Key Points
-
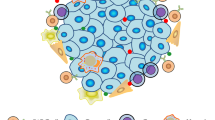
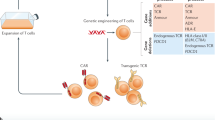
The abilities of T cells to coordinate immunity and to deliver lethal hits against diseased cells can be directed towards tumours by genetically modifying T cells.
-
Genes encoding specific antigen receptors can be inserted into T cells to enable them to recognize and respond to cancer cells.
-
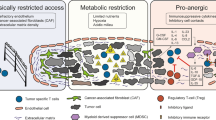
Migration of T cells towards tumours can be facilitated by the expression of specific chemokine receptors in T cells.
-
Genetic modification of T cells can enhance their proliferation and survival, leading to a sustained attack on tumours.
-
Resistance to tumour-derived immunosuppressive factors can be provided genetically to T cells so that they can maintain their activity in a hostile environment.
-
Genes that confer sensitivity to drugs can be used to enable the elimination of T cells if they exert toxicity against vital normal tissues.
Abstract
T cells have the capacity to eradicate diseased cells, but tumours present considerable challenges that render T cells ineffectual. Cancer cells often make themselves almost 'invisible' to the immune system, and they sculpt a microenvironment that suppresses T cell activity, survival and migration. Genetic engineering of T cells can be used therapeutically to overcome these challenges. T cells can be taken from the blood of cancer patients and then modified with genes encoding receptors that recognize cancer-specific antigens. Additional genes can be used to enable resistance to immunosuppression, to extend survival and to facilitate the penetration of engineered T cells into tumours. Using genetic modification, highly active, self-propagating 'slayers' of cancer cells can be generated.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Dudley, M. E. & Rosenberg, S. A. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nature Rev. Cancer 3, 666–675 (2003).
Restifo, N. P. et al. Loss of functional β 2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J. Natl Cancer Inst. 88, 100–108 (1996).
Motz, G. T. & Coukos, G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nature Rev. Immunol. 11, 702–711 (2011).
Dudley, M. E. et al. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J. Clin. Oncol. 26, 5233–5239 (2008). This is an important paper that describes increased objective response rates in patients with metastatic melanoma following ACT of TILs combined with a myeloablative treatment regimen comprising chemotherapy and total body irradiation.
Kawakami, Y. et al. Recognition of multiple epitopes in the human melanoma antigen gp100 by tumor-infiltrating T lymphocytes associated with in vivo tumor regression. J. Immunol. 154, 3961–3968 (1995).
Clay, T. M. et al. Efficient transfer of a tumor antigen-reactive TCR to human peripheral blood lymphocytes confers anti-tumor reactivity. J. Immunol. 163, 507–513 (1999).
Morgan, R. A. et al. High efficiency TCR gene transfer into primary human lymphocytes affords avid recognition of melanoma tumor antigen glycoprotein 100 and does not alter the recognition of autologous melanoma antigens. J. Immunol. 171, 3287–3295 (2003).
Weide, B. et al. Functional T cells targeting NY-ESO-1 or Melan-A are predictive for survival of patients with distant melanoma metastasis. J. Clin. Oncol. 30, 1835–1841 (2012).
Xue, S. A. et al. Elimination of human leukemia cells in NOD/SCID mice by WT1-TCR gene-transduced human T cells. Blood 106, 3062–3067 (2005).
Parkhurst, M. R. et al. T cells targeting carcinoembryonic antigen can mediate regression of metastatic colorectal cancer but induce severe transient colitis. Mol. Ther. 19, 620–626 (2011).
Stanislawski, T. et al. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nature Immunol. 2, 962–970 (2001).
Zhao, Y. et al. High-affinity TCRs generated by phage display provide CD4+ T cells with the ability to recognize and kill tumor cell lines. J. Immunol. 179, 5845–5854 (2007).
Gehring, A. J. et al. Engineering virus-specific T cells that target HBV infected hepatocytes and hepatocellular carcinoma cell lines. J. Hepatol. 55, 103–110 (2011).
Scholten, K. B. et al. Generating HPV specific T helper cells for the treatment of HPV induced malignancies using TCR gene transfer. J. Transl. Med. 9, 147 (2011).
Jurgens, L. A., Khanna, R., Weber, J. & Orentas, R. J. Transduction of primary lymphocytes with Epstein-Barr virus (EBV) latent membrane protein-specific T-cell receptor induces lysis of virus-infected cells: a novel strategy for the treatment of Hodgkin's disease and nasopharyngeal carcinoma. J. Clin. Immunol. 26, 22–32 (2006).
Pleasance, E. D. et al. A comprehensive catalogue of somatic mutations from a human cancer genome. Nature 463, 191–196 (2010).
Wei, X. et al. Exome sequencing identifies GRIN2A as frequently mutated in melanoma. Nature Genet. 43, 442–446 (2011).
Altenschmidt, U. et al. Cytolysis of tumor cells expressing the Neu/erbB-2, erbB-3, and erbB-4 receptors by genetically targeted naive T lymphocytes. Clin. Cancer. Res. 2, 1001–1008 (1996).
Brown, C. E. et al. Stem-like tumor-initiating cells isolated from IL13Rα2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin. Cancer. Res. 18, 2199–209 (2012).
Huang, G. et al. Genetically modified T cells targeting interleukin-11 receptor α-chain kill human osteosarcoma cells and induce the regression of established osteosarcoma lung metastases. Cancer Res. 72, 271–281 (2012).
Shaffer, D. R. et al. T cells redirected against CD70 for the immunotherapy of CD70-positive malignancies. Blood 117, 4304–4314 (2011).
Brentjens, R. J. et al. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nature Med. 9, 279–286 (2003). This study describes the successful eradication of tumours in mice treated with human CAR T cells following co-stimulation with CD80 and IL-15 and represents an important step prior to the translation of this approach into the clinic.
