenesis and cancer progression.
-
Aberrant expression, overexpression or signal reprogramming of GPCRs and G proteins in tumour cells can contribute to cancer development and progression. These alterations may arise from cancer-specific changes in gene copy number, as well as from other genetic, epigenetic and post-translational changes resulting in higher protein expression, thereby enhancing tumour progression and metastasis.
-
Detailed three dimensional structures of GPCRs in various activation states can now help to explain the functional impact of cancer-associated GPCR mutations, and guide the rational design of signalling-selective GPCR agonists, antagonists and allosteric modulators.
-
G proteins, GPCRs and their linked signalling circuitry represent novel therapeutic targets for cancer prevention and treatment.
Abstract
Aberrant expression and activity of G proteins and G-protein-coupled receptors (GPCRs) are frequently associated with tumorigenesis. Deep sequencing studies show that 4.2% of tumours carry activating mutations in GNAS (encoding Gαs), and that oncogenic activating mutations in genes encoding Gαq family members (GNAQ or GNA11) are present in ∼66% and ∼6% of melanomas arising in the eye and skin, respectively. Furthermore, nearly 20% of human tumours harbour mutations in GPCRs. Many human cancer-associated viruses also express constitutively active viral GPCRs. These studies indicate that G proteins, GPCRs and their linked signalling circuitry represent novel therapeutic targets for cancer prevention and treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Pierce, K. L., Premont, R. T. & Lefkowitz, R. J. Seven-transmembrane receptors. Nature Rev. Mol. Cell Biol. 3, 639–650 (2002).
Ma, P. & Zemmel, R. Value of novelty? Nature Rev. Drug Discov. 1, 571–572 (2002).
Rask-Andersen, M., Almen, M. S. & Schioth, H. B. Trends in the exploitation of novel drug targets. Nature Rev. Drug Discov. 10, 579–590 (2011).
Rosenbaum, D. M., Rasmussen, S. G. & Kobilka, B. K. The structure and function of G-protein-coupled receptors. Nature 459, 356–363 (2009).
DeWire, S. M., Ahn, S., Lefkowitz, R. J. & Shenoy, S. K. β-arrestins and cell signaling. Annu. Rev. Physiol. 69, 483–510 (2007).
Garcia-Marcos, M., Ghosh, P. & Farquhar, M. G. GIV is a nonreceptor GEF for Gαi with a unique motif that regulates Akt signaling. Proc. Natl Acad. Sci. USA 106, 3178–3183 (2009).
Marty, C. & Ye, R. D. Heterotrimeric G protein signaling outside the realm of seven transmembrane domain receptors. Mol. Pharmacol. 78, 12–18 (2010).
Knoblich, J. A. Asymmetric cell division: recent developments and their implications for tumour biology. Nature Rev. Mol. Cell Biol. 11, 849–860 (2010).
Mauser, J. F. & Prehoda, K. E. Inscuteable regulates the Pins-Mud spindle orientation pathway. PLoS ONE 7, e29611 (2012).
Salon, J. A., Lodowski, D. T. & Palczewski, K. The significance of G protein-coupled receptor crystallography for drug discovery. Pharmacol. Rev. 63, 901–937 (2011).
Xu, F. et al. Structure of an agonist-bound human A2A adenosine receptor. Science 332, 322–327 (2011).
Rasmussen, S. G. et al. Crystal structure of the β2 adrenergic receptor-Gs protein complex. Nature 477, 549–555 (2011).
Wu, B. et al. Structures of the CXCR4 chemokine GPCR with small-molecule and cyclic peptide antagonists. Science 330, 1066–1071 (2010).
Ballesteros, J. A. et al. Activation of the β2-adrenergic receptor involves disruption of an ionic lock between the cytoplasmic ends of transmembrane segments 3 and 6. J. Biol. Chem. 276, 29171–29177 (2001).
Montaner, S., Kufareva, I., Abagyan, R. & Gutkind, J. S. Molecular mechanisms deployed by virally encoded G protein-coupled receptors in human diseases. Annu. Rev. Pharmacol. Toxicol. 53, 331–354 (2013).
Smit, M. J. et al. Pharmacogenomic and structural analysis of constitutive g protein-coupled receptor activity. Annu. Rev. Pharmacol. Toxicol. 47, 53–87 (2007).
