Key Points
-
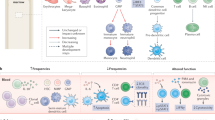
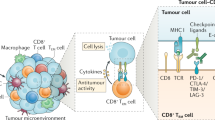
The detection of tumour-infiltrating lymphocytes (TILs) on routine histology constitutes a robust prognostic and predictive biomarker in patients with early stage breast cancer, despite the complexity of host antitumour immunity
-
Ongoing efforts to ensure reliable and reproducible reporting of TILs will facilitate their use in the routine management of breast cancer
-
Exploiting the antitumour immune response in breast cancer for therapeutic benefit is currently an area of active research
-
Early phase trials of antibodies that target programmed cell-death protein 1 (PD-1) and PD1 ligand 1 in patients with metastatic triple-negative breast cancer have shown promising and durable responses
-
Useful biomarkers to predict benefit from immunotherapy are urgently needed — TILs might fulfil this role
Abstract
The clinical relevance of the host immune system in breast cancer has long been unexplored. Studies developed over the past decade have highlighted the biological heterogeneity of breast cancer, prompting researchers to investigate whether the role of the immune system in this malignancy is similar across different molecular subtypes of the disease. The presence of high levels of lymphocytic infiltration has been consistently associated with a more-favourable prognosis in patients with early stage triple-negative and HER2-positive breast cancer. These infiltrates seem to reflect favourable host antitumour immune responses, suggesting that immune activation is important for improving survival outcomes. In this Review, we discuss the composition of the immune infiltrates observed in breast cancers, as well as data supporting the clinical relevance of host antitumour immunity, as represented by lymphocytic infiltration, and how this biomarker could be used in the clinical setting. We also discuss the rationale for enhancing immunity in breast cancer, including early data on the efficacy of T-cell checkpoint inhibition in this setting.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Underwood, J. C. Lymphoreticular infiltration in human tumours: prognostic and biological implications: a review. Br. J. Cancer 30, 538–548 (1974).
Tsuyuguchi, I., Shiratsuchi, H. & Fukuoka, M. T-lymphocyte subsets in primary lung cancer. Jpn J. Clin. Oncol. 17, 13–17 (1987).
Sistrunk, W. E. & Maccarty, W. C. Life expectancy following radical amputation for carcinoma of the breast: a clinical and pathologic study of 218 cases. Ann. Surg. 75, 61–69 (1922).
Tang, R. P. et al. Oncogene amplification correlates with dense lymphocyte infiltration in human breast cancers: a role for hematopoietic growth factor release by tumor cells? J. Cell. Biochem. 44, 189–198 (1990).
Whitford, P., George, W. D. & Campbell, A. M. Flow cytometric analysis of tumour infiltrating lymphocyte activation and tumour cell MHC class I and II expression in breast cancer patients. Cancer Lett. 61, 157–164 (1992).
Chin, Y. et al. Phenotypic analysis of tumor-infiltrating lymphocytes from human breast cancer. Anticancer Res. 12, 1463–1466 (1992).
Schwartzentruber, D. J., Topalian, S. L., Mancini, M. & Rosenberg, S. A. Specific release of granulocyte-macrophage colony-stimulating factor, tumor necrosis factor-alpha, and IFN-gamma by human tumor-infiltrating lymphocytes after autologous tumor stimulation. J. Immunol. 146, 3674–3681 (1991).
Tanaka, H., Watanabe, M., Zeniya, M. & Takahashi, H. Ultrastructure of IL2-stimulated tumor-infiltrating lymphocytes showing cytolytic activity against tumor cells. Acta Pathol. Jpn 41, 94–105 (1991).
Schwartzentruber, D. J., Solomon, D., Rosenberg, S. A. & Topalian, S. L. Characterization of lymphocytes infiltrating human breast cancer: specific immune reactivity detected by measuring cytokine secretion. J. Immunother. 12, 1–12 (1992).
Baxevanis, C. N. et al. Tumor specific cytolysis by tumor infiltrating lymphocytes in breast cancer. Cancer 74, 1275–1282 (1994).
Dadmarz, R., Sgagias, M. K., Rosenberg, S. A. & Schwartzentruber, D. J. CD4+ T lymphocytes infiltrating human breast cancer recognise autologous tumor in an MHC-class-II restricted fashion. Cancer Immunol. Immunother. 40, 1–9 (1995).
