Key Points
-
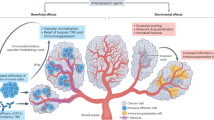
Proteins with major roles in angiogenesis can also have direct or indirect effects on components of the immune system, most often resulting in immunosuppressive outcomes
-
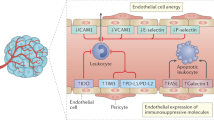
The tumour vasculature can upregulate or downregulate proteins that control the homing and trafficking of immune cells, creating a selective immune-cell barrier on endothelium
-
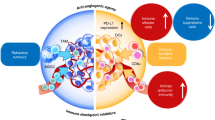
The use of anti-angiogenic drugs and other vascular-targeting agents can normalize and remodel the tortuous tumour vasculature, enabling alleviation of hypoxia and efficient tumour infiltration by effector immune cells
-
Combinations of anti-angiogenic agents with various immunotherapies, including immune-checkpoint inhibitors, have been shown preclinically to generate more-potent antitumour effects and might have clinical potential
-
Anti-angiogenic agents can alleviate immunosuppression, and conversely, immunotherapies can induce changes in the vasculature or bring about antivascular effects; thus, immunotherapy and/or anti-angiogenic therapies have the potential to create a cycle of immunostimulation and vascular remodelling within tumours
Abstract
Immunotherapies have revolutionized medical oncology following the remarkable and, in some cases, unprecedented outcomes observed in certain groups of patients with cancer. Combination with other therapeutic modalities, including anti-angiogenic agents, is one of the many strategies currently under investigation to improve the response rates and duration of immunotherapies. Such a strategy might seem counterintuitive given that anti-angiogenic agents can increase tumour hypoxia and reduce the number of blood vessels within tumours. Herein, we review the additional effects mediated by drugs targeting VEGF-dependent signalling and other pathways, such as those mediated by angiopoietin 2 or HGF, which might increase the efficacy of immunotherapies. In addition, we discuss the seldom considered possibility that immunotherapies, and immune-checkpoint inhibitors in particular, might increase the efficacy of anti-angiogenic or other types of antivascular therapies and/or promote changes in the tumour vasculature. In short, we propose that interactions between both therapeutic modalities could be considered a 'two-way street'.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996).
Krummel, M. F. & Allison, J. P. CD28 and CTLA-4 have opposing effects on the response of T cells to stimulation. J. Exp. Med. 182, 459–465 (1995).
Oleinika, K., Nibbs, R. J., Graham, G. J. & Fraser, A. R. Suppression, subversion and escape: the role of regulatory T cells in cancer progression. Clin. Exp. Immunol. 171, 36–45 (2013).
Buchbinder, E. I. & Desai, A. CTLA-4 and PD-1 pathways: similarities, differences, and implications of their inhibition. Am. J. Clin. Oncol. 39, 98–106 (2016).
Chen, L. & Han, X. Anti-PD-1/PD-L1 therapy of human cancer: past, present, and future. J. Clin. Invest. 125, 3384–3391 (2015).
Dong, H. et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Hui, E. et al. T cell costimulatory receptor CD28 is a primary target for PD-1-mediated inhibition. Science 355, 1428–1433 (2017).
Amarnath, S. et al. The PDL1-PD1 axis converts human TH1 cells into regulatory T cells. Sci. Transl Med. 3, 111ra120 (2011).
Azuma, T. et al. B7-H1 is a ubiquitous antiapoptotic receptor on cancer cells. Blood 111, 3635–3643 (2008).
Black, M. et al. Activation of the PD-1/PD-L1 immune checkpoint confers tumor cell chemoresistance associated with increased metastasis. Oncotarget 7, 10557–10567 (2016).
Ishibashi, M. et al. Myeloma drug resistance induced by binding of myeloma B7-H1 (PD-L1) to PD-1. Cancer. Immunol. Res. 4, 779–788 (2016).
Balar, A. V. et al. First-line pembrolizumab in cisplatin-ineligible patients with locally advanced and unresectable or metastatic urothelial cancer (KEYNOTE-052): a multicentre, single-arm, phase 2 study. Lancet Oncol. 18, 1483–1492 (2017).
El-Khoueiry, A. B. et al. Nivolumab in patients with advanced hepatocellular carcinoma (CheckMate 040): an open-label, non-comparative, phase 1/2 dose escalation and expansion trial. Lancet 389, 2492–2502 (2017).
Balar, A. V. et al. Atezolizumab as first-line treatment in cisplatin-ineligible patients with locally advanced and metastatic urothelial carcinoma: a single-arm, multicentre, phase 2 trial. Lancet 389, 67–76 (2017).
Apolo, A. B. et al. Avelumab, an anti-programmed death-ligand 1 antibody, in patients with refractory metastatic urothelial carcinoma: results from a multicenter, Phase Ib study. J. Clin. Oncol. 35, 2117–2124 (2017).
Hodi, F. S. et al. Bevacizumab plus ipilimumab in patients with metastatic melanoma. Cancer. Immunol. Res. 2, 632–642 (2014).
Wallin, J. J. et al. Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat. Commun. 7, 12624 (2016).
Cheever, M. A. & Higano, C. S. PROVENGE (Sipuleucel-T) in prostate cancer: the first FDA-approved therapeutic cancer vaccine. Clin. Cancer Res. 17, 3520–3526 (2011).
Huijbers, E. J. et al. Vaccination against the extra domain-B of fibronectin as a novel tumor therapy. FASEB J. 24, 4535–4544 (2010).
Wentink, M. Q. et al. Targeted vaccination against the bevacizumab binding site on VEGF using 3D-structured peptides elicits efficient antitumor activity. Proc. Natl Acad. Sci. USA 113, 12532–12537 (2016).
Zhuang, X. et al. Robo4 vaccines induce antibodies that retard tumor growth. Angiogenesis 18, 83–95 (2015).
Wentink, M. Q. et al. Vaccination approach to anti-angiogenic treatment of cancer. Biochim. Biophys. Acta 1855, 155–171 (2015).
Blumenthal, G. M. & Pazdur, R. Approvals in 2017: gene therapies and site-agnostic indications. Nat. Rev. Clin. Oncol. https://doi.org/10.1038/nrclinonc.2018.11 (2017).
Rosenberg, S. A. & Restifo, N. P. Adoptive cell transfer as personalized immunotherapy for human cancer. Science 348, 62–68 (2015).
Hegde, P. S., Karanikas, V. & Evers, S. The where, the when, and the how of immune monitoring for cancer immunotherapies in the era of checkpoint inhibition. Clin. Cancer Res. 22, 1865–1874 (2016).
Oelkrug, C. & Ramage, J. M. Enhancement of T cell recruitment and infiltration into tumours. Clin. Exp. Immunol. 178, 1–8 (2014).
Motz, G. T. & Coukos, G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat. Rev. Immunol. 11, 702–711 (2011).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Simons, M., Gordon, E. & Claesson-Welsh, L. Mechanisms and regulation of endothelial VEGF receptor signalling. Nat. Rev. Mol. Cell. Biol. 17, 611–625 (2016).
Gabrilovich, D. I. et al. Production of vascular endothelial growth factor by human tumors inhibits the functional maturation of dendritic cells. Nat. Med. 2, 1096–1103 (1996).
Mimura, K., Kono, K., Takahashi, A., Kawaguchi, Y. & Fujii, H. Vascular endothelial growth factor inhibits the function of human mature dendritic cells mediated by VEGF receptor-2. Cancer Immunol. Immunother. 56, 761–770 (2007).
Oyama, T. et al. Vascular endothelial growth factor affects dendritic cell maturation through the inhibition of nuclear factor-κB activation in hemopoietic progenitor cells. J. Immunol. 160, 1224–1232 (1998).
Oussa, N. A. et al. VEGF requires the receptor NRP-1 to inhibit lipopolysaccharide-dependent dendritic cell maturation. J. Immunol. 197, 3927–3935 (2016).
Boissel, N. et al. Defective blood dendritic cells in chronic myeloid leukemia correlate with high plasmatic VEGF and are not normalized by imatinib mesylate. Leukemia 18, 1656–1661 (2004).
Lissoni, P. et al. Abnormally enhanced blood concentrations of vascular endothelial growth factor (VEGF) in metastatic cancer patients and their relation to circulating dendritic cells, IL-12 and endothelin-1. J. Biol. Regul. Homeost. Agents 15, 140–144 (2001).
Strauss, L., Volland, D., Kunkel, M. & Reichert, T. E. Dual role of VEGF family members in the pathogenesis of head and neck cancer (HNSCC): possible link between angiogenesis and immune tolerance. Med. Sci. Monit. 11, BR280–BR292 (2005).
Alfaro, C. et al. Influence of bevacizumab, sunitinib and sorafenib as single agents or in combination on the inhibitory effects of VEGF on human dendritic cell differentiation from monocytes. Br. J. Cancer 100, 1111–1119 (2009).
Osada, T. et al. The effect of anti-VEGF therapy on immature myeloid cell and dendritic cells in cancer patients. Cancer Immunol. Immunother. 57, 1115–1124 (2008).
Curiel, T. J. et al. Blockade of B7-H1 improves myeloid dendritic cell-mediated antitumor immunity. Nat. Med. 9, 562–567 (2003).
Dikov, M. M. et al. Differential roles of vascular endothelial growth factor receptors 1 and 2 in dendritic cell differentiation. J. Immunol. 174, 215–222 (2005).
Lin, Y. L., Liang, Y. C. & Chiang, B. L. Placental growth factor down-regulates type 1 T helper immune response by modulating the function of dendritic cells. J. Leukoc. Biol. 82, 1473–1480 (2007).
Ohm, J. E. et al. VEGF inhibits T-cell development and may contribute to tumor-induced immune suppression. Blood 101, 4878–4886 (2003).
Gavalas, N. G. et al. VEGF directly suppresses activation of T cells from ascites secondary to ovarian cancer via VEGF receptor type 2. Br. J. Cancer 107, 1869–1875 (2012).
Voron, T. et al. VEGF-A modulates expression of inhibitory checkpoints on CD8+ T cells in tumors. J. Exp. Med. 212, 139–148 (2015).
Wada, J. et al. The contribution of vascular endothelial growth factor to the induction of regulatory T-cells in malignant effusions. Anticancer Res. 29, 881–888 (2009).
Adotevi, O. et al. A decrease of regulatory T cells correlates with overall survival after sunitinib-based antiangiogenic therapy in metastatic renal cancer patients. J. Immunother. 33, 991–998 (2010).
Terme, M. et al. VEGFA-VEGFR pathway blockade inhibits tumor-induced regulatory T-cell proliferation in colorectal cancer. Cancer Res. 73, 539–549 (2013).
Hansen, W. et al. Neuropilin 1 deficiency on CD4+Foxp3+ regulatory T cells impairs mouse melanoma growth. J. Exp. Med. 209, 2001–2016 (2012).
Barleon, B. et al. Migration of human monocytes in response to vascular endothelial growth factor (VEGF) is mediated via the VEGF receptor flt-1. Blood 87, 3336–3343 (1996).
Huang, Y. et al. Distinct roles of VEGFR-1 and VEGFR-2 in the aberrant hematopoiesis associated with elevated levels of VEGF. Blood 110, 624–631 (2007).
Kusmartsev, S. et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 181, 346–353 (2008).
Serafini, P., Mgebroff, S., Noonan, K. & Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 68, 5439–5449 (2008).
Lee, J. Y., Park, S., Min, W. S. & Kim, H. J. Restoration of natural killer cell cytotoxicity by VEGFR-3 inhibition in myelogenous leukemia. Cancer Lett. 354, 281–289 (2014).
Le, D. T. et al. PD-1 blockade in tumors with mismatch-repair deficiency. N. Engl. J. Med. 372, 2509–2520 (2015).
Pogue-Geile, K. et al. Defective mismatch repair and benefit from bevacizumab for colon cancer: findings from NSABP C-08. J. Natl Cancer Inst. 105, 989–992 (2013).
Hochster, H. S. et al. Efficacy and safety of atezolizumab (atezo) and bevacizumab (bev) in a phase Ib study of microsatellite instability (MSI)-high metastatic colorectal cancer (mCRC). JCO 35, 673–673 (2017).
Augustin, H. G., Koh, G. Y., Thurston, G. & