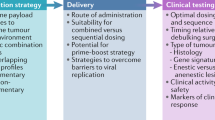
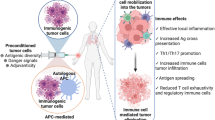
Abstract
Recent clinical data have emphatically shown the capacity of our immune systems to eradicate even advanced cancers. Although oncolytic viruses (OVs) were originally designed to function as tumour-lysing therapeutics, they have now been clinically shown to initiate systemic antitumour immune responses. Cell signalling pathways that are activated and promote the growth of tumour cells also favour the growth and replication of viruses within the cancer. The ability to engineer OVs that express immune-stimulating 'cargo', the induction of immunogenic tumour cell death by OVs and the selective targeting of OVs to tumour beds suggests that they are the ideal reagents to enhance antitumour immune responses. Coupling of OV therapy with tumour antigen vaccination, immune checkpoint inhibitors and adoptive cell therapy seems to be ready to converge towards a new generation of multimodal therapeutics to improve outcomes for cancer patients.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Glenney, J. R. Jr., Zokas, L. & Kamps, M. P. Monoclonal antibodies to phosphotyrosine. J. Immunol. Methods 109, 277–285 (1988).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nature Rev. Cancer 12, 252–264 (2012).
Kreiter, S., Castle, J. C., Tureci, O. & Sahin, U. Targeting the tumor mutanome for personalized vaccination therapy. Oncoimmunology 1, 768–769 (2012).
Russell, S. J., Peng, K. W. & Bell, J. C. Oncolytic virotherapy. Nature Biotech. 30, 658–670 (2012).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Kelly, E. & Russell, S. J. History of oncolytic viruses: genesis to genetic engineering. Mol. Ther. 15, 651–659 (2007).
Parato, K. A. et al. The oncolytic poxvirus JX-594 selectively replicates in and destroys cancer cells driven by genetic pathways commonly activated in cancers. Mol. Ther. 4, 749–758 (2011).
Stojdl, D. F. et al. VSV strains with defects in their ability to shutdown innate immunity are potent systemic anti-cancer agents. Cancer Cell 4, 263–275 (2003).
Breitbach, C. J. et al. Intravenous delivery of a multi-mechanistic cancer-targeted oncolytic poxvirus in humans. Nature 477, 99–102 (2011).
Liu, T. C., Hwang, T., Park, B. H., Bell, J. & Kirn, D. H. The targeted oncolytic poxvirus JX-594 demonstrates antitumoral, antivascular, and anti-HBV activities in patients with hepatocellular carcinoma. Mol. Ther. 16, 1637–1642 (2008).
Breitbach, C. J. et al. Oncolytic vaccinia virus disrupts tumor-associated vasculature in humans. Cancer Res. 73, 1265–1275 (2013).
Breitbach, C. J. et al. Targeted inflammation during oncolytic virus therapy severely compromises tumor blood flow. Mol. Ther. 15, 1686–1693 (2007).
Breitbach, C. J. et al. Targeting tumor vasculature with an oncolytic virus. Mol. Ther. 19, 886–894 (2011).
Liu, T. C., Galanis, E. & Kirn, D. Clinical trial results with oncolytic virotherapy: a century of promise, a decade of progress. Nature Clin. Pract. Oncol. 4, 101–117 (2007).
Vacchelli, E. et al. Trial watch: oncolytic viruses for cancer therapy. Oncoimmunology 2, e24612 (2013).
Miest, T. S. & Cattaneo, R. New viruses for cancer therapy: meeting clinical needs. Nature Rev. Microbiol. 12, 23–34 (2013).
Atherton, M. J. & Lichty, B. D. Evolution of oncolytic viruses: novel strategies for cancer treatment. Immunotherapy 5, 1191–1206 (2013).
Kaufman, H. L. & Bines, S. D. OPTIM trial: a Phase III trial of an oncolytic herpes virus encoding GM-CSF for unresectable stage III or IV melanoma. Future Oncol. 6, 941–949 (2010).
Garber, K. China approves world's first oncolytic virus therapy for cancer treatment. J. Natl Cancer Inst. 98, 298–300 (2006).
Senzer, N. N. et al. Phase II clinical trial of a granulocyte-macrophage colony-stimulating factor-encoding, second-generation oncolytic herpesvirus in patients with unresectable metastatic melanoma. J. Clin. Oncol. 27, 5763–5771 (2009).
Nemunaitis, J. et al. Intravenous infusion of a replication-selective adenovirus (ONYX-015) in cancer patients: safety, feasibility and biological activity. Gene Ther. 8, 746–759 (2001).
Park, B. H. et al. Use of a targeted oncolytic poxvirus, JX-594, in patients with refractory primary or metastatic liver cancer: a phase I trial. Lancet Oncol. 9, 533–542 (2008).
Heo, J. et al. Randomized dose-finding clinical trial of oncolytic immunotherapeutic vaccinia JX-594 in liver cancer. Nature Med. 19, 329–336 (2013).
Ingemar Andtbacka, R. H. et al. OPTiM: A randomized phase III trial of talimogene laherparepvec (T-VEC) versus subcutaneous (SC) granulocyte-macrophage colony-stimulating factor (GM-CSF) for the treatment (tx) of unresected stage IIIB/C & IV melanoma. J. Clin. Oncol. 31, abstract LBA9008 (2013).
Harrington, K. J. et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 16, 4005–4015 (2010).
Harrington, K. J. et al. Two-stage phase I dose-escalation study of intratumoral reovirus type 3 dearing and palliative radiotherapy in patients with advanced cancers. Clin. Cancer Res. 16, 3067–3077 (2010).
Heo, J. et al. Sequential therapy with JX-594, a targeted oncolytic poxvirus, followed by sorafenib in hepatocellular carcinoma: preclinical and clinical demonstration of combination efficacy. Mol. Ther. 19, 1170–1179 (2011).
Cerullo, V. et al. Immunological effects of low-dose cyclophosphamide in cancer patients treated with oncolytic adenovirus. Mol. Ther. 19, 1737–1746 (2011).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. New Engl. J. Med. 363, 411–422 (2010).
Small, E. J. et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 24, 3089–3094 (2006).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. New Engl. J. Med. 363, 711–723 (2010).
Qureshi, O. S. et al. Trans-endocytosis of CD80 and CD86: a molecular basis for the cell-extrinsic function of CTLA-4. Science 332, 600–603 (2011).
Dudley, M. E. & Rosenberg, S. A. Adoptive-cell-transfer therapy for the treatment of patients with cancer. Nature Rev. Cancer 3, 666–675 (2003).
Kershaw, M. H., Westwood, J. A. & Darcy, P. K. Gene-engineered T cells for cancer therapy. Nature Rev. Cancer 13, 525–541 (2013).
Adair, R. A. et al. Cell carriage, delivery, and selective replication of an oncolytic virus in tumor in patients. Sci. Transl. Med. 4, 138ra77 (2012).
Kottke, T. et al. Treg depletion-enhanced IL-2 treatment facilitates therapy of established tumors using systemically delivered oncolytic virus. Mol. Ther. 16, 1217–1226 (2008).
Diaz, R. M. et al. Oncolytic immunovirotherapy for melanoma using vesicular stomatitis virus. Cancer Res. 67, 2840–2848 (2007).
Liu, B. L. et al. ICP34.5 deleted herpes simplex virus with enhanced oncolytic, immune stimulating, and anti-tumour properties. Gene Ther. 10, 292–303 (2003).
Moehler, M. H. et al. Parvovirus H-1-induced tumor cell death enhances human immune response in vitro via increased phagocytosis, maturation, and cross-presentation by dendritic cells. Hum. Gene Ther. 16, 996–1005 (2005).
Mastrangelo, M. J. et al. Intratumoral recombinant GM-CSF-encoding virus as gene therapy in patients with cutaneous melanoma. Cancer Gene Ther. 6, 409–422 (1999).
Hwang, T. H. et al. A mechanistic proof-of-concept clinical trial with JX-594, a targeted multi-mechanistic oncolytic poxvirus, in patients with metastatic melanoma. Mol. Ther. 19, 1913–1922 (2011).
Kim, M. K. et al. Oncolytic and immunotherapeutic vaccinia induces antibody-mediated complement-dependent cancer cell lysis in humans. Sci. Transl. Med. 5, 185ra63 (2013).
Sukkurwala, A. Q. et al. Immunogenic calreticulin exposure occurs through a phylogenetically conserved stress pathway involving the chemokine CXCL8. Cell Death Differ. 21, 59–68 (2014).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nature Med. 15, 1170–1178 (2009).
Zelenay, S. et al. The dendritic cell receptor DNGR-1 controls endocytic handling of necrotic cell antigens to favor cross-priming of CTLs in virus-infected mice. J. Clin. Invest. 122, 1615–1627 (2012).
Lee, B. H. et al. Activation of P2X(7) receptor by ATP plays an important role in regulating inflammatory responses during acute viral infection. PLoS ONE 7, e35812 (2012).
Huang, B., Sikorski, R., Kirn, D. H. & Thorne, S. H. Synergistic anti-tumor effects between oncolytic vaccinia virus and paclitaxel are mediated by the IFN response and HMGB1. Gene Ther. 18, 164–172 (2011).
Miyamoto, S. et al. Coxsackievirus B3 is an oncolytic virus with immunostimulatory properties that is active against lung adenocarcinoma. Cancer Res. 72, 2609–2621 (2012).
Postow, M. A. et al. Immunologic correlates of the abscopal effect in a patient with melanoma. N. Engl. J. Med. 366, 925–931 (2012).
Galivo, F. et al. Interference of CD40L-mediated tumor immunotherapy by oncolytic vesicular stomatitis virus. Hum. Gene Ther. 21, 439–450 (2010).
Schirrmacher, V., Griesbach, A. & Ahlert, T. Antitumor effects of Newcastle disease virus in vivo: local versus systemic effects. Int. J. Oncol. 18, 945–952 (2001).
Diallo, J. S. et al. A high-throughput pharmacoviral approach identifies novel oncolytic virus sensitizers. Mol. Ther. 18, 1123–1129 (2010).
Nguyen, T. L. et al. Chemical targeting of the innate antiviral response by histone deacetylase inhibitors renders refractory cancers sensitive to viral oncolysis. Proc. Natl Acad. Sci. USA 105, 14981–14986 (2008).
Jha, B. K., Dong, B., Nguyen, C. T., Polyakova, I. & Silverman, R. H. Suppression of antiviral innate immunity by sunitinib enhances oncolytic virotherapy. Mol. Ther. 21, 1749–1757 (2013).
Bose, A. et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int. J. Cancer 129, 2158–2170 (2011).
Beug, S. T. et al. Smac mimetics and innate immune stimuli synergize to promote tumor death. Nature Biotech. 32, 182–190 (2014).
Hu, J. C. et al. A phase I study of OncoVEXGM-CSF, a second-generation oncolytic herpes simplex virus expressing granulocyte macrophage colony-stimulating factor. Clin. Cancer Res. 12, 6737–6747 (2006).
Curran, M. A. & Allison, J. P. Tumor vaccines expressing flt3 ligand synergize with ctla-4 blockade to reject preimplanted tumors. Cancer Res. 69, 7747–7755 (2009).
Epardaud, M. et al. Interleukin-15/interleukin-15Rα complexes promote destruction of established tumors by reviving tumor-resident CD8+ T cells. Cancer Res. 68, 2972–2983 (2008).
Liu, R. B. et al. IL-15 in tumor microenvironment causes rejection of large established tumors by T cells in a noncognate T cell receptor-dependent manner. Proc. Natl Acad. Sci. USA 110, 8158–8163 (2013).
Onu, A., Pohl, T., Krause, H. & Bulfone-Paus, S. Regulation of IL-15 secretion via the leader peptide of two IL-15 isoforms. J. Immunol. 158, 255–262 (1997).
Yu, F. et al. T-cell engager-armed oncolytic vaccinia virus significantly enhances antitumor therapy. Mol. Ther. 22, 102–111 (2014).
Amato, R. J. et al. Vaccination of renal cell cancer patients with modified vaccinia ankara delivering tumor antigen 5T4 (TroVax) administered with interleukin 2: a phase II trial. Clin. Cancer Res. 14, 7504–7510 (2008).
Harrop, R. et al. Vaccination of colorectal cancer patients with modified vaccinia Ankara delivering the tumor antigen 5T4 (TroVax) induces immune responses which correlate with disease control: a phase I/II trial. Clin. Cancer Res. 12, 3416–3424 (2006).
Horig, H. et al. Phase I clinical trial of a recombinant canarypoxvirus (ALVAC) vaccine expressing human carcinoembryonic antigen and the B7.1 co-stimulatory molecule. Cancer Immunol. Immunother. 49, 504–514 (2000).
Jager, E. et al. Recombinant vaccinia/fowlpox NY-ESO-1 vaccines induce both humoral and cellular NY-ESO-1-specific immune responses in cancer patients. Proc. Natl Acad. Sci. USA 103, 14453–14458 (2006).
Kaufman, H. L. et al. Combination chemotherapy and ALVAC-CEA/B7.1 vaccine in patients with metastatic colorectal cancer. Clin. Cancer Res. 14, 4843–4849 (2008).
Madan, R. A. et al. Ipilimumab and a poxviral vaccine targeting prostate-specific antigen in metastatic castration-resistant prostate cancer: a phase 1 dose-escalation trial. Lancet Oncol. 13, 501–508 (2012).
Morse, M. A. et al. Novel adenoviral vector induces T-cell responses despite anti-adenoviral neutralizing antibodies in colorectal cancer patients. Cancer Immunol. Immunother. 62, 1293–1301 (2013).
Odunsi, K. et al. Efficacy of vaccination with recombinant vaccinia and fowlpox vectors expressing NY-ESO-1 antigen in ovarian cancer and melanoma patients. Proc. Natl Acad. Sci. USA 109, 5797–5802 (2012).
Harrop, R., John, J. & Carroll, M. W. Recombinant viral vectors: cancer vaccines. Adv. Drug Deliv. Rev. 58, 931–947 (2006).
Elzey, B. D., Siemens, D. R., Ratliff, T. L. & Lubaroff, D. M. Immunization with type 5 adenovirus recombinant for a tumor antigen in combination with recombinant canarypox virus (ALVAC) cytokine gene delivery induces destruction of established prostate tumors. Int. J. Cancer 94, 842–849 (2001).
Hodge, J. W., McLaughlin, J. P., Kantor, J. A. & Schlom, J. Diversified prime and boost protocols using recombinant vaccinia virus and recombinant non-replicating avian pox virus to enhance T-cell immunity and antitumor responses. Vaccine 15, 759–768 (1997).
Hodge, J. W. et al. Modified vaccinia virus ankara recombinants are as potent as vaccinia recombinants in diversified prime and boost vaccine regimens to elicit therapeutic antitumor responses. Cancer Res. 63, 7942–7949 (2003).
Irvine, K. R. et al. Enhancing efficacy of recombinant anticancer vaccines with prime/boost regimens that use two different vectors. J. Natl Cancer Inst. 89, 1595–1601 (1997).
Marshall, J. L. et al. Phase I study in advanced cancer patients of a diversified prime-and-boost vaccination protocol using recombinant vaccinia virus and recombinant nonreplicating avipox virus to elicit anti-carcinoembryonic antigen immune responses. J. Clin. Oncol. 18, 3964–3973 (2000).
Naslund, T. I. et al. Comparative prime-boost vaccinations using Semliki Forest virus, adenovirus, and ALVAC vectors demonstrate differences in the generation of a protective central memory CTL response against the P815 tumor. J. Immunol. 178, 6761–6769 (2007).
Bridle, B. W. et al. Potentiating cancer immunotherapy using an oncolytic virus. Mol. Ther. 18, 1430–1439 (2010).
Vigil, A., Martinez, O., Chua, M. A. & Garcia-Sastre, A. Recombinant Newcastle disease virus as a vaccine vector for cancer therapy. Mol. Ther. 16, 1883–1890 (2008).
Pol, J. G. et al. Maraba virus as a potent oncolytic vaccine vector. Mol. Ther. 22, 420–429 (2014).
Loi, S. Tumor-infiltrating lymphocytes, breast cancer subtypes and therapeutic efficacy. Oncoimmunology 2, e24720 (2013).
Zamarin, D. et al. Localized oncolytic virotherapy overcomes systemic tumor resistance to immune checkpoint blockade immunotherapy. Sci. Transl. Med. 6, 226ra32 (2014).
Puzanov, I. et al. Primary analysis of a phase 1b multicenter trial to evaluate safety and efficacy of talimogene laherparepvec (T-VEC) and ipilimumab (ipi) in previously untreated, unresected stage IIIB-IV melanoma. J Clin. Oncol. 32 (suppl; abstr 9029^) (2014).
Pule, M. A. et al. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nature Med. 14, 1264–1270 (2008).
Martins, I. et al. Restoration of the immunogenicity of cisplatin-induced cancer cell death by endoplasmic reticulum stress. Oncogene 30, 1147–1158 (2011).
Galluzzi, L. & Kroemer, G. Autophagy mediates the metabolic benefits of endurance training. Circ. Res. 110, 1276–1278 (2012).
Kaczmarek, A., Vandenabeele, P. & Krysko, D. V. Necroptosis: the release of damage-associated molecular patterns and its physiological relevance. Immunity 38, 209–223 (2013).
Krysko, D. V. et al. Immunogenic cell death and DAMPs in cancer therapy. Nature Rev. Cancer 12, 860–875 (2012).
Kaufman, H. L. et al. Local and distant immunity induced by intralesional vaccination with an oncolytic herpes virus encoding GM-CSF in patients with stage IIIc and IV melanoma. Ann. Surg. Oncol. 17, 718–730 (2010).
Kim, J. H. et al. Systemic armed oncolytic and immunologic therapy for cancer with JX-594, a targeted poxvirus expressing GM-CSF. Mol. Ther. 14, 361–370 (2006).
Lee, J. H. et al. Oncolytic and immunostimulatory efficacy of a targeted oncolytic poxvirus expressing human GM-CSF following intravenous administration in a rabbit tumor model. Cancer Gene Ther. 17, 73–79 (2010).
Cerullo, V. et al. Oncolytic adenovirus coding for granulocyte macrophage colony-stimulating factor induces antitumoral immunity in cancer patients. Cancer Res. 70, 4297–4309 (2010).
Chang, J. et al. A Phase I study of KH901, a conditionally replicating granulocyte-macrophage colony-stimulating factor: armed oncolytic adenovirus for the treatment of head and neck cancers. Cancer Biol. Ther. 8, 676–682 (2009).
Robinson, M. et al. Novel immunocompetent murine tumor model for evaluation of conditionally replication-competent (oncolytic) murine adenoviral vectors. J. Virol. 83, 3450–3462 (2009).
Vigil, A. et al. Use of reverse genetics to enhance the oncolytic properties of Newcastle disease virus. Cancer Res. 67, 8285–8292 (2007).
Grote, D., Cattaneo, R. & Fielding, A. K. Neutrophils contribute to the measles virus-induced antitumor effect: enhancement by granulocyte macrophage colony-stimulating factor expression. Cancer Res. 63, 6463–6468 (2003).
Bergman, I., Griffin, J. A., Gao, Y. & Whitaker-Dowling, P. Treatment of implanted mammary tumors with recombinant vesicular stomatitis virus targeted to Her2/neu. Int. J. Cancer 121, 425–430 (2007).
Bernt, K. M., Ni, S., Tieu, A. T. & Lieber, A. Assessment of a combined, adenovirus-mediated oncolytic and immunostimulatory tumor therapy. Cancer Res. 65, 4343–4352 (2005).
Ramakrishna, E. et al. Antitumoral immune response by recruitment and expansion of dendritic cells in tumors infected with telomerase-dependent oncolytic viruses. Cancer Res. 69, 1448–1458 (2009).
Leveille, S., Goulet, M. L., Lichty, B. D. & Hiscott, J. Vesicular stomatitis virus oncolytic treatment interferes with tumor-associated dendritic cell functions and abrogates tumor antigen presentation. J. Virol. 85, 12160–12169 (2011).