Han, X., Tachado, S. D., Koziel, H. & Boisvert, W. A. Leu1283.43 (L128) and Val2476.40 (V247) of CXCR1 are critical amino acid residues for G protein coupling and receptor activation. PLoS ONE 7, e42765 (2012).
Young, D., Waitches, G., Birchmeier, C., Fasano, O. & Wigler, M. Isolation and characterization of a new cellular oncogene encoding a protein with multiple potential transmembrane domains. Cell 45, 711–719 (1986). This ground-breaking work was the first study to identify a gene that encodes a seven-transmembrane receptor, named MAS1 , as an oncogene.
Julius, D., Livelli, T. J., Jessell, T. M. & Axel, R. Ectopic expression of the serotonin 1c receptor and the triggering of malignant transformation. Science 244, 1057–1062 (1989).
Gutkind, J. S., Novotny, E. A., Brann, M. R. & Robbins, K. C. Muscarinic acetylcholine receptor subtypes as agonist-dependent oncogenes. Proc. Natl Acad. Sci. USA 88, 4703–4707 (1991). This study revealed the transforming potential of normal GPCRs when stimulated by the unrestricted availability of their ligands, and that G protein coupling-specificity is a major determinant of the oncogenic activity of GPCRs.
Allen, L. F., Lefkowitz, R. J., Caron, M. G. & Cotecchia, S. G-protein-coupled receptor genes as protooncogenes: constitutively activating mutation of the α1B-adrenergic receptor enhances mitogenesis and tumorigenicity. Proc. Natl Acad. Sci. USA 88, 11354–11358 (1991).
Parma, J. et al. Somatic mutations in the thyrotropin receptor gene cause hyperfunctioning thyroid adenomas. Nature 365, 649–651 (1993). This study provided the first description of a mutant GPCR that is involved in tumorigenesis.
Dhanasekaran, N., Heasley, L. E. & Johnson, G. L. G protein-coupled receptor systems involved in cell growth and oncogenesis. Endocr. Rev. 16, 259–270 (1995).
Dorsam, R. T. & Gutkind, J. S. G-protein-coupled receptors and cancer. Nature Rev. Cancer 7, 79–94 (2007).
Weinstein, L. S. et al. Activating mutations of the stimulatory G protein in the McCune-Albright syndrome. N. Engl. J. Med. 325, 1688–1695 (1991).
Landis, C. A. et al. GTPase inhibiting mutations activate the α chain of Gs and stimulate adenylyl cyclase in human pituitary tumours. Nature 340, 692–696 (1989). This seminal manuscript provided a causal link between the mutational activation of a G protein and human tumours.
Lyons, J. et al. Two G protein oncogenes in human endocrine tumors. Science 249, 655–659 (1990).
Kalinec, G., Nazarali, A. J., Hermouet, S., Xu, N. & Gutkind, J. S. Mutated α subunit of the Gq protein induces malignant transformation in NIH 3T3 cells. Mol. Cell. Biol. 12, 4687–4693 (1992).
Xu, N., Bradley, L., Ambdukar, I. & Gutkind, J. S. A mutant α subunit of G12 potentiates the eicosanoid pathway and is highly oncogenic in NIH 3T3 cells. Proc. Natl Acad. Sci. USA 90, 6741–6745 (1993).
Xu, N., Voyno-Yasenetskaya, T. & Gutkind, J. S. Potent transforming activity of the G13 α subunit defines a novel family of oncogenes. Biochem. Biophys. Res. Commun. 201, 603–609 (1994).
Chan, A. M. et al. Expression cDNA cloning of a transforming gene encoding the wild-type Gα12 gene product. Mol. Cell. Biol. 13, 762–768 (1993).
Drews, R. T., Gravel, R. A. & Collu, R. Identification of G protein α subunit mutations in human growth hormone (GH)- and GH/prolactin-secreting pituitary tumors by single-strand conformation polymorphism (SSCP) analysis. Mol. Cell Endocrinol. 87, 125–129 (1992).
Wilson, C. H., McIntyre, R. E., Arends, M. J. & Adams, D. J. The activating mutation R201C in GNAS promotes intestinal tumourigenesis in ApcMin./+ mice through activation of Wnt and ERK1/2 MAPK pathways. Oncogene 29, 4567–4575 (2010).