Degnim, A. C. et al. Immune cell quantitation in normal breast tissue lobules with and without lobulitis. Breast Cancer Res. Treat. 144, 539–549 (2014).
Gouon-Evans, V., Rothenberg, M. E. & Pollard, J. W. Postnatal mammary gland development requires macrophages and eosinophils. Development 127, 2269–2282 (2000).
Tuaillon, E. et al. Human milk-derived B cells: a highly activated switched memory cell population primed to secrete antibodies. J. Immunol. 182, 7155–7162 (2009).
Ruffell, B. et al. Leukocyte composition of human breast cancer. Proc. Natl Acad. Sci. USA 109, 2796–2801 (2012).
Willard-Gallo, K. et al. The significance of tumor infiltrating lymphocyte density, subset composition and organization in breast cancer. [abstract] Cancer Res. 75, aPD1-3 (2015).
Yamaguchi, R. et al. Tumor-infiltrating lymphocytes are important pathologic predictors for neoadjuvant chemotherapy in patients with breast cancer. Hum. Pathol. 43, 1688–1694 (2012).
Loi, S. et al. Prognostic and predictive value of tumor-infiltrating lymphocytes in a Phase III randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98. J. Clin. Oncol. 31, 860–867 (2013).
Ali, H. R. et al. Association between CD8+ T-cell infiltration and breast cancer survival in 12 439 patients. Ann. Oncol. 25, 1536–1543 (2014).
Schmidt, M. et al. A comprehensive analysis of human gene expression profiles identifies stromal immunoglobulin κ C as a compatible prognostic marker in human solid tumors. Clin. Cancer Res. 18, 2695–2703 (2012).
Ignatiadis, M. et al. Gene modules and response to neoadjuvant chemotherapy in breast cancer subtypes: a pooled analysis. J. Clin. Oncol. 30, 1996–2004 (2012).
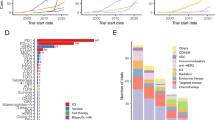
Salgado, R. et al. The evaluation of tumor-infiltrating lymphocytes (TILs) in breast cancer: recommendations by an International TILs Working Group 2014. Ann. Oncol. 26, 259–271 (2014).
Gu-Trantien, C. et al. CD4+ follicular helper T cell infiltration predicts breast cancer survival. J. Clin. Invest. 123, 2873–2892 (2013).
Denkert, C. et al. Tumor-associated lymphocytes as an independent predictor of response to neoadjuvant chemotherapy in breast cancer. J. Clin. Oncol. 28, 105–113 (2010).
Nawaz, S., Heindl, A., Koelble, K. & Yuan, Y. Beyond immune density: critical role of spatial heterogeneity in estrogen receptor-negative breast cancer. Mod. Pathol. 28, 766–777 (2015).
Loi, S. Host antitumor immunity plays a role in the survival of patients with newly diagnosed triple-negative breast cancer. J. Clin. Oncol. 32, 2935–2937 (2014).
Polley, M.-Y. C. et al. An international Ki67 reproducibility study. J. Natl Cancer Inst. 105, 1897–1906 (2013).
Van de Pavert, S. A. & Mebius, R. E. New insights into the development of lymphoid tissues. Nat. Rev. Immunol. 10, 664–674 (2010).
Brown, M. & Wittwer, C. Flow cytometry: principles and clinical applications in hematology. Clin. Chem. 46, 1221–1229 (2000).
Stack, E. C., Wang, C., Roman, K. A. & Hoyt, C. C. Multiplexed immunohistochemistry, imaging, and quantitation: a review, with an assessment of Tyramide signal amplification, multispectral imaging and multiplex analysis. Methods 70, 46–58 (2014).
Denkert, C. et al. Tumor-infiltrating lymphocytes and response to neoadjuvant chemotherapy with or without carboplatin in human epidermal growth factor receptor 2-positive and triple-negative primary breast cancers. J. Clin. Oncol. 33, 983–991 (2015).
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015).
Cortazar, P. et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet 384, 164–172 (2014).
Tan, M. C. et al. Predictors of complete pathological response after neoadjuvant systemic therapy for breast cancer. Am. J. Surg. 198, 520–525 (2009).
Kraus, J. A. et al. Predictors of pathologic complete response after standard neoadjuvant chemotherapy in triple-negative breast carcinoma. Appl. Immunohistochem. Mol. Morphol. 20, 334–339 (2012).
Kurozumi, S. et al. ER, PgR, Ki67, 27Kip1, and histological grade as predictors of pathological complete response in patients with HER2-positive breast cancer receiving neoadjuvant chemotherapy using taxanes followed by fluorouracil, epirubicin, and cyclophosphamide conco. BMC Cancer 15, 622 (2015).
Simon, R. M., Paik, S. & Hayes, D. F. Use of archived specimens in evaluation of prognostic and predictive biomarkers. J. Natl Cancer Inst. 101, 1446–1452 (2009).
Loi, S. et al. Tumor infiltrating lymphocytes are prognostic in triple negative breast cancer and predictive for trastuzumab benefit in early breast cancer: results from the FinHER trial. Ann. Oncol. 25, 1544–1550 (2014).
Adams, S. et al. Prognostic value of tumor-infiltrating lymphocytes in triple-negative breast cancers from two phase III randomized adjuvant breast cancer trials: ECOG 2197 and ECOG 1199. J. Clin. Oncol. 32, 2959–2966 (2014).
Salgado, R. et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab. JAMA Oncol. 1, 448 (2015).
Obeid, M. et al. Calreticulin exposure dictates the immunogenicity of cancer cell death. Nat. Med. 13, 54–61 (2007).
Mattarollo, S. R. et al. Pivotal role of innate and adaptive immunity in anthracycline chemotherapy of established tumors. Cancer Res. 71, 4809–4820 (2011).
Issa-Nummer, Y. et al. Prospective validation of immunological infiltrate for prediction of response to neoadjuvant chemotherapy in HER2-negative breast cancer — a substudy of the neoadjuvant GeparQuinto trial. PLoS ONE 8, e79775 (2013).
West, N. R. et al. Tumor-infiltrating lymphocytes predict response to anthracycline-based chemotherapy in estrogen receptor-negative breast cancer. Breast Cancer Res. 13, R126 (2011).
Dieci, M. et al. Tumor infiltrating lymphocytes and correlation with outcome in the Cher-LOB study. [abstract] Cancer Res. 75, aPD1-1 (2015).
Loi, S. et al. Tumor infiltrating lymphocytes (TILs) indicate trastuzumab benefit in early-stage HER2-positive breast cancer (HER2+ BC). [abstract] Cancer Res. 73, aS1-05 (2013).
Demaria, S. et al. Development of tumor-infiltrating lymphocytes in breast cancer after neoadjuvant paclitaxel chemotherapy. Clin. Cancer Res. 7, 3025–3030 (2001).
Horlock, C. et al. The effects of trastuzumab on the CD4+CD25+FoxP3+ and CD4+IL17A+ T-cell axis in patients with breast cancer. Br. J. Cancer 100, 1061–1067 (2009).
Ladoire, S. et al. Pathologic complete response to neoadjuvant chemotherapy of breast carcinoma is associated with the disappearance of tumor-infiltrating foxp3+ regulatory T cells. Clin. Cancer Res. 14, 2413–2420 (2008).
Dieci, M. V. et al. Prognostic value of tumor-infiltrating lymphocytes on residual disease after primary chemotherapy for triple-negative breast cancer: a retrospective multicenter study. Ann. Oncol. 25, 1–8 (2014).
Loi, S. et al. RAS/MAPK activation is associated with reduced tumor-infiltrating lymphocytes in triple- negative breast cancer: therapeutic cooperation between MEK and PD-1/PD-L1 immune checkpoint inhibitors. Clin. Cancer Res. http://dx.doi.org/10.1158/1078-0432.CCR-15-1125 (2015).
García-Martínez, E. et al. Tumor-infiltrating immune cell profiles and their change after neoadjuvant chemotherapy predict response and prognosis of breast cancer. Breast Cancer Res. 16, 488 (2014).
Robbins, P. F. et al. Mining exomic sequencing data to identify mutated antigens recognized by adoptively transferred tumor-reactive T cells. Nat. Med. 19, 747–752 (2013).
Linnemann, C. et al. High-throughput epitope discovery reveals frequent recognition of neo-antigens by CD4+ T cells in human melanoma. Nat. Med. 21, 81–85 (2014).
Tran, E. et al. Cancer immunotherapy based on mutation-specific CD4+ T cells in a patient with epithelial cancer. Science 344, 641–645 (2014).
Schreiber, R. D., Old, L. J. & Smyth, M. J. Cancer immunoediting: integrating immunity's roles in cancer suppression and promotion. Science 331, 1565–1570 (2011).