Lapteva, N. et al. Targeting the intratumoral dendritic cells by the oncolytic adenoviral vaccine expressing RANTES elicits potent antitumor immunity. J. Immunother. 32, 145–156 (2009).
Li, J. et al. Chemokine expression from oncolytic vaccinia virus enhances vaccine therapies of cancer. Mol. Ther. 19, 650–657 (2011).
Carew, J. F. et al. A novel approach to cancer therapy using an oncolytic herpes virus to package amplicons containing cytokine genes. Mol. Ther. 4, 250–256 (2001).
Zhao, H., Janke, M., Fournier, P. & Schirrmacher, V. Recombinant Newcastle disease virus expressing human interleukin-2 serves as a potential candidate for tumor therapy. Virus Res. 136, 75–80 (2008).
Post, D. E. et al. Targeted cancer gene therapy using a hypoxia inducible factor dependent oncolytic adenovirus armed with interleukin-4. Cancer Res. 67, 6872–6881 (2007).
Terada, K., Wakimoto, H., Tyminski, E., Chiocca, E. A. & Saeki, Y. Development of a rapid method to generate multiple oncolytic HSV vectors and their in vivo evaluation using syngeneic mouse tumor models. Gene Ther. 13, 705–714 (2006).
Choi, I. K. et al. Oncolytic adenovirus co-expressing IL-12 and IL-18 improves tumor-specific immunity via differentiation of T cells expressing IL-12Rβ2 or IL-18Rα. Gene Ther. 18, 898–909 (2011).
Lee, Y. S. et al. Enhanced antitumor effect of oncolytic adenovirus expressing interleukin-12 and B7-1 in an immunocompetent murine model. Clin. Cancer Res. 12, 5859–5868 (2006).
Derubertis, B. G. et al. Cytokine-secreting herpes viral mutants effectively treat tumor in a murine metastatic colorectal liver model by oncolytic and T-cell-dependent mechanisms. Cancer Gene Ther. 14, 590–597 (2007).
Varghese, S. et al. Enhanced therapeutic efficacy of IL-12, but not GM-CSF, expressing oncolytic herpes simplex virus for transgenic mouse derived prostate cancers. Cancer Gene Ther. 13, 253–265 (2006).
Shin, E. J. et al. Interleukin-12 expression enhances vesicular stomatitis virus oncolytic therapy in murine squamous cell carcinoma. Laryngoscope 117, 210–214 (2007).
Gaston, D. C. et al. Production of bioactive soluble interleukin-15 in complex with interleukin-15 receptor alpha from a conditionally-replicating oncolytic HSV-1. PLoS ONE 8, e81768 (2013).
Stephenson, K. B., Barra, N. G., Davies, E., Ashkar, A. A. & Lichty, B. D. Expressing human interleukin-15 from oncolytic vesicular stomatitis virus improves survival in a murine metastatic colon adenocarcinoma model through the enhancement of anti-tumor immunity. Cancer Gene Ther. 19, 238–246 (2012).
van Rikxoort, M. et al. Oncolytic effects of a novel influenza A virus expressing interleukin-15 from the NS reading frame. PLoS ONE 7, e36506 (2012).
Fukuhara, H., Ino, Y., Kuroda, T., Martuza, R. L. & Todo, T. Triple gene-deleted oncolytic herpes simplex virus vector double-armed with interleukin 18 and soluble B7-1 constructed by bacterial artificial chromosome-mediated system. Cancer Res. 65, 10663–10668 (2005).
Ino, Y., Saeki, Y., Fukuhara, H. & Todo, T. Triple combination of oncolytic herpes simplex virus-1 vectors armed with interleukin-12, interleukin-18, or soluble B7-1 results in enhanced antitumor efficacy. Clin. Cancer Res. 12, 643–652 (2006).
Kirn, D. H., Wang, Y., Le Boeuf, F., Bell, J. & Thorne, S. H. Targeting of interferon-beta to produce a specific, multi-mechanistic oncolytic vaccinia virus. PLoS Med. 4, e353 (2007).
Li, H., Peng, K. W., Dingli, D., Kratzke, R. A. & Russell, S. J. Oncolytic measles viruses encoding interferon β and the thyroidal sodium iodide symporter gene for mesothelioma virotherapy. Cancer Gene Ther. 17, 550–558 (2010).