Furukawa, T. et al. Whole-exome sequencing uncovers frequent GNAS mutations in intraductal papillary mucinous neoplasms of the pancreas. Sci. Rep. 1, 161 (2011).
Wu, J. et al. Recurrent GNAS mutations define an unexpected pathway for pancreatic cyst development. Sci. Transl Med. 3, 92ra66 (2011).
Ong, C. K. et al. Exome sequencing of liver fluke-associated cholangiocarcinoma. Nature Genet. 44, 690–693 (2012).
Castellone, M. D., Teramoto, H., Williams, B. O., Druey, K. M. & Gutkind, J. S. Prostaglandin E2 promotes colon cancer cell growth through a Gs-axin-β-catenin signaling axis. Science 310, 1504–1510 (2005).
Gupta, R. A. & Dubois, R. N. Colorectal cancer prevention and treatment by inhibition of cyclooxygenase-2. Nature Rev. Cancer 1, 11–21 (2001).
Chan, A. T., Ogino, S. & Fuchs, C. S. Aspirin and the risk of colorectal cancer in relation to the expression of COX-2. N. Engl. J. Med. 356, 2131–2142 (2007).
Wood, L. D. et al. The genomic landscapes of human breast and colorectal cancers. Science 318, 1108–1113 (2007).
Berman, D. M., Wilkie, T. M. & Gilman, A. G. GAIP and RGS4 are GTPase-activating proteins for the Gi subfamily of G protein α subunits. Cell 86, 445–452 (1996).
Van Raamsdonk, C. D. et al. Mutations in GNA11 in uveal melanoma. N. Engl. J. Med. 363, 2191–2199 (2010).
Kusters-Vandevelde, H. V. et al. Improved discrimination of melanotic schwannoma from melanocytic lesions by combined morphological and GNAQ mutational analysis. Acta Neuropathol. 120, 755–764 (2010).
Van Raamsdonk, C. D. et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 (2009). This work identified the first human malignancy driven primarily by the oncogenic activation of genes encoding members of the Gα q family of heterotrimeric G proteins.
Garcia-Marcos, M., Ghosh, P. & Farquhar, M. G. Molecular basis of a novel oncogenic mutation in GNAO1. Oncogene 30, 2691–2696 (2011).
Kan, Z. et al. Diverse somatic mutation patterns and pathway alterations in human cancers. Nature 466, 869–873 (2010). This ground-breaking study revealed the unexpected high frequency of somatic mutations in genes encoding GPCRs in most highly prevalent human malignancies.
Schuijers, J. & Clevers, H. Adult mammalian stem cells: the role of Wnt, Lgr5 and R-spondins. EMBO J. 31, 2685–2696 (2012).
Epstein, E. H. Basal cell carcinomas: attack of the hedgehog. Nature Rev. Cancer 8, 743–754 (2008).
Rubin, L. L. & de Sauvage, F. J. Targeting the Hedgehog pathway in cancer. Nature Rev. Drug Discov. 5, 1026–1033 (2006).
Zhao, Y., Tong, C. & Jiang, J. Hedgehog regulates smoothened activity by inducing a conformational switch. Nature 450, 252–258 (2007).
Lum, L. & Beachy, P. A. The Hedgehog response network: sensors, switches, and routers. Science 304, 1755–1759 (2004).
Xie, J. et al. Activating Smoothened mutations in sporadic basal-cell carcinoma. Nature 391, 90–92 (1998).
Brastianos, P. K. et al. Genomic sequencing of meningiomas identifies oncogenic SMO and AKT1 mutations. Nature Genet. 45, 285–289. (2013).
Clark, V. E. et al. Genomic analysis of non-NF2 meningiomas reveals mutations in TRAF7, KLF4, AKT1, and SMO. Science 339, 1077–1080 (2013).
Scales, S. J. & de Sauvage, F. J. Mechanisms of Hedgehog pathway activation in cancer and implications for therapy. Trends Pharmacol. Sci. 30, 303–312 (2009).
Mitra, D. et al. An ultraviolet-radiation-independent pathway to melanoma carcinogenesis in the red hair/fair skin background. Nature 491, 449–453 (2012).
Bjarnadottir, T. K. et al. The human and mouse repertoire of the adhesion family of G-protein-coupled receptors. Genomics 84, 23–33 (2004).
