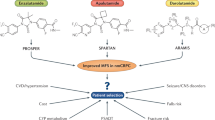
Abstract
Castration-resistant prostate cancer (CRPC) has a poor prognosis and remains a significant therapeutic challenge. Before 2010, only docetaxel-based chemotherapy improved survival in patients with CRPC compared with mitoxantrone. Our improved understanding of the underlying biology of CRPC has heralded a new era in molecular anticancer drug development, with a myriad of novel anticancer drugs for CRPC entering the clinic. These include the novel taxane cabazitaxel, the vaccine sipuleucel-T, the CYP17 inhibitor abiraterone, the novel androgen-receptor antagonist MDV-3100 and the radioisotope alpharadin. With these developments, the management of patients with CRPC is changing. In this Review, we discuss these promising therapies along with other novel agents that are demonstrating early signs of activity in CRPC. We propose a treatment pathway for patients with CRPC and consider strategies to optimize the use of these agents, including the incorporation of predictive and intermediate end point biomarkers, such as circulating tumor cells.
Key Points
-
Castration-resistant prostate cancer (CRPC) is associated with a poor prognosis and remains a significant therapeutic challenge
-
Before 2010, there was an urgent unmet clinical need for more-effective and well-tolerated therapies for CRPC
-
Improved understanding of the underlying biology of CRPC heralded a new era in molecular anticancer drug development
-
New treatments for CRPC imparting an overall survival benefit include cabazitaxel, sipuleucel-T, abiraterone and alpharadin
-
The management of patients with CRPC is changing; therefore, we propose a novel treatment pathway including the incorporation of predictive and intermediate end point biomarkers
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Jemal, A., Siegel, R., Xu, J. & Ward, E. Cancer statistics, 2010. CA Cancer J. Clin. 60, 277–300 (2010).
Ferlay, J., Parkin, D. M. & Steliarova-Foucher, E. Estimates of cancer incidence and mortality in Europe in 2008. Eur. J. Cancer 46, 765–781 (2010).
Pienta, K. J. & Bradley, D. Mechanisms underlying the development of androgen-independent prostate cancer. Clin. Cancer Res. 12, 1665–1671 (2006).
Yap, T. A., Carden, C. P., Attard, G. & de Bono, J. S. Targeting CYP17: established and novel approaches in prostate cancer. Curr. Opin. Pharmacol. 8, 449–457 (2008).
[No authors listed]. Maximum androgen blockade in advanced prostate cancer: an overview of the randomised trials. Prostate Cancer Trialists' Collaborative Group. Lancet 355, 1491–1498 (2000).
Figg, W. D. et al. Prostate specific antigen decline following the discontinuation of flutamide in patients with stage D2 prostate cancer. Am. J. Med. 98, 412–414 (1995).
Nishimura, K. et al. Low doses of oral dexamethasone for hormone-refractory prostate carcinoma. Cancer 89, 2570–2576 (2000).
Small, E. J. et al. Antiandrogen withdrawal alone or in combination with ketoconazole in androgen-independent prostate cancer patients: a phase III trial (CALGB 9583). J. Clin. Oncol. 22, 1025–1033 (2004).
Tannock, I. F. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer. N. Engl. J. Med. 351, 1502–1512 (2004).
Petrylak, D. P. et al. Docetaxel and estramustine compared with mitoxantrone and prednisone for advanced refractory prostate cancer. N. Engl. J. Med. 351, 1513–1520 (2004).
Attard, G. et al. Improving the outcome of patients with castration-resistant prostate cancer through rational drug development. Br. J. Cancer 95, 767–774 (2006).
Chen, Y., Clegg, N. J. & Scher, H. I. Anti-androgens and androgen-depleting therapies in prostate cancer: new agents for an established target. Lancet Oncol. 10, 981–991 (2009).
Yamaoka, M., Hara, T. & Kusaka, M. Overcoming persistent dependency on androgen signaling after progression to castration-resistant prostate cancer. Clin. Cancer Res. 16, 4319–4324 (2010).
Bruno, R. D. & Njar, V. C. Targeting cytochrome P450 enzymes: a new approach in anti-cancer drug development. Bioorg. Med. Chem. 15, 5047–5060 (2007).
Labrie, C., Cusan, L., Plante, M., Lapointe, S. & Labrie, F. Analysis of the androgenic activity of synthetic “progestins” currently used for the treatment of prostate cancer. J. Steroid Biochem. 28, 379–384 (1987).
Titus, M. A., Schell, M. J., Lih, F. B., Tomer, K. B. & Mohler, J. L. Testosterone and dihydrotestosterone tissue levels in recurrent prostate cancer. Clin. Cancer Res. 11, 4653–4657 (2005).
He, B. et al. Structural basis for androgen receptor interdomain and coactivator interactions suggests a transition in nuclear receptor activation function dominance. Mol. Cell 16, 425–438 (2004).
Taplin, M. E. et al. A phase II study of mifepristone (RU-486) in castration-resistant prostate cancer, with a correlative assessment of androgen-related hormones. BJU Int. 101, 1084–1089 (2008).
Chen, C. D. et al. Molecular determinants of resistance to antiandrogen therapy. Nat. Med. 10, 33–39 (2004).
Harris, W. P., Mostaghel, E. A., Nelson, P. S. & Montgomery, B. Androgen deprivation therapy: progress in understanding mechanisms of resistance and optimizing androgen depletion. Nat. Clin. Pract. Urol. 6, 76–85 (2009).
Venkitaraman, R. et al. Efficacy of low-dose dexamethasone in castration-refractory prostate cancer. BJU Int. 101, 440–443 (2008).
Tannock, I. F. et al. Chemotherapy with mitoxantrone plus prednisone or prednisone alone for symptomatic hormone-resistant prostate cancer: a Canadian randomized trial with palliative end points. J. Clin. Oncol. 14, 1756–1764 (1996).
Kantoff, P. W. et al. Hydrocortisone with or without mitoxantrone in men with hormone-refractory prostate cancer: results of the cancer and leukemia group B 9182 study. J. Clin. Oncol. 17, 2506–2513 (1999).
Nishimura, K. et al. Potential mechanism for the effects of dexamethasone on growth of androgen-independent prostate cancer. J. Natl Cancer Inst. 93, 1739–1746 (2001).
Morioka, M. et al. Prostate-specific antigen levels and prognosis in patients with hormone-refractory prostate cancer treated with low-dose dexamethasone. Urol. Int. 68, 10–15 (2002).
Saika, T. et al. Treatment of androgen-independent prostate cancer with dexamethasone: a prospective study in stage D2 patients. Int. J. Urol. 8, 290–294 (2001).
Berry, W., Dakhil, S., Modiano, M., Gregurich, M. & Asmar, L. Phase III study of mitoxantrone plus low dose prednisone versus low dose prednisone alone in patients with asymptomatic hormone refractory prostate cancer. J. Urol. 168, 2439–2443 (2002).
Fossa, S. D. et al. Weekly docetaxel and prednisolone versus prednisolone alone in androgen-independent prostate cancer: a randomized phase II study. Eur. Urol. 52, 1691–1698 (2007).
Chang, C. Y., Walther, P. J. & McDonnell, D. P. Glucocorticoids manifest androgenic activity in a cell line derived from a metastatic prostate cancer. Cancer Res. 61, 8712–8717 (2001).
Fousteris, M. A. et al. 20-Aminosteroids as a novel class of selective and complete androgen receptor antagonists and inhibitors of prostate cancer cell growth. Bioorg. Med. Chem. 18, 6960–6969 (2010).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Scher, H. I. et al. Antitumour activity of MDV3100 in castration-resistant prostate cancer: a phase 1–2 study. Lancet 375, 1437–1446 (2010).
US National Library of Medicine. ClinicalTrials.gov [online], (2011).
Jarman, M., Barrie, S. E. & Llera, J. M. The 16, 17-double bond is needed for irreversible inhibition of human cytochrome p45017alpha by abiraterone (17-(3-pyridyl) androsta-5, 16-dien-3beta-ol) and related steroidal inhibitors. J. Med. Chem. 41, 5375–5381 (1998).
Rowlands, M. G. et al. Esters of 3-pyridylacetic acid that combine potent inhibition of 17 alpha-hydroxylase/C17, 20-lyase (cytochrome P45017 alpha) with resistance to esterase hydrolysis. J. Med. Chem. 38, 4191–4197 (1995).
Potter, G. A., Barrie, S. E., Jarman, M. & Rowlands, M. G. Novel steroidal inhibitors of human cytochrome P45017 alpha (17 alpha-hydroxylase-C17, 20-lyase): potential agents for the treatment of prostatic cancer. J. Med. Chem. 38, 2463–2471 (1995).
Barrie, S. E. et al. Pharmacology of novel steroidal inhibitors of cytochrome P450(17) alpha (17 alpha-hydroxylase/C17–20 lyase). J. Steroid Biochem. Mol. Biol. 50, 267–273 (1994).
Bianchini, D., Zivi, A., Sandhu, S. & de Bono, J. S. Horizon scanning for novel therapeutics for the treatment of prostate cancer. Expert Opin. Investig. Drugs 19, 1487–1502 (2010).
O'Donnell, A. et al. Hormonal impact of the 17alpha-hydroxylase/C(17, 20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br. J. Cancer 90, 2317–2325 (2004).
Attard, G. et al. Phase I clinical trial of a selective inhibitor of CYP17, abiraterone acetate, confirms that castration-resistant prostate cancer commonly remains hormone driven. J. Clin. Oncol. 26, 4563–4571 (2008).
Attard, G. et al. Selective inhibition of CYP17 with abiraterone acetate is highly active in the treatment of castration-resistant prostate cancer. J. Clin. Oncol. 27, 3742–3748 (2009).
Reid, A. H. et al. Significant and sustained antitumor activity in post-docetaxel, castration-resistant prostate cancer with the CYP17 inhibitor abiraterone acetate. J. Clin. Oncol. 28, 1489–1495 (2010).
Ryan, C. J. et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J. Clin. Oncol. 28, 1481–1488 (2010).
Danila, D. C. et al. Phase II multicenter study of abiraterone acetate plus prednisone therapy in patients with docetaxel-treated castration-resistant prostate cancer. J. Clin. Oncol. 28, 1496–1501 (2010).
de Bono, J. S. et al. Abiraterone and increased survival in metastatic prostate cancer. N. Engl. J. Med. 364, 1995–2005 (2011).
FDA. Abiraterone Acetate [online], (2011).
Dreicer, R. et al. Safety, pharmacokinetics, and efficacy of TAK-700 in metastatic castration-resistant prostrate cancer: A phase I/II, open-label study [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a3084 (2010).
Handratta, V. D. et al. Novel C-17-heteroaryl steroidal CYP17 inhibitors/antiandrogens: synthesis, in vitro biological activity, pharmacokinetics, and antitumor activity in the LAPC4 human prostate cancer xenograft model. J. Med. Chem. 48, 2972–2984 (2005).
Tokai Pharmaceuticals. Tokai pharmaceuticals initiates ARMOR clinical development program for TOK-001; first ever multi-target investigational drug for prostate cancer [online], (2009).
Berthold, D. R. et al. Docetaxel plus prednisone or mitoxantrone plus prednisone for advanced prostate cancer: updated survival in the TAX 327 study. J. Clin. Oncol. 26, 242–245 (2008).
Scher, H. I. et al. Docetaxel (D) plus high-dose calcitriol versus D plus prednisone (P) for patients (Pts) with progressive castration-resistant prostate cancer (CRPC): Results from the phase III ASCENT2 trial [abstract]. J. Clin. Oncol. 28, (15 Suppl.), a4509 (2010).
Small, E. et al. A phase III trial of GVAX immunotherapy for prostate cancer in combination with docetaxel versus docetaxel plus prednisone in symptomatic, castration-resistant prostate cancer (CRPC) [abstract]. Genitourinary Cancers Symp. a7 (2009).
Kelly, W. K. et al. A randomized, double-blind, placebo-controlled phase III trial comparing docetaxel, prednisone, and placebo with docetaxel, prednisone, and bevacizumab in men with metastatic castration-resistant prostate cancer (mCRPC): Survival results of CALGB 90401 [abstract]. J. Clin. Oncol. 28 (18 Suppl.), LBA4511 (2010).
Franke, R. M., Carducci, M. A., Rudek, M. A., Baker, S. D. & Sparreboom, A. Castration-dependent pharmacokinetics of docetaxel in patients with prostate cancer. J. Clin. Oncol. 28, 4562–4567 (2010).
Ansari, J. et al. Docetaxel chemotherapy for metastatic hormone refractory prostate cancer as first-line palliative chemotherapy and subsequent re-treatment: Birmingham experience. Oncol. Rep. 20, 891–896 (2008).
Eymard, J. C. et al. Docetaxel reintroduction in patients with metastatic castration-resistant docetaxel-sensitive prostate cancer: a retrospective multicentre study. BJU Int. 106, 974–978 (2010).
Bracarda, S., Logothetis, C., Sternberg, C. N. & Oudard, S. Current and emerging treatment modalities for metastatic castration-resistant prostate cancer. BJU Int. 107 (Suppl. 2), 13–20 (2011).
Sartor, A. O. et al. Satraplatin in patients with advanced hormone-refractory prostate cancer (HRPC): Overall survival (OS) results from the phase III satraplatin and prednisone against refractory cancer (SPARC) trial [abstract]. J. Clin. Oncol. 26, a5003 (2008).
Kelland, L. R. et al. Preclinical antitumor evaluation of bis-acetato-ammine-dichloro-cyclohexylamine platinum(IV): an orally active platinum drug. Cancer Res. 53, 2581–2586 (1993).
Yap, T. A., Sandhu, S. K., Carden, C. P. & de Bono, J. S. Poly(ADP-Ribose) polymerase (PARP) inhibitors: Exploiting a synthetic lethal strategy in the clinic. CA Cancer J. Clin. 61, 31–49 (2011).
de Bono, J. S. et al. Prednisone plus cabazitaxel or mitoxantrone for metastatic castration-resistant prostate cancer progressing after docetaxel treatment: a randomised open-label trial. Lancet 376, 1147–1154 (2010).
Attard, G., Greystoke, A., Kaye, S. & De Bono, J. Update on tubulin-binding agents. Pathol. Biol. (Paris) 54, 72–84 (2006).
Aller, A. W., Kraus, L. A. & Bissery, M.-C. In vitro activity of TXD258 in chemotherapeutic resistant tumor cell lines [abstract 1923]. Proc. Am. Assoc. Cancer Res. 41, 303 (2000).
Bissery, M.-C. et al. Preclinical evaluation of TXD258, a new taxoid [abstract 1364]. Proc. Am. Assoc. Cancer Res. 41, 214 (2000).
Mita, A. C. et al. Phase I and pharmacokinetic study of XRP6258 (RPR 116258A), a novel taxane, administered as a 1-hour infusion every 3 weeks in patients with advanced solid tumors. Clin. Cancer Res. 15, 723–730 (2009).
Pivot, X. et al. A multicenter phase II study of XRP6258 administered as a 1-h i.v. infusion every 3 weeks in taxane-resistant metastatic breast cancer patients. Ann. Oncol. 19, 1547–1552 (2008).
FDA. Cabazitaxel [online], (2010).
Rosenberg, J. E. et al. Activity of second-line chemotherapy in docetaxel-refractory hormone-refractory prostate cancer patients: randomized phase 2 study of ixabepilone or mitoxantrone and prednisone. Cancer 110, 556–563 (2007).
Beardsley, E. K. et al. A phase II study of patupilone in patients (pts) with metastatic castration- resistant prostate cancer (CRPC) who have progressed after docetaxel [abstract 5139]. J. Clin. Oncol. 27 (15 Suppl.), a5139 (2009).
Ribas, A. & Flaherty, K. T. BRAF targeted therapy changes the treatment paradigm in melanoma. Nat. Rev. Clin. Oncol. 8, 426–433 (2011).
Sanda, M. G. et al. Demonstration of a rational strategy for human prostate cancer gene therapy. J. Urol. 151, 622–628 (1994).
Wang, X. et al. Autoantibody signatures in prostate cancer. N. Engl. J. Med. 353, 1224–1235 (2005).
Drake, C. G. Prostate cancer as a model for tumour immunotherapy. Nat. Rev. Immunol. 10, 580–93 (2010).
Di Lorenzo, G., Buonerba, C. & Kantoff, P. W. Immunotherapy for the treatment of prostate cancer. Nat. Rev. Clin. Oncol. doi:10.1038/nrclinonc.2011.72.
Brahmer, J. R. et al. Phase I study of single-agent anti-programmed death-1 (MDX-1106) in refractory solid tumors: safety, clinical activity, pharmacodynamics, and immunologic correlates. J. Clin. Oncol. 28, 3167–3175 (2010).
Small, E. J. et al. Placebo-controlled phase III trial of immunologic therapy with sipuleucel-T (APC8015) in patients with metastatic, asymptomatic hormone refractory prostate cancer. J. Clin. Oncol. 24, 3089–3094 (2006).
Higano, C. S. et al. Integrated data from 2 randomized, double-blind, placebo-controlled, phase 3 trials of active cellular immunotherapy with sipuleucel-T in advanced prostate cancer. Cancer 115, 3670–3679 (2009).
Kantoff, P. W. et al. Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N. Engl. J. Med. 363, 411–422 (2010).
Higano, C. S. et al. Sipuleucel-T. Nat. Rev. Drug Discov. 9, 513–514 (2010).
Nilsson, S., O' Bryan-Tear, C. G., Bolstad, B., Lokna, A. & Parker, C. C. Alkaline phosphatase (ALP) normalization and overall survival in patients with bone metastases from castration-resistant prostate cancer (CRPC) treated with radium-223 [abstract]. J. Clin. Oncol. 29 (Suppl.), a4620 (2011).
Bayer. Alpharadin significantly improves overall survival in phase III trial in patients with castration-resistant prostate cancer that has spread to the bone [online], (2011).
James, N. D. et al. Final safety and efficacy analysis of the specific endothelin A receptor antagonist zibotentan (ZD4054) in patients with metastatic castration-resistant prostate cancer and bone metastases who were pain-free or mildly symptomatic for pain: a double-blind, placebo-controlled, randomized phase II trial. BJU Int. 106, 966–973 (2010).
Nelson, J. B. et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer 113, 2478–2487 (2008).
Carducci, M. A. et al. A phase 3 randomized controlled trial of the efficacy and safety of atrasentan in men with metastatic hormone-refractory prostate cancer. Cancer 110, 1959–1966 (2007).
Kummar, S., Gutierrez, M., Doroshow, J. H. & Murgo, A. J. Drug development in oncology: classical cytotoxics and molecularly targeted agents. Br. J. Clin. Pharmacol. 62, 15–26 (2006).
Biomarkers Definitions Working Group. Biomarkers and surrogate endpoints: preferred definitions and conceptual framework. Clin. Pharmacol. Ther. 69, 89–95 (2001).
Yap, T. A., Sandhu, S. K., Workman, P. & de Bono, J. S. Envisioning the future of early anticancer drug development. Nat. Rev. Cancer 10, 514–523 (2010).
Attard, G. et al. Characterization of ERG, AR and PTEN gene status in circulating tumor cells from patients with castration-resistant prostate cancer. Cancer Res. 69, 2912–2918 (2009).
Petrovics, G. et al. Frequent overexpression of ETS-related gene-1 (ERG1) in prostate cancer transcriptome. Oncogene 24, 3847–3852 (2005).
Tomlins, S. A. et al. Recurrent fusion of TMPRSS2 and ETS transcription factor genes in prostate cancer. Science 310, 644–648 (2005).
Attard, G. et al. Duplication of the fusion of TMPRSS2 to ERG sequences identifies fatal human prostate cancer. Oncogene 27, 253–263 (2008).
Narod, S. A., Seth, A. & Nam, R. Fusion in the ETS gene family and prostate cancer. Br. J. Cancer 99, 847–851 (2008).
Bubley, G. J. et al. Eligibility and response guidelines for phase II clinical trials in androgen-independent prostate cancer: recommendations from the Prostate-Specific Antigen Working Group. J. Clin. Oncol. 17, 3461–3467 (1999).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl Cancer Inst. 92, 205–216 (2000).
Scher, H. I. et al. Design and end points of clinical trials for patients with progressive prostate cancer and castrate levels of testosterone: recommendations of the Prostate Cancer Clinical Trials Working Group. J. Clin. Oncol. 26, 1148–1159 (2008).
Yap, T. A., Sarker, D., Kaye, S. B. & de Bono, J. S in Imaging in Oncology 3rd edn Ch. 61 (eds Husband, J. E. & Reznek, R. H.) 1366–1383 (Informa Healthcare, New York, USA 2010).
Scher, H. I., Warren, M. & Heller, G. The association between measures of progression and survival in castrate-metastatic prostate cancer. Clin. Cancer Res. 13, 1488–1492 (2007).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Lilja, H., Ulmert, D. & Vickers, A. J. Prostate-specific antigen and prostate cancer: prediction, detection and monitoring. Nat. Rev. Cancer 8, 268–278 (2008).
D'Amico, A. V. et al. Predictors of mortality after prostate-specific antigen failure. Int. J. Radiat. Oncol. Biol. Phys. 65, 656–660 (2006).
Attard, G. & de Bono, J. S. Prostate cancer: PSA as an intermediate end point in clinical trials. Nat. Rev. Urol. 6, 473–475 (2009).
Attard, G. & de Bono, J. S. Utilizing circulating tumor cells: challenges and pitfalls. Curr. Opin. Genet. Dev. 21, 50–58 (2010).
Defeo, E. M., Wu, C. L., McDougal, W. S. & Cheng, L. L. A decade in prostate cancer: from NMR to metabolomics. Nat. Rev. Urol. 8, 301–311 (2011).
FDA. CellSearch™ Epithelial Cell Kit/ CellSpotter™ Analyzer—K031588 [online], (2009).
Zhu, M. L. et al. Tubulin-targeting chemotherapy impairs androgen receptor activity in prostate cancer. Cancer Res. 70, 7992–8002 (2010).
Sternberg, C. N. et al. Multinational, double-blind, phase III study of prednisone and either satraplatin or placebo in patients with castrate-refractory prostate cancer progressing after prior chemotherapy: the SPARC trial. J. Clin. Oncol. 27, 5431–5438 (2009).
Sternberg, C. N. et al. Docetaxel plus oblimersen sodium (Bcl-2 antisense oligonucleotide): an EORTC multicenter, randomized phase II study in patients with castration-resistant prostate cancer. Ann. Oncol. 20, 1264–1269 (2009).
Sonpavde, G. et al. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann. Oncol. 21, 319–324 (2009).
Cathomas, R. et al. Cetuximab in combination with docetaxel in patients (pts) with metastatic castration resistant (mCRPC) and docetaxel-refractory prostate cancer: A multicenter phase II trial (SAKK 08/07) [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a4666 (2010).
Small, E. et al. Randomized phase II study comparing 4 monthly doses of ipilimumab (MDX-010) as a single agent or in combination with a single dose of docetaxel in patients with hormone-refractory prostate cancer [abstract]. J. Clin. Oncol. 24 (18 Suppl.), a4609 (2006).
Beer, T. M. et al. Randomized, double-blind, phase III trial to compare the efficacy of ipilimumab (Ipi) versus placebo in asymptomatic or minimally symptomatic patients (pts) with metastatic chemotherapy-naïve castration-resistant prostate cancer (CRPC) [abstract]. J Clin. Oncol. 29 (Suppl.), TPS182 (2011).
Drake, C. G. et al. A randomized, double-blind, phase III trial comparing ipilimumab versus placebo following radiotherapy (RT) in patients (pts) with castration-resistant prostate cancer (CRPC) who have received prior treatment with docetaxel (D) [abstract]. J. Clin. Oncol. 29 (Suppl.), TPS181 (2011).
Tollefson, M. K. et al. A randomized phase II study of ipilimumab with androgen ablation compared with androgen ablation alone in patients with advanced prostate cancer [abstract]. Genitourinary Cancers Symp. a168 (2010).
Granberg, C. F. et al. Conversion of advanced prostate cancer to organ-confined minimal residual disease using CTLA-4 blockade (ipilimumab) immunotherapy [abstract]. Genitourinary Cancers Symp. a33 (2010).
Mohebtash, M. et al. Phase I trial of targeted therapy with PSA-TRICOM vaccine (V) and ipilimumab (ipi) in patients (pts) with metastatic castration-resistant prostate cancer (mCRPC) [abstract]. J. Clin. Oncol. 27 (15 Suppl.), a5144 (2009).
Madan, R. A. et al. Overall survival (OS) analysis of a phase l trial of a vector-based vaccine (PSA-TRICOM) and ipilimumab (Ipi) in the treatment of metastatic castration-resistant prostate cancer (mCRPC) [abstract]. Genitourinary Cancers Symp. a38 (2010).
Fong, L. et al. Potentiating endogenous antitumor immunity to prostate cancer through combination immunotherapy with CTLA4 blockade and GM-CSF. Cancer Res. 69, 609–615 (2009).
Kantoff, P. W. et al. Overall survival analysis of a phase II randomized controlled trial of a Poxviral-based PSA-targeted immunotherapy in metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 1099–1105 (2010).
Heath, E. I. et al. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin. Prostate Cancer 4, 138–141 (2005).
Heath, E. I. et al. A phase II trial of 17-allylamino-17-demethoxygeldanamycin in patients with hormone-refractory metastatic prostate cancer. Clin. Cancer Res. 14, 7940–7946 (2008).
Chi, K. N. et al. Randomized phase II study of docetaxel and prednisone with or without OGX-011 in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 4247–4254 (2010).
Chi, K. N. et al. A phase I dose-finding study of combined treatment with an antisense Bcl-2 oligonucleotide (Genasense) and mitoxantrone in patients with metastatic hormone-refractory prostate cancer. Clin. Cancer Res. 7, 3920–3927 (2001).
Tolcher, A. W. Preliminary phase I results of G3139 (bcl-2 antisense oligonucleotide) therapy in combination with docetaxel in hormone-refractory prostate cancer. Semin. Oncol. 28, 67–70 (2001).
Liu, G. et al. An open-label, multicenter, phase I/II study of single-agent AT-101 in men with castrate-resistant prostate cancer. Clin. Cancer Res. 15, 3172–3176 (2009).
Tolcher, A. W. et al. Phase I and pharmacokinetic study of YM155, a small-molecule inhibitor of survivin. J. Clin. Oncol. 26, 5198–5203 (2008).
Tolcher, A. W. et al. Phase I/II open-label study of YM155 plus docetaxel and prednisone in men with hormone refractory prostate cancer (HRPC) [abstract]. Genitourinary Cancers Symp. a214 (2009).
Fong, P. C. et al. Inhibition of poly(ADP-ribose) polymerase in tumors from BRCA mutation carriers. N. Engl. J. Med. 361, 123–134 (2009).
Sonpavde, G. et al. Azacitidine favorably modulates PSA kinetics correlating with plasma DNA LINE-1 hypomethylation in men with chemonaive castration-resistant prostate cancer. Urol. Oncol. doi:10.1016/j.urolonc.2009.09.015.
Sonpavde, G. et al. Phase II study of azacitidine to restore responsiveness of prostate cancer to hormonal therapy. Clin. Genitourin. Cancer 5, 457–459 (2007).
Bradley, D. et al. Vorinostat in advanced prostate cancer patients progressing on prior chemotherapy (National Cancer Institute Trial 6862): trial results and interleukin-6 analysis: a study by the Department of Defense Prostate Cancer Clinical Trial Consortium and University of Chicago Phase 2 Consortium. Cancer 115, 5541–5549 (2009).
Schneider, B. J. et al. Phase I study of vorinostat (suberoylanilide hydroxamic acid, NSC 701852) in combination with docetaxel in patients with advanced and relapsed solid malignancies. Invest. New Drugs doi:10.1007/s10637-010-9503-6.
Molife, L. R. et al. Phase II, two-stage, single-arm trial of the histone deacetylase inhibitor (HDACi) romidepsin in metastatic castration-resistant prostate cancer (CRPC). Ann. Oncol. 21, 109–113 (2009).
Picus, J. et al. A phase 2 study of estramustine, docetaxel, and bevacizumab in men with castrate-resistant prostate cancer: results from Cancer and Leukemia Group B Study 90006. Cancer 117, 525–533 (2011).
Ning, Y. M. et al. Phase II trial of bevacizumab, thalidomide, docetaxel, and prednisone in patients with metastatic castration-resistant prostate cancer. J. Clin. Oncol. 28, 2070–2076 (2010).
Dahut, W. L. et al. A phase II clinical trial of sorafenib in androgen-independent prostate cancer. Clin. Cancer Res. 14, 209–214 (2008).
Chi, K. N. et al. A phase II study of sorafenib in patients with chemo-naive castration-resistant prostate cancer. Ann. Oncol. 19, 746–751 (2008).
Steinbild, S. et al. A clinical phase II study with sorafenib in patients with progressive hormone-refractory prostate cancer: a study of the CESAR Central European Society for Anticancer Drug Research-EWIV. Br. J. Cancer 97, 1480–1485 (2007).
Sonpavde, G. et al. Sunitinib malate for metastatic castration-resistant prostate cancer following docetaxel-based chemotherapy. Ann. Oncol. 21, 319–324 (2010).
Tew, W. P. et al. Phase 1 study of aflibercept administered subcutaneously to patients with advanced solid tumors. Clin. Cancer Res. 16, 358–366 (2010).
Adelberg, D. et al. A phase II study of cediranib in post-docetaxel, castration-resistant prostate cancer (CRPC) [abstract]. Genitourinary Cancers Symp. a63 (2010).
Horti, J. et al. A randomized, double-blind, placebo-controlled phase II study of vandetanib plus docetaxel/prednisolone in patients with hormone-refractory prostate cancer. Cancer Biother. Radiopharm. 24, 175–180 (2009).
Tiffany, N. M., Wersinger, E. M., Garzotto, M. & Beer, T. M. Imatinib mesylate and zoledronic acid in androgen-independent prostate cancer. Urology 63, 934–939 (2004).
Mathew, P. et al. Platelet-derived growth factor receptor inhibition and chemotherapy for castration-resistant prostate cancer with bone metastases. Clin. Cancer Res. 13, 5816–5824 (2007).
Baselga, J. et al. Phase I safety, pharmacokinetics, and inhibition of SRC activity study of saracatinib in patients with solid tumors. Clin. Cancer Res. 16, 4876–4883 (2010).
Yu, E. Y. et al. Once-daily dasatinib: expansion of phase II study evaluating safety and efficacy of dasatinib in patients with metastatic castration-resistant prostate cancer. Urology 77, 1166–1171 (2011).
Armstrong, A. J. et al. A phase I-II study of docetaxel and atrasentan in men with castration-resistant metastatic prostate cancer. Clin. Cancer Res. 14, 6270–6276 (2008).
James, N. D. et al. Safety and efficacy of the specific endothelin-A receptor antagonist ZD4054 in patients with hormone-resistant prostate cancer and bone metastases who were pain free or mildly symptomatic: a double-blind, placebo-controlled, randomised, phase 2 trial. Eur. Urol. 55, 1112–1123 (2009).
Slovin, S. F. et al. Anti-epidermal growth factor receptor monoclonal antibody cetuximab plus Doxorubicin in the treatment of metastatic castration-resistant prostate cancer. Clin. Genitourin. Cancer 7, E77–E82 (2009).
Boccardo, F. et al. Prednisone plus gefitinib versus prednisone plus placebo in the treatment of hormone-refractory prostate cancer: a randomized phase II trial. Oncology 74, 223–228 (2008).
Small, E. J. et al. A phase II trial of gefitinib in patients with non-metastatic hormone-refractory prostate cancer. BJU Int. 100, 765–769 (2007).
Canil, C. M. et al. Randomized phase II study of two doses of gefitinib in hormone-refractory prostate cancer: a trial of the National Cancer Institute of Canada-Clinical Trials Group. J. Clin. Oncol. 23, 455–460 (2005).