Rajasagi, M. et al. Systematic identification of personal tumor-specific neoantigens in chronic lymphocytic leukemia. Blood 124, 453–462 (2014).
Wang, Y. et al. Clonal evolution in breast cancer revealed by single nucleus genome sequencing. Nature 512, 1–15 (2014).
Yadav, M. et al. Predicting immunogenic tumour mutations by combining mass spectrometry and exome sequencing. Nature 515, 572–576 (2014).
Granados, D. P. et al. Impact of genomic polymorphisms on the repertoire of human MHC class I-associated peptides. Nat. Commun. 5, 3600 (2014).
The Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 490, 61–70 (2012).
Denizot, F., Wilson, A., Battye, F., Berke, G. & Shortman, K. Clonal expansion of T cells: a cytotoxic T-cell response in vivo that involves precursor cell proliferation. Proc. Natl Acad. Sci. USA 83, 6089–6092 (1986).
Langhoff, E. & Steinman, R. M. Clonal expansion of human T lymphocytes initiated by dendritic cells. J. Exp. Med. 169, 315–320 (1989).
Marchingo, J. M. et al. Antigen affinity, costimulation, and cytokine inputs sum linearly to amplify T cell expansion. Science 346, 1123–1127 (2014).
Mlecnik, B. et al. Functional network pipeline reveals genetic determinants associated with in situ lymphocyte proliferation and survival of cancer patients. Sci. Transl. Med. 6, 228ra37 (2014).
Ye, J. et al. Specific recruitment of γδ regulatory T cells in human breast cancer. Cancer Res. 73, 6137–6148 (2013).
Diamond, M. S. et al. Type I interferon is selectively required by dendritic cells for immune rejection of tumors. J. Exp. Med. 208, 1989–2003 (2011).
Fuertes, M. B. et al. Host type I IFN signals are required for antitumor CD8+ T cell responses through CD8α+ dendritic cells. J. Exp. Med. 208, 2005–2016 (2011).
Woo, S.-R. et al. The STING pathway mediates innate immune sensing of immunogenic tumors. Immunity 41, 830–842 (2014).
Hansen, M. H., Nielsen, H. V. & Ditzel, H. J. Translocation of an intracellular antigen to the surface of medullary breast cancer cells early in apoptosis allows for an antigen-driven antibody response elicited by tumor-infiltrating B cells. J. Immunol. 169, 2701–2711 (2002).
Zitvogel, L., Apetoh, L., Ghiringhelli, F. & Kroemer, G. Immunological aspects of cancer chemotherapy. Nat. Rev. Immunol. 8, 59–73 (2008).
Apetoh, L. et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat. Med. 13, 1050–1059 (2007).
Ghiringhelli, F. et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1β-dependent adaptive immunity against tumors. Nat. Med. 15, 1170–1178 (2009).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl. Med. 5, 200ra116 (2013).
Gros, A. et al. PD-1 identifies the patient-specific CD8+ tumor-reactive repertoire infiltrating human tumors. J. Clin. Invest. 124, 2246–2259 (2014).
Read, S., Malmström, V. & Powrie, F. Cytotoxic T lymphocyte-associated antigen 4 plays an essential role in the function of CD25+CD4+ regulatory cells that control intestinal inflammation. J. Exp. Med. 192, 295–302 (2000).
Krummel, M. F. & Allison, J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Hodi, F. S. et al. Improved survival with ipilimumab in patients with metastatic melanoma. N. Engl. J. Med. 363, 711–723 (2010).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N. Engl. J. Med. 372, 320–330 (2015).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Schalper, K. A. et al. In situ tumor PD-L1 mRNA expression is associated with increased TILs and better outcome in breast carcinomas. Clin. Cancer Res. 20, 2773–2782 (2014).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Gramaglia, I., Weinberg, A. D., Lemon, M. & Croft, M. Ox-40 ligand: a potent costimulatory molecule for sustaining primary CD4 T cell responses. J. Immunol. 161, 6510–6517 (1998).
Vu, M. D. et al. OX40 costimulation turns off Foxp3+ Tregs. Blood 110, 2501–2510 (2007).
Ruby, C. E. et al. Cutting edge: OX40 agonists can drive regulatory T cell expansion if the cytokine milieu is right. J. Immunol. 183, 4853–4857 (2009).
Curti, B. D. et al. OX40 is a potent immune-stimulating target in late-stage cancer patients. Cancer Res. 73, 7189–7198 (2013).
Monney, L. et al. Th1-specific cell surface protein Tim-3 regulates macrophage activation and severity of an autoimmune disease. Nature 415, 536–541 (2002).
Sakuishi, K. et al. Targeting Tim-3 and PD-1 pathways to reverse T cell exhaustion and restore anti-tumor immunity. J. Exp. Med. 207, 2187–2194 (2010).
Ngiow, S. F. et al. Anti-TIM3 antibody promotes T cell IFN-γ-mediated antitumor immunity and suppresses established tumors. Cancer Res. 71, 3540–3551 (2011).
Sakuishi, K. et al. TIM3+FOXP3+ regulatory T cells are tissue-specific promoters of T-cell dysfunction in cancer. Oncoimmunology 2, e23849 (2013).
Huang, Y.-H. et al. CEACAM1 regulates TIM-3-mediated tolerance and exhaustion. Nature 517, 386–390 (2014).
Mellor, A. L., Keskin, D. B., Johnson, T., Chandler, P. & Munn, D. H. Cells expressing indoleamine 2,3-dioxygenase inhibit T cell responses. J. Immunol. 168, 3771–3776 (2002).
Qian, F. et al. Efficacy of levo-1-methyl tryptophan and dextro-1-methyl tryptophan in reversing indoleamine-2,3-dioxygenase-mediated arrest of T-cell proliferation in human epithelial ovarian cancer. Cancer Res. 69, 5498–5504 (2009).
US National Library of Medicine. NCT01792050. ClinicalTrials.gov [online], (2015).
US National Library of Medicine. NCT02048709. ClinicalTrials.gov [online], (2015).
Antonioli, L., Blandizzi, C., Pacher, P. & Haskó, G. Immunity, inflammation and cancer: a leading role for adenosine. Nat. Rev. Cancer 13, 842–857 (2013).
Loi, S. et al. CD73 promotes anthracycline resistance and poor prognosis in triple negative breast cancer. Proc. Natl Acad. Sci. USA 110, 11091–11096 (2013).
Stagg, J. et al. Anti-CD73 antibody therapy inhibits breast tumor growth and metastasis. Proc. Natl Acad. Sci. USA 107, 1547–1552 (2010).
Beavis, P. A. et al. Blockade of A2A receptors potently suppresses the metastasis of CD73+ tumors. Proc. Natl Acad. Sci. USA 110, 14711–14716 (2013).
Beavis, P. A. et al. Adenosine receptor 2A blockade increases the efficacy of anti-PD-1 through enhanced antitumor T-cell responses. Cancer Immunol. Res. 3, 506–517 (2015).
Hauser, R. A. et al. Tozadenant (SYN115) in patients with Parkinson's disease who have motor fluctuations on levodopa: a phase 2b, double-blind, randomised trial. Lancet Neurol. 13, 767–776 (2014).
Emens, L. et al. Inhibition of PD-L1 by MPDL3280A leads to clinical activity in patients with metastatic triple-negative breast cancer. [abstract] Cancer Res. 75, aPD1-6 (2015).
Wolchok, J. D. et al. Guidelines for the evaluation of immune therapy activity in solid tumors: immune-related response criteria. Clin. Cancer Res. 15, 7412–7420 (2009).
US National Library of Medicine. NCT02425891. ClinicalTrials.gov [online], (2015).
Nanda, R. et al. A Phase Ib study of pembrolizumab (MK-3475) in patients with advanced triple-negative breast cancer. [abstract] Cancer Res. 75, aS1-09 (2015).
US National Library of Medicine. NCT02447003. ClinicalTrials.gov [online], (2015).
Vonderheide, R. H. et al. Tremelimumab in combination with exemestane in patients with advanced breast cancer and treatment-associated modulation of inducible costimulator expression on patient T cells. Clin. Cancer Res. 16, 3485–3494 (2010).
Postow, M. A., Callahan, M. K. & Wolchok, J. D. Immune checkpoint blockade in cancer therapy. J. Clin. Oncol. 33, 1974–1982 (2015).
Stagg, J. et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc. Natl Acad. Sci. USA 108, 7142–7147 (2011).
US National Library of Medicine. NCT02129556. ClinicalTrials.gov [online], (2015).
Verbrugge, I. et al. Radiotherapy increases the permissiveness of established mammary tumors to rejection by immunomodulatory antibodies. Cancer Res. 72, 3163–3174 (2012).
Dewan, M. Z. et al. Fractionated but not single-dose radiotherapy induces an immune-mediated abscopal effect when combined with anti-CTLA-4 antibody. Clin. Cancer Res. 15, 5379–5388 (2009).
Twyman-Saint Victor, C. et al. Radiation and dual checkpoint blockade activate non-redundant immune mechanisms in cancer. Nature 520, 373–377 (2015).
US National Library of Medicine. NCT02303366. ClinicalTrials.gov [online], (2015).
Eggermont, A. M. et al. Ipilimumab versus placebo after complete resection of stage III melanoma: initial efficacy and safety results from the EORTC 18071 phase III trial. [abstract] J. Clin. Oncol. 32, aLBA9008 (2014).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Tumeh, P. C. et al. PD-1 blockade induces responses by inhibiting adaptive immune resistance. Nature 515, 568–571 (2014).
Velcheti, V. et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab. Invest. 94, 107–116 (2014).
Larkin, J. et al. Combined nivolumab and ipilimumab or monotherapy in untreated melanoma. N. Engl. J. Med. 373, 23–34 (2015).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Quezada, S. A. et al. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J. Exp. Med. 205, 2125–2138 (2008).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Van Allen, E. M. et al. Genomic correlates of response to CTLA-4 blockade in metastatic melanoma. Science 350, 207–211 (2015).
Savas, P. S. et al. Lack of correlation of neoantigens arising from tumor somatic mutations with tumor infiltrating lymphocytes (TILs) or survival in HER2-positive breast cancer (HER2+ BC). [abstract] J. Clin. Oncol. 33, a613 (2015).
Cimino-Mathews, A., Ye, X., Meeker, A., Argani, P. & Emens, L. A. Metastatic triple-negative breast cancers at first relapse have fewer tumor-infiltrating lymphocytes than their matched primary breast tumors: a pilot study. Hum. Pathol. 44, 2055–2063 (2013).
Kawai, O. et al. Predominant infiltration of macrophages and CD8+ T cells in cancer nests is a significant predictor of survival in stage IV nonsmall cell lung cancer. Cancer 113, 1387–1395 (2008).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Acknowledgements
The work of S.L. is supported by the Cancer Council Victoria, the National Breast Cancer Foundation (NBCF) Australia, the Breast Cancer Research Foundation (BCRF), New York, and the National Health and Medical Council of Australia (NHMRC). The work of P.S. is supported by the NHMRC. The work of C.D. is supported by the German Cancer Consortium (DKTK), as well as the European Commission (FP7, RESPONSIFY project and TRANSCAN UGI1 project). The work of M.J.S. is supported by the NHMRC and a Susan Komen for the Cure Grant. The work of P.K.D. is supported by an NHMRC Senior Research Fellowship
Author information
Authors and Affiliations
Contributions
P.S., R.S., C.D. and S.L. researched data for the article. P.S., R.S., C.D., P.K.D., M.J.S. and S.L. contributed to discussing the article's content. All authors wrote, reviewed and edited the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
S.L. receives research support from Merck, Novartis, Pfizer and Roche-Genentech, and co-chairs the PANACEA and BOSTON-II studies. M.J.S. has received research support from Bristol-Myers Squibb, and is a consultant for Amgen, F-star and Kymab. C.D. is the cofounder and shareholder of Sividon Diagnostics, Cologne, Germany. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Savas, P., Salgado, R., Denkert, C. et al. Clinical relevance of host immunity in breast cancer: from TILs to the clinic. Nat Rev Clin Oncol 13, 228–241 (2016). https://doi.org/10.1038/nrclinonc.2015.215
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2015.215
This article is cited by
-
Exploring the underlying correlation between microbiota, immune system, hormones, and inflammation with breast cancer and the role of probiotics, prebiotics and postbiotics
Archives of Microbiology (2024)
-
The expression profiles of signature genes from CD103+LAG3+ tumour-infiltrating lymphocyte subsets predict breast cancer survival
BMC Medicine (2023)
-
TIMELESS upregulates PD-L1 expression and exerts an immunosuppressive role in breast cancer
Journal of Translational Medicine (2023)
-
The prognostic value of tumor mutation burden (TMB) and its relationship with immune infiltration in breast cancer patients
European Journal of Medical Research (2023)
-
Radiotherapy versus low-dose tamoxifen following breast-conserving surgery for low-risk and estrogen receptor-positive breast ductal carcinoma in situ: an international open-label randomized non-inferiority trial (TBCC-ARO DCIS Trial)
BMC Cancer (2023)