Lagerstrom, M. C. & Schioth, H. B. Structural diversity of G protein-coupled receptors and significance for drug discovery. Nature Rev. Drug Discov. 7, 339–357 (2008).
Paavola, K. J. & Hall, R. A. Adhesion G protein-coupled receptors: signaling, pharmacology, and mechanisms of activation. Mol. Pharmacol. 82, 777–783 (2012).
McMillan, D. R. & White, P. C. Studies on the very large G protein-coupled receptor: from initial discovery to determining its role in sensorineural deafness in higher animals. Adv. Exp. Med. Biol. 706, 76–86 (2010).
Shiratsuchi, T., Nishimori, H., Ichise, H., Nakamura, Y. & Tokino, T. Cloning and characterization of BAI2 and BAI3, novel genes homologous to brain-specific angiogenesis inhibitor 1 (BAI1). Cytogenet. Cell Genet. 79, 103–108 (1997).
Park, D. et al. BAI1 is an engulfment receptor for apoptotic cells upstream of the ELMO/Dock180/Rac module. Nature 450, 430–434 (2007).
Cork, S. M. & Van Meir, E. G. Emerging roles for the BAI1 protein family in the regulation of phagocytosis, synaptogenesis, neurovasculature, and tumor development. J. Mol. Med. 89, 743–752 (2011).
Nishimura, T., Honda, H. & Takeichi, M. Planar cell polarity links axes of spatial dynamics in neural-tube closure. Cell 149, 1084–1097 (2012).
Caddy, J. et al. Epidermal wound repair is regulated by the planar cell polarity signaling pathway. Dev. Cell 19, 138–147 (2010).
Arac, D. et al. A novel evolutionarily conserved domain of cell-adhesion GPCRs mediates autoproteolysis. EMBO J. 31, 1364–1378 (2012).
Prickett, T. D. et al. Exon capture analysis of G protein-coupled receptors identifies activating mutations in GRM3 in melanoma. Nature Genet. 43, 1119–1126 (2011).
Teh, J. L. & Chen, S. Glutamatergic signaling in cellular transformation. Pigment Cell. Melanoma Res. 25, 331–342 (2012).
Muller, A. et al. Involvement of chemokine receptors in breast cancer metastasis. Nature 410, 50–56 (2001).
Zlotnik, A., Burkhardt, A. M. & Homey, B. Homeostatic chemokine receptors and organ-specific metastasis. Nature Rev. Immunol. 11, 597–606 (2011).
Maceyka, M., Harikumar, K. B., Milstien, S. & Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 22, 50–60 (2012).
Houben, A. J. & Moolenaar, W. H. Autotaxin and LPA receptor signaling in cancer. Cancer Metastasis Rev. 30, 557–565 (2011).
Yagi, H. et al. A synthetic biology approach reveals a CXCR4-G13-Rho signaling axis driving transendothelial migration of metastatic breast cancer cells. Sci Signal. 4, ra60 (2011).
Haass, N. K. & Herlyn, M. Normal human melanocyte homeostasis as a paradigm for understanding melanoma. J. Investig. Dermatol. Symp. Proc. 10, 153–163 (2005).
Prenzel, N. et al. EGF receptor transactivation by G-protein-coupled receptors requires metalloproteinase cleavage of proHB-EGF. Nature 402, 884–888 (1999).
Vaque, J. P. et al. A genome-wide RNAi screen reveals a Trio-regulated Rho GTPase circuitry transducing mitogenic signals initiated by G protein-coupled receptors. Mol. Cell 49, 94–108 (2013).
Yu, F. X. et al. Regulation of the Hippo-YAP pathway by G-protein-coupled receptor signaling. Cell 150, 780–791 (2012)
Martin, D. & Gutkind, J. S. Human tumor-associated viruses and new insights into the molecular mechanisms of cancer. Oncogene 27 (Suppl. 2), S31–S42 (2008).
Arvanitakis, L., Geras-Raaka, E., Varma, A., Gershengorn, M. C. & Cesarman, E. Human herpesvirus KSHV encodes a constitutively active G-protein-coupled receptor linked to cell proliferation. Nature 385, 347–350 (1997).
Wall, M. A. et al. The structure of the G protein heterotrimer Giα1β1γ2 . Cell 83, 1047–1058 (1995).
Rasmussen, S. G. et al. Structure of a nanobody-stabilized active state of the β2 adrenoceptor. Nature 469, 175–180 (2011).
Waldo, G. L. et al. Kinetic scaffolding mediated by a phospholipase C-β and Gq signaling complex. Science 330, 974–980 (2010).
Forbes, S. A. et al. COSMIC: mining complete cancer genomes in the Catalogue of Somatic Mutations in Cancer. Nucleic Acids Res. 39, D945–D950 (2011). This article describes the COSMIC database, which provides a wealth of information on somatic mutations in cancers, including the identification of specific gene mutations and their frequency in tumours of various tissues.
Acknowledgements
The mutation data were obtained from the Wellcome Trust Sanger Institute COSMIC web site. This work was supported by the Intramural Research Program of the US National Institutes of Health and the US National Institute of Dental and Craniofacial Research (to J.S.G. and M.O.), extramural grants U01 GM094612 and partial funding from R01 GM071872 and U54 GM094618 (to T.M.H. and I.K.) and grant 152434 from Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) (to J.V.P.).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
This work was supported by the Intramural Research Program of the US National Institutes of Health, the US National Institute of Dental and Craniofacial Research (to J.S.G. and M.O.) as well as extramural grants U01 GM094612, and partial funding from R01 GM071872, and U54 GM094618 (to T.M.H. and I.K.); and grant 152434 from Consejo Nacional de Ciencia y Tecnología (CONACyT, Mexico) (to J.V.P.).
Supplementary information
Supplementary information S1 (table)
Frequency of somatic mutation in G protein subunits and G protein coupled receptors (GPCRs). (XLS 275 kb)
Supplementary information S2 (table)
G protein and G protein coupled receptors (GPCRs) genes with a significant number of mutations relative to their respective tissue type background mutation rate. (XLS 69 kb)
Supplementary information S3 (table)
G Protein and G protein coupled receptor (GPCR) hotspot mutations. (XLS 121 kb)
Supplementary information S4 (table)
Complete list of G Protein and G protein coupled receptor (GPCR) somatic mutants reported in cancer. (XLSX 1546 kb)
Glossary
- G-protein-coupled receptors
-
(GPCRs). A family of receptor proteins that have seven-transmembrane domains, an extracellular amino terminus and an intracellular carboxyl terminus. They respond to stimuli outside the cell and transduce signals into the cells through interactions with intracellular signalling proteins, including G proteins.
- G proteins
-
A family of guanine-nucleotide-binding proteins that are important for signal transduction. Their activity is regulated by binding and hydrolysing GTP such that the active state is GTP-bound, whereas the inactive form is in a GDP-bound state. Heterotrimeric G proteins consist of α-, β- and γ-subunits.
- Regulators of G protein signalling
-
(RGS). GTPase-accelerating proteins that lead to heterotrimeric G protein inactivation by promoting hydrolysis of the GTP of G to GDP.
- Arrestins
-
A family of proteins that interact with the carboxyl termini of G-protein-coupled receptors and help to mediate receptor desensitization, internalization, recycling and signalling.
- Guanine-nucleotide exchange factors
-
(GEFs). Proteins that stimulate the release of GDP to allow exchange for GTP, thereby promoting G protein activation.
Rights and permissions
About this article
Cite this article
O'Hayre, M., Vázquez-Prado, J., Kufareva, I. et al. The emerging mutational landscape of G proteins and G-protein-coupled receptors in cancer. Nat Rev Cancer 13, 412–424 (2013). https://doi.org/10.1038/nrc3521
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3521
This article is cited by
-
Coexistence of meningioma and craniofacial fibrous dysplasia: a case series of clinicopathological study and literature review
Orphanet Journal of Rare Diseases (2024)
-
Choroidal biopsies; a review and optimised approach
Eye (2023)
-
Activation of melanocortin-1 receptor signaling in melanoma cells impairs T cell infiltration to dampen antitumor immunity
Nature Communications (2023)
-
Isoform- and ligand-specific modulation of the adhesion GPCR ADGRL3/Latrophilin3 by a synthetic binder
Nature Communications (2023)
-
Hypermethylated GPR135 gene expression is a favorable independent prognostic factor in nasopharyngeal carcinoma
Holistic Integrative Oncology (2023)