Teng, M. W., Kershaw, M. H., Moeller, M., Smyth, M. J. & Darcy, P. K. Immunotherapy of cancer using systemically delivered gene-modified human T lymphocytes. Hum. Gene Ther. 15, 699–708 (2004).
Chmielewski, M. et al. T cells that target carcinoembryonic antigen eradicate orthotopic pancreatic carcinomas without inducing autoimmune colitis in mice. Gastroenterology 143, 1095–1107 (2012).
Westwood, J. A. et al. The Lewis-Y carbohydrate antigen is expressed by many human tumors and can serve as a target for genetically redirected T cells despite the presence of soluble antigen in serum. J. Immunother. 32, 292–301 (2009).
Lamers, C. H. et al. Immune responses to transgene and retroviral vector in patients treated with ex vivo-engineered T cells. Blood 117, 72–82 (2011).
Kershaw, M. H. et al. A phase I study on adoptive immunotherapy using gene-modified T cells for ovarian cancer. Clin. Cancer Res. 12, 6106–6115 (2006). This study represents one of the earliest reports of using adoptively transferred CAR T cells in a patient setting.
Westwood, J. A. et al. Adoptive transfer of T cells modified with a humanized chimeric receptor gene inhibits growth of Lewis-Y-expressing tumors in mice. Proc. Natl Acad. Sci. USA 102, 19051–19056 (2005).
Morgan, R. A. et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene Ther. 23, 1043–1053 (2012).
Robbins, P. F. et al. Single and dual amino acid substitutions in TCR CDRs can enhance antigen-specific T cell functions. J. Immunol. 180, 6116–6131 (2008).
Mehrotra, S. et al. A coreceptor-independent transgenic human TCR mediates anti-tumor and anti-self immunity in mice. J. Immunol. 189, 1627–1638 (2012).
Kuball, J. et al. Facilitating matched pairing and expression of TCR chains introduced into human T cells. Blood 109, 2331–2338 (2007).
Cohen, C. J. et al. Enhanced antitumor activity of T cells engineered to express T-cell receptors with a second disulfide bond. Cancer Res. 67, 3898–903 (2007).
Aggen, D. H. et al. Single-chain VαVβ T-cell receptors function without mispairing with endogenous TCR chains. Gene Ther. 19, 365–374 (2012).
Willemsen, R. A. et al. Grafting primary human T lymphocytes with cancer-specific chimeric single chain and two chain TCR. Gene Ther. 7, 1369–1377 (2000).
Cohen, C. J., Zhao, Y., Zheng, Z., Rosenberg, S. A. & Morgan, R. A. Enhanced antitumor activity of murine-human hybrid T-cell receptor (TCR) in human lymphocytes is associated with improved pairing and TCR/CD3 stability. Cancer Res. 66, 8878–8886 (2006).
Davis, J. L. et al. Development of human anti-murine T-cell receptor antibodies in both responding and nonresponding patients enrolled in TCR gene therapy trials. Clin. Cancer Res. 16, 5852–5861 (2010).
Okamoto, S. et al. Improved expression and reactivity of transduced tumor-specific TCRs in human lymphocytes by specific silencing of endogenous TCR. Cancer Res. 69, 9003–9011 (2009).
Provasi, E. et al. Editing T cell specificity towards leukemia by zinc finger nucleases and lentiviral gene transfer. Nature Med. 18, 807–815 (2012).
Moeller, M. et al. A functional role for CD28 costimulation in tumor recognition by single-chain receptor-modified T cells. Cancer Gene Ther. 11, 371–379 (2004).
Brentjens, R. J. et al. Genetically targeted T cells eradicate systemic acute lymphoblastic leukemia xenografts. Clin. Cancer Res. 13, 5426–5435 (2007).
Zhao, Y. et al. A herceptin-based chimeric antigen receptor with modified signaling domains leads to enhanced survival of transduced T lymphocytes and antitumor activity. J. Immunol. 183, 5563–5574 (2009).
Cheadle, E. J. et al. Ligation of the CD2 co-stimulatory receptor enhances IL-2 production from first-generation chimeric antigen receptor T cells. Gene Ther. 19, 1114–1120 (2012).
Carpenito, C. et al. Control of large, established tumor xenografts with genetically retargeted human T cells containing CD28 and CD137 domains. Proc. Natl Acad. Sci. USA 106, 3360–3365 (2009).
Zhong, X. S., Matsushita, M., Plotkin, J., Riviere, I. & Sadelain, M. Chimeric antigen receptors combining 4-1BB and CD28 signaling domains augment PI3kinase/AKT/Bcl-XL activation and CD8+ T cell-mediated tumor eradication. Mol. Ther. 18, 413–420 (2010).
Wang, L. X. et al. Tumor ablation by gene-modified T cells in the absence of autoimmunity. Cancer Res. 70, 9591–9598 (2010). This is one of the first reports to demonstrate efficacy and safety of CAR-modified T cells in a self-antigen immunocompetent setting.
Stephan, M. T. et al. T cell-encoded CD80 and 4-1BBL induce auto- and transcostimulation, resulting in potent tumor rejection. Nature Med. 13, 1440–1449 (2007).
Duong, C. P. et al. Engineering T cell function using chimeric antigen receptors identified using a DNA library approach. PLoS ONE 8, e63037 (2013).
Haso, W. et al. Anti-CD22-chimeric antigen receptors targeting B cell precursor acute lymphoblastic leukemia. Blood 121, 1165–1174 (2012).
Kunii, N. et al. Enhanced function of redirected human t cells expressing linker for activation of t cells that is resistant to ubiquitylation. Hum. Gene. Ther. 24, 27–37 (2012).