Salzberg, M. et al. An open-label, noncomparative phase II trial to evaluate the efficacy and safety of docetaxel in combination with gefitinib in patients with hormone-refractory metastatic prostate cancer. Onkologie 30, 355–360 (2007).
Ziada, A. et al. The use of trastuzumab in the treatment of hormone refractory prostate cancer; phase II trial. Prostate 60, 332–337 (2004).
Small, E. J., Bok, R., Reese, D. M., Sudilovsky, D. & Frohlich, M. Docetaxel, estramustine, plus trastuzumab in patients with metastatic androgen-independent prostate cancer. Semin. Oncol. 28, 71–76 (2001).
Sridhar, S. S. et al. A multicenter phase II clinical trial of lapatinib (GW572016) in hormonally untreated advanced prostate cancer. Am. J. Clin. Oncol. 33, 609–613 (2010).
Dreicer, R. et al. Oral enzastaurin in prostate cancer: A two-cohort phase II trial in patients with PSA progression in the non-metastatic castrate state and following docetaxel-based chemotherapy for castrate metastatic disease. Invest. New Drugs doi:10.1007/s10637-010-9428-0.
Higano, C. et al. A phase II study evaluating the efficacy and safety of single agent IMC A12, a monoclonal antibody (MAb), against the insulin-like growth factor-1 receptor (IGF-IR), as monotherapy in patients with metastastic, asymptomatic castration-resistant prostate cancer (CRPC) [abstract]. J. Clin. Oncol. 27 (15 Suppl.), a5142 (2009).
Chi, K. N. et al. A phase II study of preoperative figitumumab (F) in patients (pts) with localized prostate cancer (PCa) [abstract]. J. Clin. Oncol. 28 (15 Suppl.), a4662 (2010).
Molife, L. R. et al. The insulin-like growth factor-I receptor inhibitor figitumumab (CP-751,871) in combination with docetaxel in patients with advanced solid tumours: results of a phase Ib dose-escalation, open-label study. Br. J. Cancer 103, 332–339 (2010).
Dorff, T. B. et al. Clinical and correlative results of SWOG S0354: a phase II trial of CNTO328 (siltuximab), a monoclonal antibody against interleukin-6, in chemotherapy-pretreated patients with castration-resistant prostate cancer. Clin. Cancer Res. 16, 3028–3034 (2010).
Hussain, M. et al. Cabozantinib (XL184) in metastatic castration-resistant prostate cancer (mCRPC): Results from a phase II randomized discontinuation trial [abstract]. J. Clin. Oncol. 29 (Suppl.) a4516 (2011).
Yap, T. A. et al. Phase I trial of a selective c-MET inhibitor ARQ 197 incorporating proof of mechanism pharmacodynamic studies. J. Clin. Oncol. 29, 1271–1279 (2011).
Mekhail, T. et al. Final results: A dose escalation phase I study of ARQ 197, a selective c-Met inhibitor, in patients with metastatic solid tumors [abstract]. J. Clin. Oncol. 27 (15 Suppl.), a3548 (2009).
Holzbeierlein, J. et al. Gene expression analysis of human prostate carcinoma during hormonal therapy identifies androgen-responsive genes and mechanisms of therapy resistance. Am. J. Pathol. 164, 217–227 (2004).
Stanbrough, M. et al. Increased expression of genes converting adrenal androgens to testosterone in androgen-independent prostate cancer. Cancer Res. 66, 2815–2825 (2006).
Edwards, J., Krishna, N. S., Grigor, K. M. & Bartlett, J. M. Androgen receptor gene amplification and protein expression in hormone refractory prostate cancer. Br. J. Cancer 89, 552–556 (2003).
Brooke, G. N., Parker, M. G. & Bevan, C. L. Mechanisms of androgen receptor activation in advanced prostate cancer: differential co-activator recruitment and gene expression. Oncogene 27, 2941–2950 (2008).
Hara, T. et al. Novel mutations of androgen receptor: a possible mechanism of bicalutamide withdrawal syndrome. Cancer Res. 63, 149–153 (2003).
Elo, J. P. et al. Mutated human androgen receptor gene detected in a prostatic cancer patient is also activated by estradiol. J. Clin. Endocrinol. Metab. 80, 3494–3500 (1995).
Tan, J. et al. Dehydroepiandrosterone activates mutant androgen receptors expressed in the androgen-dependent human prostate cancer xenograft CWR22 and LNCaP cells. Mol. Endocrinol. 11, 450–459 (1997).
Schweizer, L. et al. The androgen receptor can signal through Wnt/beta-catenin in prostate cancer cells as an adaptation mechanism to castration levels of androgens. BMC Cell Biol. 9, 4 (2008).
Marcelli, M. et al. Androgen receptor mutations in prostate cancer. Cancer Res. 60, 944–949 (2000).
Taplin, M. E. et al. Mutation of the androgen-receptor gene in metastatic androgen-independent prostate cancer. N. Engl. J. Med. 332, 1393–1398 (1995).
Fenton, M. A. et al. Functional characterization of mutant androgen receptors from androgen-independent prostate cancer. Clin. Cancer Res. 3, 1383–1388 (1997).
Sartor, A. O. et al. Antiandrogen withdrawal in castrate-refractory prostate cancer: a Southwest Oncology Group trial (SWOG 9426). Cancer 112, 2393–2400 (2008).
Small, E. J. & Srinivas, S. The antiandrogen withdrawal syndrome. Experience in a large cohort of unselected patients with advanced prostate cancer. Cancer 76, 1428–1434 (1995).
Nadiminty, N. et al. Aberrant activation of the androgen receptor by NF-kappaB2/p52 in prostate cancer cells. Cancer Res. 70, 3309–3319 (2010).
Kim, H. J. & Lee, W. J. Ligand-independent activation of the androgen receptor by insulin-like growth factor-I and the role of the MAPK pathway in skeletal muscle cells. Mol. Cells 28, 589–593 (2009).
Wegiel, B. et al. Molecular pathways in the progression of hormone-independent and metastatic prostate cancer. Curr. Cancer Drug Targets 10, 392–401 (2010).
Mahajan, N. P. et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proc. Natl Acad. Sci. USA 104, 8438–8443 (2007).
Gao, S., Liu, G. Z. & Wang, Z. Modulation of androgen receptor-dependent transcription by resveratrol and genistein in prostate cancer cells. Prostate 59, 214–225 (2004).
Whitaker, H. C. & Neal, D. E. RAS pathways in prostate cancer—mediators of hormone resistance? Curr. Cancer Drug Targets 10, 834–839 (2010).
Carver, B. S. et al. Reciprocal feedback regulation of PI3K and androgen receptor signaling in PTEN-deficient prostate cancer. Cancer Cell 19, 575–586 (2011).
Chandarlapaty, S. et al. AKT inhibition relieves feedback suppression of receptor tyrosine kinase expression and activity. Cancer Cell 19, 58–71 (2011).
Mulholland, D. J. et al. Cell autonomous role of PTEN in regulating castration-resistant prostate cancer growth. Cancer Cell 19, 792–804 (2011).
Wu, J. D. et al. Interaction of IGF signaling and the androgen receptor in prostate cancer progression. J. Cell Biochem. 99, 392–401 (2006).
Wen, Y. et al. HER-2/neu promotes androgen-independent survival and growth of prostate cancer cells through the Akt pathway. Cancer Res. 60, 6841–6845 (2000).
Verras, M. et al. The androgen receptor negatively regulates the expression of c-Met: implications for a novel mechanism of prostate cancer progression. Cancer Res. 67, 967–975 (2007).
Leotoing, L. et al. Influence of nucleophosmin/B23 on DNA binding and transcriptional activity of the androgen receptor in prostate cancer cell. Oncogene 27, 2858–2867 (2008).
Desai, S. J., Ma, A. H., Tepper, C. G., Chen, H. W. & Kung, H. J. Inappropriate activation of the androgen receptor by nonsteroids: involvement of the Src kinase pathway and its therapeutic implications. Cancer Res. 66, 10449–10459 (2006).
Comuzzi, B. et al. The transcriptional co-activator cAMP response element-binding protein-binding protein is expressed in prostate cancer and enhances androgen- and anti-androgen-induced androgen receptor function. Am. J. Pathol. 162, 233–241 (2003).
Zhou, X. E. et al. Identification of SRC3/AIB1 as a preferred coactivator for hormone-activated androgen receptor. J. Biol. Chem. 285, 9161–9171 (2010).
Sadar, M. D. Small molecule inhibitors targeting the “achilles' heel” of androgen receptor activity. Cancer Res 71, 1208–1213 (2011).
Author information
Authors and Affiliations
Contributions
All authors contributed to the research, discussion of content, writing, editing and reviewing of this manuscript.
Corresponding author
Ethics declarations
Competing interests
T. A. Yap, A. Zivi, A. Omlin and J. S. de Bono are employees of The Institute of Cancer Research, where abiraterone was first designed and synthesized and which has a commercial interest in abiraterone. J. S. de Bono has received consulting fees from Ortho Biotech Oncology Research and Development (a unit of Cougar Biotechnology), consulting fees and travel support from Amgen, Astellas, AstraZeneca, Boehringer Ingelheim, Bristol-Myers Squibb, Dendreon, Enzon, Exelixis, Genentech, GlaxoSmithKline, Medivation, Merck, Novartis, Pfizer, Roche, Sanofi-Aventis, Supergen, and Takeda, and grant support from AstraZeneca.
Supplementary information
Supplementary Table 1
Ongoing clinical trials in chemotherapy-naive patients with CRPC (DOC 100 kb)
Supplementary Table 2
Ongoing clinical trials in CRPC previously treated with chemotherapy (DOC 75 kb)
Rights and permissions
About this article
Cite this article
Yap, T., Zivi, A., Omlin, A. et al. The changing therapeutic landscape of castration-resistant prostate cancer. Nat Rev Clin Oncol 8, 597–610 (2011). https://doi.org/10.1038/nrclinonc.2011.117
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2011.117
This article is cited by
-
Spatio-temporal analysis of prostate tumors in situ suggests pre-existence of treatment-resistant clones
Nature Communications (2022)
-
Targeting dual-specificity tyrosine phosphorylation-regulated kinase 2 with a highly selective inhibitor for the treatment of prostate cancer
Nature Communications (2022)
-
Prognostic impact of bone metastatic volume beyond vertebrae and pelvis in patients with metastatic hormone-sensitive prostate cancer
International Journal of Clinical Oncology (2021)
-
Atorvastatin and Caffeine in Combination Regulates Apoptosis, Migration, Invasion and Tumorspheres of Prostate Cancer Cells
Pathology & Oncology Research (2020)
-
Oncolytic vaccinia virus as a vector for therapeutic sodium iodide symporter gene therapy in prostate cancer
Gene Therapy (2016)