Shashkova, E. V., Spencer, J. F., Wold, W. S. & Doronin, K. Targeting interferon-α increases antitumor efficacy and reduces hepatotoxicity of E1A-mutated spread-enhanced oncolytic adenovirus. Mol. Ther. 15, 598–607 (2007).
Shashkova, E. V., Kuppuswamy, M. N., Wold, W. S. & Doronin, K. Anticancer activity of oncolytic adenovirus vector armed with IFN-α and ADP is enhanced by pharmacologically controlled expression of TRAIL. Cancer Gene Ther. 15, 61–72 (2008).
Willmon, C. L. et al. Expression of IFN-β enhances both efficacy and safety of oncolytic vesicular stomatitis virus for therapy of mesothelioma. Cancer Res. 69, 7713–7720 (2009).
Su, C. et al. Immune gene-viral therapy with triplex efficacy mediated by oncolytic adenovirus carrying an interferon-γ gene yields efficient antitumor activity in immunodeficient and immunocompetent mice. Mol. Ther. 13, 918–927 (2006).
Choi, K. J. et al. Concurrent delivery of GM-CSF and B7-1 using an oncolytic adenovirus elicits potent antitumor effect. Gene Ther. 13, 1010–1020 (2006).
Todo, T., Martuza, R. L., Dallman, M. J. & Rabkin, S. D. In situ expression of soluble B7-1 in the context of oncolytic herpes simplex virus induces potent antitumor immunity. Cancer Res. 61, 153–161 (2001).
Huang, J. H. et al. Therapeutic and tumor-specific immunity induced by combination of dendritic cells and oncolytic adenovirus expressing IL-12 and 4-1BBL. Mol. Ther. 18, 264–274 (2010).
Kim, H. S., Kim-Schulze, S., Kim, D. W. & Kaufman, H. L. Host lymphodepletion enhances the therapeutic activity of an oncolytic vaccinia virus expressing 4-1BB ligand. Cancer Res. 69, 8516–8525 (2009).
Li, J. L. et al. A phase I trial of intratumoral administration of recombinant oncolytic adenovirus overexpressing HSP70 in advanced solid tumor patients. Gene Ther. 16, 376–382 (2009).
Yoo, J. Y. et al. Tumor suppression by apoptotic and anti-angiogenic effects of mortalin-targeting adeno-oncolytic virus. J. Gene Med. 12, 586–595 (2010).
Hu, Z. B. et al. A simplified system for generating oncolytic adenovirus vector carrying one or two transgenes. Cancer Gene Ther. 15, 173–182 (2008).
Acknowledgements
B.D.L., D.F.S. and J.C.B. are supported by Ontario Institute for Cancer Research, Terry Fox Foundation. D.F.S. and J.C.B. are supported by the Canadian Institutes of Health Research (CIHR), and the Canadian Cancer Society Research Institute (CCSRI). B.D.L. is supported by the Canadian Breast Cancer Foundation (CBCF).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
Employment: SillaJen Biotherapeutics (C.J.B.). B.D.L., D.F.S. and J.C.B. declare no competing interests.
Related links
DATABASES
Rights and permissions
About this article
Cite this article
Lichty, B., Breitbach, C., Stojdl, D. et al. Going viral with cancer immunotherapy. Nat Rev Cancer 14, 559–567 (2014). https://doi.org/10.1038/nrc3770
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrc3770
This article is cited by
-
A combination of genetically engineered oncolytic virus and melittin-CpG for cancer viro-chemo-immunotherapy
BMC Medicine (2023)
-
Oncolytic virotherapy evolved into the fourth generation as tumor immunotherapy
Journal of Translational Medicine (2023)
-
Dual peptides-modified cationic liposomes for enhanced Lung cancer gene therapy by a gap junction regulating strategy
Journal of Nanobiotechnology (2023)
-
Mesenchymal stem cell-released oncolytic virus: an innovative strategy for cancer treatment
Cell Communication and Signaling (2023)
-
Intratumoral microbiota: roles in cancer initiation, development and therapeutic efficacy
Signal Transduction and Targeted Therapy (2023)