Turatti, F. et al. Redirected activity of human antitumor chimeric immune receptors is governed by antigen and receptor expression levels and affinity of interaction. J. Immunother. 30, 684–693 (2007).
Chmielewski, M., Hombach, A., Heuser, C., Adams, G. P. & Abken, H. T cell activation by antibody-like immunoreceptors: increase in affinity of the single-chain fragment domain above threshold does not increase T cell activation against antigen-positive target cells but decreases selectivity. J. Immunol. 173, 7647–7653 (2004).
Guest, R. D. et al. The role of extracellular spacer regions in the optimal design of chimeric immune receptors: evaluation of four different scFvs and antigens. J. Immunother. 28, 203–211 (2005).
Bridgeman, J. S., Hawkins, R. E., Hombach, A. A., Abken, H. & Gilham, D. E. Building better chimeric antigen receptors for adoptive T cell therapy. Curr. Gene Ther. 10, 77–90 (2010).
Maher, J., Brentjens, R. J., Gunset, G., Riviere, I. & Sadelain, M. Human T-lymphocyte cytotoxicity and proliferation directed by a single chimeric TCRzeta /CD28 receptor. Nature Biotechnol. 20, 70–75 (2002).
Kershaw, M. H. et al. Redirecting migration of T cells to chemokine secreted from tumors by genetic modification with CXCR2. Hum. Gene Ther. 13, 1971–1980 (2002).
Di Stasi, A. et al. T lymphocytes coexpressing CCR4 and a chimeric antigen receptor targeting CD30 have improved homing and antitumor activity in a Hodgkin tumor model. Blood 113, 6392–6402 (2009). This study demonstrates that genetic modification of CAR-modified T cells with a chemokine receptor can substantially improve their homing and antitumour activity in vivo.
Craddock, J. A. et al. Enhanced tumor trafficking of GD2 chimeric antigen receptor T cells by expression of the chemokine receptor CCR2b. J. Immunother. 33, 780–788 (2010).
Chinnasamy, D. et al. Local delivery of interleukin-12 using T cells targeting VEGF receptor-2 eradicates multiple vascularized tumors in mice. Clin. Cancer Res. 18, 1672–1683 (2012). This study demonstrates that local delivery of the cytokine IL-12 by gene-modified T cells can alter the microenvironment to increase efficacy of ACT immunotherapy.
Legler, D. F., Johnson-Leger, C., Wiedle, G., Bron, C. & Imhof, B. A. The α v β 3 integrin as a tumor homing ligand for lymphocytes. Eur. J. Immunol. 34, 1608–1616 (2004).
Buckanvich, R. J. Endothelin (B) receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nature Med. 14, 28–36 (2008).
Ganss, R., Ryschich, E., Klar, E., Arnold, B. & Hammerling, G. J. Combination of T-cell therapy and trigger of inflammation induces remodeling of the vasculature and tumor eradication. Cancer Res. 62, 1462–1470 (2002).
Emtage, P. C. et al. Second-generation anti-carcinoembryonic antigen designer T cells resist activation-induced cell death, proliferate on tumor contact, secrete cytokines, and exhibit superior antitumor activity in vivo: a preclinical evaluation. Clin. Cancer Res. 14, 8112–8122 (2008).
Savoldo, B. et al. CD28 costimulation improves expansion and persistence of chimeric antigen receptor-modified T cells in lymphoma patients. J. Clin. Invest. 121, 1822–1826 (2011). This is the first study demonstrating the superiority of CARs containing the CD28 endodomain in a patient setting.
Song, D. G. et al. CD27 costimulation augments the survival and antitumor activity of redirected human T cells in vivo. Blood 119, 696–706 (2012).
Dotti, G. et al. Human cytotoxic T lymphocytes with reduced sensitivity to Fas-induced apoptosis. Blood 105, 4677–4684 (2005).
Lei, X. Y. et al. Knockdown of human bid gene expression enhances survival of CD8+ T cells. Immunol. Lett. 122, 30–36 (2009).
Eaton, D., Gilham, D. E., O'Neill, A. & Hawkins, R. E. Retroviral transduction of human peripheral blood lymphocytes with Bcl-X(L) promotes in vitro lymphocyte survival in pro-apoptotic conditions. Gene Ther. 9, 527–535 (2002).
Charo, J. et al. Bcl-2 overexpression enhances tumor-specific T-cell survival. Cancer Res. 65, 2001–2008 (2005).
Shin, J. H. et al. Positive conversion of negative signaling of CTLA4 potentiates antitumor efficacy of adoptive T-cell therapy in murine tumor models. Blood 119, 5678–5687 (2012).
Gattinoni, L., Klebanoff, C. A. & Restifo, N. P. Paths to stemness: building the ultimate antitumour T cell. Nature Rev. Cancer 12, 671–684 (2012).
Klebanoff, C. A. et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 17, 5343–5352 (2011).
Zou, Z. et al. Cytotoxic T lymphocyte trafficking and survival in an augmented fibrin matrix carrier. PLoS ONE 7, e34652 (2012).
Vatakis, D. N. et al. Antitumor activity from antigen-specific CD8 T cells generated in vivo from genetically engineered human hematopoietic stem cells. Proc. Natl Acad. Sci. USA 108, E1408–E1416 (2011).
Vizcardo, R. et al. Regeneration of human tumor antigen-specific T cells from iPSCs derived from mature CD8+ T cells. Cell Stem Cell 12, 31–36 (2013).
Pule, M. A. et al. A chimeric T cell antigen receptor that augments cytokine release and supports clonal expansion of primary human T cells. Mol. Ther. 12, 933–941 (2005).
Wang, J. et al. Optimizing adoptive polyclonal T cell immunotherapy of lymphomas, using a chimeric T cell receptor possessing CD28 and CD137 costimulatory domains. Hum. Gene Ther. 18, 712–725 (2007).
Lo, A. S. et al. Harnessing the tumour-derived cytokine, CSF-1, to co-stimulate T-cell growth and activation. Mol. Immunol. 45, 1276–1287 (2008).
Jonnalagadda, M. et al. Efficient selection of genetically modified human T cells using methotrexate-resistant human dihydrofolate reductase. Gene. Ther. http://dx.doi.org/10.1038/gt.2012.97 (2013).
Stromnes, I. M. et al. Abrogating Cbl-b in effector CD8+ T cells improves the efficacy of adoptive therapy of leukemia in mice. J. Clin. Invest. 120, 3722–3734 (2010). This preclinical study describes an elegant approach for enhancing the efficacy of adoptive T cell immunotherapy against disseminated leukaemia by abrogating CBLB, a negative regulator of T cell signalling.
Stromnes, I. M. et al. Abrogation of SRC homology region 2 domain-containing phosphatase 1 in tumor-specific T cells improves efficacy of adoptive immunotherapy by enhancing the effector function and accumulation of short-lived effector T cells in vivo. J. Immunol. 189, 1812–1825 (2012).
Cooper, L. J. et al. Enhanced antilymphoma efficacy of CD19-redirected influenza MP1-specific CTLs by cotransfer of T cells modified to present influenza MP1. Blood 105, 1622–1631 (2005).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Med. 14, 1264–1270 (2008).
Langerman, A., Callender, G. G. & Nishimura, M. I. Retroviral transduction of peptide stimulated t cells can generate dual t cell receptor-expressing (bifunctional) t cells reactive with two defined antigens. J. Transl. Med. 2, 42 (2004).
Kershaw, M. H., Westwood, J. A. & Hwu, P. Dual-specific T cells combine proliferation and antitumor activity. Nature Biotechnol. 20, 1221–1227 (2002). This landmark study describes a novel concept for enhancing adoptive T cell immunotherapy by the generation of dual-specific T cells that could effectively respond to both TAA and immunogen.
Murphy, A. et al. Antitumor activity of dual-specific T cells and influenza virus. Cancer Gene Ther. 14, 499–508 (2007).
Dossett, M. L. et al. Adoptive immunotherapy of disseminated leukemia with TCR-transduced, CD8+ T cells expressing a known endogenous TCR. Mol. Ther. 17, 742–749 (2009).
Loskog, A. et al. Addition of the CD28 signaling domain to chimeric T-cell receptors enhances chimeric T-cell resistance to T regulatory cells. Leukemia 20, 1819–1828 (2006).
Lee, J. C. et al. In vivo inhibition of human CD19-targeted effector T cells by natural T regulatory cells in a xenotransplant murine model of B cell malignancy. Cancer Res. 71, 2871–2881 (2011).
Koehler, H., Kofler, D., Hombach, A. & Abken, H. CD28 costimulation overcomes transforming growth factor-β-mediated repression of proliferation of redirected human CD4+ and CD8+ T cells in an antitumor cell attack. Cancer Res. 67, 2265–2273 (2007).
Sun, J. et al. T cells expressing constitutively active Akt resist multiple tumor-associated inhibitory mechanisms. Mol. Ther. 18, 2006–2017 (2010).
Bollard, C. M. et al. Adapting a transforming growth factor β-related tumor protection strategy to enhance antitumor immunity. Blood 99, 3179–3187 (2002).
Gorelik, L. & Flavell, R. A. Transforming growth factor-β in T-cell biology. Nature Rev. Immunol. 2, 46–53 (2002).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N. Engl. J. Med. 366, 2443–2454 (2012).
Borkner, L. et al. RNA interference targeting programmed death receptor-1 improves immune functions of tumor-specific T cells. Cancer Immunol. Immunother. 59, 1173–1183 (2010).
Prosser, M. E., Brown, C. E., Shami, A. F., Forman, S. J. & Jensen, M. C. Tumor PD-L1 co-stimulates primary human CD8+ cytotoxic T cells modified to express a PD1:CD28 chimeric receptor. Mol. Immunol. 51, 263–272 (2012).
Spear, P., Barber, A., Rynda-Apple, A. & Sentman, C. L. Chimeric antigen receptor T cells shape myeloid cell function within the tumor microenvironment through IFN-γ and GM-CSF. J. Immunol. 188, 6389–6398 (2012).
Kerkar, S. P. et al. Tumor-specific CD8+ T cells expressing interleukin-12 eradicate established cancers in lymphodepleted hosts. Cancer Res. 70, 6725–6734 (2010).
Pegram, H. J. et al. Tumor-targeted T cells modified to secrete IL-12 eradicate systemic tumors without need for prior conditioning. Blood 119, 4133–4141 (2012).
Chmielewski, M., Kopecky, C., Hombach, A. A. & Abken, H. IL-12 release by engineered T cells expressing chimeric antigen receptors can effectively Muster an antigen-independent macrophage response on tumor cells that have shut down tumor antigen expression. Cancer Res. 71, 5697–706 (2011).
Kerkar, S. P. et al. IL-12 triggers a programmatic change in dysfunctional myeloid-derived cells within mouse tumors. J. Clin. Invest. 121, 4746–4757 (2012).
Duval, L. et al. Adoptive transfer of allogeneic cytotoxic T lymphocytes equipped with a HLA-A2 restricted MART-1 T-cell receptor: a phase I trial in metastatic melanoma. Clin. Cancer Res. 12, 1229–1236 (2006).
Morgan, R. A. et al. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science 314, 126–129 (2006). This seminal paper describes the first successful trial in patients using TCR gene-modified T cells against metastatic melanoma.
Burns, W. R., Zheng, Z., Rosenberg, S. A. & Morgan, R. A. Lack of specific γ-retroviral vector long terminal repeat promoter silencing in patients receiving genetically engineered lymphocytes and activation upon lymphocyte restimulation. Blood 114, 2888–2899 (2009).
Dudley, M. E. et al. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J. Clin. Oncol. 23, 2346–2357 (2005).
Johnson, L. A. et al. Gene therapy with human and mouse T-cell receptors mediates cancer regression and targets normal tissues expressing cognate antigen. Blood 114, 535–546 (2009).
Robbins, P. F. et al. Tumor regression in patients with metastatic synovial cell sarcoma and melanoma using genetically engineered lymphocytes reactive with NY-ESO-1. J. Clin. Oncol. 29, 917–924 (2011).
Warren, R. S. et al. Clinical studies of regional and systemic gene therapy with autologous CC49-z modified T cells in colorectal cancer metastatic to the liver. (Abstract, 7th International Conference on Gene Therapy of Cancer). Cancer Gene Ther. 5, S1–S2 (1998).
Ma, Q., Gonzalo-Daganzo, R. M. & Junghans, R. P. Genetically engineered T cells as adoptive immunotherapy of cancer. Cancer Chemother. Biol. Response Modif. 20, 315–341 (2002).
Park, J. R. et al. Adoptive transfer of chimeric antigen receptor re-directed cytolytic T lymphocyte clones in patients with neuroblastoma. Mol. Ther. 15, 825–833 (2007).
Lamers, C. H. et al. Treatment of metastatic renal cell carcinoma with autologous T-lymphocytes genetically retargeted against carbonic anhydrase IX: first clinical experience. J. Clin. Oncol. 24, e20–e22 (2006).
Louis, C. U. et al. Antitumor activity and long-term fate of chimeric antigen receptor-positive T cells in patients with neuroblastoma. Blood 118, 6050–6056 (2011).
Kochenderfer, J. N. et al. B-cell depletion and remissions of malignancy along with cytokine-associated toxicity in a clinical trial of anti-CD19 chimeric-antigen-receptor-transduced T cells. Blood 119, 2709–2720 (2012).
Brentjens, R. J. et al. Safety and persistence of adoptively transferred autologous CD19-targeted T cells in patients with relapsed or chemotherapy refractory B-cell leukemias. Blood 118, 4817–4828 (2011).
Kalos, M. et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci. Transl. Med. 3, 95ra73 (2011).
Jensen, M. C. et al. Antitransgene rejection responses contribute to attenuated persistence of adoptively transferred CD20/CD19-specific chimeric antigen receptor redirected T cells in humans. Biol. Blood Marrow Transplant. 16, 1245–1256 (2010).
Kochenderfer, J. N. et al. Eradication of B-lineage cells and regression of lymphoma in a patient treated with autologous T cells genetically engineered to recognize CD19. Blood 116, 4099–4102 (2010).
Porter, D. L., Levine, B. L., Kalos, M., Bagg, A. & June, C. H. Chimeric antigen receptor-modified T cells in chronic lymphoid leukemia. N. Engl. J. Med. 365, 725–733 (2011). This landmark paper highlights the enormous potential of ACT immunotherapy using genetically modified T cells, with long-term remission reported in patients with chronic lymphocytic leukaemia.
Davila, M. L., Brentjens, R., Wang, X., Riviere, I. & Sadelain, M. How do CARs work?: Early insights from recent clinical studies targeting CD19. Oncoimmunology 1, 1577–1583 (2012).
Grupp, S. A. et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N. Engl. J. Med. 368, 1509–1518 (2013).
Till, B. G. et al. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood 112, 2261–2271 (2008).
Till, B. G. et al. CD20-specific adoptive immunotherapy for lymphoma using a chimeric antigen receptor with both CD28 and 4-1BB domains: pilot clinical trial results. Blood 119, 3940–3950 (2012).
Lipowska-Bhalla, G., Gilham, D. E., Hawkins, R. E. & Rothwell, D. G. Targeted immunotherapy of cancer with CAR T cells: achievements and challenges. Cancer Immunol. Immunother. 61, 953–962 (2012).
Palmer, D. C. et al. Effective tumor treatment targeting a melanoma/melanocyte-associated antigen triggers severe ocular autoimmunity. Proc. Natl Acad. Sci. USA 105, 8061–8066 (2008).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 (2013).
Linette, G. P. et al. Cardiovascular toxicity and titin cross-reactivity of affinity enhanced T cells in myeloma and melanoma. Blood http://dx.doi.org/10.1182/blood-2013-03-490565 (2013).
Morgan, R. A. et al. Case report of a serious adverse event following the administration of T cells transduced with a chimeric antigen receptor recognizing ERBB2. Mol. Ther. 18, 843–851 (2010).
Ertl, H. C. et al. Considerations for the clinical application of chimeric antigen receptor T cells: observations from a recombinant DNA Advisory Committee Symposium held June 15, 2010. Cancer Res. 71, 3175–3181 (2011).
Stewart-Jones, G. et al. Rational development of high-affinity T-cell receptor-like antibodies. Proc. Natl Acad. Sci. USA 106, 5784–5788 (2009).
Schmidt, P. et al. Eradication of melanomas by targeted elimination of a minor subset of tumor cells. Proc. Natl Acad. Sci. USA 108, 2474–2479 (2011).
Rainusso, N. et al. Immunotherapy targeting HER2 with genetically modified T cells eliminates tumor-initiating cells in osteosarcoma. Cancer Gene Ther. 19, 212–217 (2012).
Alkins, R. D. et al. Focused ultrasound delivers targeted immune cells to metastatic brain tumors. Cancer Res. 73, 1892–1899 (2013).
Marcus, A., Waks, T. & Eshhar, Z. Redirected tumor-specific allogeneic T cells for universal treatment of cancer. Blood 118, 975–983 (2011).
Torikai, H. et al. A foundation for universal T-cell based immunotherapy: T cells engineered to express a CD19-specific chimeric-antigen-receptor and eliminate expression of endogenous TCR. Blood 119, 5697–705 (2012).
Tamada, K. et al. Redirecting gene-modified T cells toward various cancer types using tagged antibodies. Clin. Cancer Res. 18, 6436–6445 (2012).
Urbanska, K. et al. A universal strategy for adoptive immunotherapy of cancer through use of a novel T-cell antigen receptor. Cancer Res. 72, 1844–1852 (2012).
Petrausch, U. et al. Re-directed T cells for the treatment of fibroblast activation protein (FAP)-positive malignant pleural mesothelioma (FAPME-1). BMC Cancer 12, 615 (2012).
Lucas, S. & Coulie, P. G. About human tumor antigens to be used in immunotherapy. Semin. Immunol. 20, 301–307 (2008).
Scott, A. M. et al. Construction, production, and characterization of humanized anti-Lewis Y monoclonal antibody 3S193 for targeted immunotherapy of solid tumors. Cancer Res. 60, 3254–3261 (2000).
Ramos, C. A. & Dotti, G. Chimeric antigen receptor (CAR)-engineered lymphocytes for cancer therapy. Expert Opin. Biol. Ther. 11, 855–873 (2011).
Uttenthal, B. J., Chua, I., Morris, E. C. & Stauss, H. J. Challenges in T cell receptor gene therapy. J. Gene Med. 14, 386–399 (2012).
Dall, P. et al. In vivo cervical cancer growth inhibition by genetically engineered cytotoxic T cells. Cancer Immunol. Immunother. 54, 51–60 (2005).
Katz, S. C. et al. Anti-KIT designer T cells for the treatment of gastrointestinal stromal tumor. J. Transl. Med. 11, 46 (2013).
Hudecek, M. et al. The B-cell tumor-associated antigen ROR1 can be targeted with T cells modified to express a ROR1-specific chimeric antigen receptor. Blood 116, 4532–4541 (2010).
Mihara, K. et al. Synergistic and persistent effect of T-cell immunotherapy with anti-CD19 or anti-CD38 chimeric receptor in conjunction with rituximab on B-cell non-Hodgkin lymphoma. Br. J. Haematol. 151, 37–46 (2010).
Barber, A. et al. Chimeric NKG2D receptor-expressing T cells as an immunotherapy for multiple myeloma. Exp. Hematol. 36, 1318–1328 (2008).
Nagai, K. et al. Aurora kinase A-specific T-cell receptor gene transfer redirects T lymphocytes to display effective antileukemia reactivity. Blood 119, 368–376 (2012).
van Loenen, M. M. et al. Optimization of the HA-1-specific T-cell receptor for gene therapy of hematologic malignancies. Haematologica 96, 477–481 (2011).
Heemskerk, M. H. et al. Reprogramming of virus-specific T cells into leukemia-reactive T cells using T cell receptor gene transfer. J. Exp. Med. 199, 885–894 (2004).
Spranger, S. et al. TCR-transgenic lymphocytes specific for HMMR/Rhamm limit tumor outgrowth in vivo. Blood 119, 3440–3449 (2012).
Burns, W. R. et al. A high molecular weight melanoma-associated antigen-specific chimeric antigen receptor redirects lymphocytes to target human melanomas. Cancer Res. 70, 3027–3033 (2010).
Willemsen, R. A., Ronteltap, C., Chames, P., Debets, R. & Bolhuis, R. L. T cell retargeting with MHC class I-restricted antibodies: the CD28 costimulatory domain enhances antigen-specific cytotoxicity and cytokine production. J. Immunol. 174, 7853–7858 (2005).
Koya, R. C. et al. Kinetic phases of distribution and tumor targeting by T cell receptor engineered lymphocytes inducing robust antitumor responses. Proc. Natl Acad. Sci. USA 107, 14286–14291 (2010).
Chinnasamy, N. et al. A TCR targeting the HLA-A*0201-restricted epitope of MAGE-A3 recognizes multiple epitopes of the MAGE-A antigen superfamily in several types of cancer. J. Immunol. 186, 685–696 (2011).
Willemsen, R., Ronteltap, C., Heuveling, M., Debets, R. & Bolhuis, R. Redirecting human CD4+ T lymphocytes to the MHC class I-restricted melanoma antigen MAGE-A1 by TCR αβ gene transfer requires CD8α. Gene Ther. 12, 140–146 (2005).
Song, D. G. et al. In vivo persistence, tumor localization, and antitumor activity of CAR-engineered T cells is enhanced by costimulatory signaling through CD137 (4-1BB). Cancer Res. 71, 4617–4627 (2011).
Hillerdal, V., Nilsson, B., Carlsson, B., Eriksson, F. & Essand, M. T cells engineered with a T cell receptor against the prostate antigen TARP specifically kill HLA-A2+ prostate and breast cancer cells. Proc. Natl Acad. Sci. USA 109, 15877–15881 (2012).
Leisegang, M. et al. T-cell receptor gene-modified T cells with shared renal cell carcinoma specificity for adoptive T-cell therapy. Clin. Cancer Res. 16, 2333–2343 (2010).
Kershaw, M. H. et al. Generation of gene-modified T cells reactive against the angiogenic kinase insert domain-containing receptor (KDR) found on tumor vasculature. Hum. Gene Ther. 11, 2445–2452 (2000).
Cheung, N. K., Guo, H. F., Modak, S. & Cheung, I. Y. Anti-idiotypic antibody facilitates scFv chimeric immune receptor gene transduction and clonal expansion of human lymphocytes for tumor therapy. Hybrid Hybridom. 22, 209–218 (2003).
Leisegang, M. et al. MHC-restricted fratricide of human lymphocytes expressing survivin-specific transgenic T cell receptors. J. Clin. Invest. 120, 3869–3877 (2010).
Shirakura, Y. et al. T-cell receptor gene therapy targeting melanoma-associated antigen-A4 inhibits human tumor growth in non-obese diabetic/SCID/γcnull mice. Cancer Sci. 103, 17–25 (2012).
Berger, C., Flowers, M. E., Warren, E. H. & Riddell, S. R. Analysis of transgene-specific immune responses that limit the in vivo persistence of adoptively transferred HSV-TK-modified donor T cells after allogeneic hematopoietic cell transplantation. Blood 107, 2294–302 (2006).
Bonini, C. et al. HSV-TK gene transfer into donor lymphocytes for control of allogeneic graft-versus-leukemia. Science 276, 1719–1724 (1997). This is the first study to report successful control of graft-versus-host disease by adoptively transferred donor T cells through genetic modification with the HSV-TK suicide gene.
Straathof, K. C. et al. An inducible caspase 9 safety switch for T-cell therapy. Blood 105, 4247–4254 (2005).
Thomis, D. C. et al. A Fas-based suicide switch in human T cells for the treatment of graft-versus-host disease. Blood 97, 1249–1257 (2001).
Vogler, I. et al. An improved bicistronic CD20/tCD34 vector for efficient purification and in vivo depletion of gene-modified T cells for adoptive immunotherapy. Mol. Ther. 18, 1330–1338 (2010).
Wang, X. et al. A transgene-encoded cell surface polypeptide for selection, in vivo tracking, and ablation of engineered cells. Blood 118, 1255–1263 (2011).
Kieback, E., Charo, J., Sommermeyer, D., Blankenstein, T. & Uckert, W. A safeguard eliminates T cell receptor gene-modified autoreactive T cells after adoptive transfer. Proc. Natl Acad. Sci. USA 105, 623–628 (2008).
Jin, Z. et al. The hyperactive Sleeping Beauty transposase SB100X improves the genetic modification of T cells to express a chimeric antigen receptor. Gene Ther. 18, 849–856 (2011).
Riet, T. et al. Nonviral RNA transfection to transiently modify T cells with chimeric antigen receptors for adoptive therapy. Methods Mol. Biol. 969, 187–201 (2013).
Danke, C. et al. Adjusting transgene expression levels in lymphocytes with a set of inducible promoters. J. Gene Med. 12, 501–515 (2010).
Duong, C. P., Westwood, J. A., Berry, L. J., Darcy, P. K. & Kershaw, M. H. Enhancing the specificity of T-cell cultures for adoptive immunotherapy of cancer. Immunotherapy 3, 33–48 (2011).
Kloss, C. C., Condomines, M., Cartellieri, M., Bachmann, M. & Sadelain, M. Combinatorial antigen recognition with balanced signaling promotes selective tumor eradication by engineered T cells. Nature Biotechnol. 31, 71–75 (2012). This elegant study describes a method for increasing the specificity of CAR-redirected T cells by assigning signal 1 and signal 2 to two different CARs.
Wilkie, S. et al. Dual targeting of ErbB2 and MUC1 in breast cancer using chimeric antigen receptors engineered to provide complementary signaling. J. Clin. Immunol. 32, 1059–1070 (2012).
Cheadle, E. J. et al. Eradication of established B-cell lymphoma by CD19-specific murine T cells is dependent on host lymphopenic environment and can be mediated by CD4+ and CD8+ T cells. J. Immunother. 32, 207–218 (2009).
Cheadle, E. J. et al. Natural expression of the CD19 antigen impacts the long-term engraftment but not antitumor activity of CD19-specific engineered T cells. J. Immunol. 184, 1885–1896 (2010).
Kochenderfer, J. N., Yu, Z., Frasheri, D., Restifo, N. P. & Rosenberg, S. A. Adoptive transfer of syngeneic T cells transduced with a chimeric antigen receptor that recognizes murine CD19 can eradicate lymphoma and normal B cells. Blood 116, 3875–3886 (2010).
Milone, M. C. et al. Chimeric receptors containing CD137 signal transduction domains mediate enhanced survival of T cells and increased antileukemic efficacy in vivo. Mol. Ther. 17, 1453–1464 (2009).
Maliar, A. et al. Redirected T cells that target pancreatic adenocarcinoma antigens eliminate tumors and metastases in mice. Gastroenterology 143, 1375–1384 (2012).
Ahmed, N. et al. Immunotherapy for osteosarcoma: genetic modification of T cells overcomes low levels of tumor antigen expression. Mol. Ther. 17, 1779–1787 (2009).
Yvon, E. et al. Immunotherapy of metastatic melanoma using genetically engineered GD2-specific T cells. Clin. Cancer Res. 15, 5852–5860 (2009).
Hwu, P. et al. In vivo antitumor activity of T cells redirected with chimeric antibody/T-cell receptor genes. Cancer Res. 55, 3369–3373 (1995).
Ahmadi, M. et al. CD3 limits the efficacy of TCR gene therapy in vivo. Blood 118, 3528–3537 (2011).
de Witte, M. A. et al. TCR gene therapy of spontaneous prostate carcinoma requires in vivo T cell activation. J. Immunol. 181, 2563–2571 (2008).
Brentjens, R. J. et al. CD19-targeted T cells rapidly induce molecular remissions in adults with chemotherapy-refractory acute lymphoblastic leukemia. Sci. Transl. Med. 5, 177ra38 (2013).
Lamers, C. H. et al. Treatment of metastatic renal cell carcinoma with CAIX CAR-engineered t cells: clinical evaluation and management of on-target toxicity. Mol. Ther. 21, 904–912 (2013).
Rosenberg, S. A. Of mice, not men: no evidence for graft-versus-host disease in humans receiving T-cell receptor-transduced autologous T cells. Mol. Ther. 18, 1744–1745 (2010).
Ritchie, D. S. et al. Persistence and efficacy of second generation CAR-T cell against the LeY antigen in acute myeloid leukemia. Mol. Ther. http://dx.doi.org/10.1038/mt.2013.154(2013).
Rapoport, A. P. et al. Adoptive transfer of gene-modified t-cells engineered to express high-affinity TCRs for cancer-testis antigens (CTAs) NY-ESO-1 or Lage-1, in MM patients post auto-SCT. ASH Annual Meeting Abstr. 120, 472 (2010).
Acknowledgements
The authors wish to acknowledge funding from the National Health and Medical Research Council of Australia.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Major histocompatibility complex
-
(MHC). A family of polymorphic molecules that are expressed on the surface of cells that associate with peptide antigens. T cells are activated by interaction between their T cell receptor and MHC peptide.
- Tumour-infiltrating lymphocytes
-
(TILs). Various lymphocytes exist, including T cells, B cells and natural killer (NK) cells. These types of cells can be found within tumours.
- Macrophages
-
A type of white blood cell that is able to engulf particulate matter or even whole cells.
- Granulocytes
-
A type of white blood cell with a lobular nucleus. There are three main types of granulocyte: neutrophil, basophil and eosinophil.
- Immune tolerance
-
A state in which autoreactive T cells and B cells are rendered non-responsive by the deletion or withdrawal of growth factors and survival signals. Tolerance can be induced at an early stage of cell development in a process termed central tolerance, or at later stages of cell development in a process termed peripheral tolerance.
- Allogeneic
-
From another individual of the same species.
- HLA type
-
The array of major histocompatibility complex genes in an individual.
- Humanized
-
Much of the molecular framework of mouse T cell receptors and antibodies is replaced with similar regions from their human molecular counterparts, while retaining the antigen-binding regions of the mouse molecules.
- CD4+ T helper cells
-
T cells that express CD4 and that normally interact with antigen in the context of major histocompatibility complex class II. On engagement with antigen, CD4+ T cells secrete cytokines that can stimulate ('help') CD8+ cytotoxic T lymphocytes to enable their optimal activation and proliferation.
- Co-stimulatory molecules
-
Molecules that are expressed on the surface of T cells and that transmit signals on engagement with their ligands expressed by tumour cells or antigen-presenting cells. These signals are transmitted, in addition to the primary activation signal mediated by the T cell receptor (TCR), to amplify the TCR signal, leading to the full activation of T cells.
- Cytotoxic T lymphocytes
-
(CTLs). A type of T cell that usually expresses CD8 and that recognizes antigen in the context of major histocompatibility complex class I. Following interaction between the T cell receptor and antigen, CD8+ T cells deliver cytotoxic hits against target cells through the secretion of lytic molecules.
- Central memory
-
T cells that express CCR7 and CD62L and that recirculate among lymphoid tissue. They have a greater capacity to secrete cytokines than stem cell memory T cells but a relatively lower ability to self-renew.
- Effector memory
-
T cells that lack expression of CD62L and CCR7 and that are predominantly found in peripheral tissues. Their ability to respond to antigen by secreting cytokines and carrying out cytolysis is increased above that of central memory T cells, but their capacity to self-renew is relatively low.
- Stem cell memory T cells
-
Derived from naive T cells and express CCR7 and CD62L, which enable homing to the lymphoid compartment. They have a low capacity to engage in cytolysis and secretion of cytokines in response to antigen, but have the greatest capacity to self-renew and are able to generate the other subsets of memory and effector T cells.
- Myeloid-derived suppressor cells
-
(MDSCs). A granulocyte-like cell, expressing CD11b and GR1, that can inhibit T cells through the production of TGFβ and the amino acid-depleting enzyme arginase.
- T regulatory cells
-
(TReg cells). A type of T cell, usually expressing CD4, CD25 and FOXP3, which can inhibit the function of other T cells through a number of mechanisms including consumption of IL-2, production of TGFβ and inhibition of antigen-presenting cells.
- Immune checkpoint receptors
-
Cell-surface molecules that are expressed by T cells and the normal function of which is to maintain self-tolerance and regulate the magnitude and duration of immune responses. Checkpoint receptors, including PD1 and TIM3, can be co-opted by tumours to inhibit antitumour immune responses.
- Objective responses
-
A reduction in tumour burden that satisfies the criteria for a complete or partial response.
- Partial responses
-
Usually refers to at least a 30% decrease in the sum of the longest diameters of all target lesions.
- Complete response
-
The disappearance of all target lesions.
- Colitis
-
Inflammation of the large intestine (colon) that can lead to bleeding, ulceration and loss of bowel function.
- Universal CARs
-
Have extracellular antibody domains specific for a fluorochrome or biotin. T cells bearing these CARs could be used in combination with a fluoresceinated or biotin-coupled antibody specific for an antigen on any type of tumour.
Rights and permissions
About this article
Cite this article
Kershaw, M., Westwood, J. & Darcy, P. Gene-engineered T cells for cancer therapy. Nat Rev Cancer 13, 525–541 (2013). https://doi.org/10.1038/nrc3565
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3565
This article is cited by
-
Ray of dawn: Anti-PD-1 immunotherapy enhances the chimeric antigen receptor T-cell therapy in Lymphoma patients
BMC Cancer (2023)
-
Combination therapy for pancreatic cancer: anti-PD-(L)1-based strategy
Journal of Experimental & Clinical Cancer Research (2022)
-
Adverse effects in hematologic malignancies treated with chimeric antigen receptor (CAR) T cell therapy: a systematic review and Meta-analysis
BMC Cancer (2022)
-
The new progress in cancer immunotherapy
Clinical and Experimental Medicine (2022)
-
Immunotherapy and Biomarkers in Sarcoma
Current Treatment Options in Oncology (2022)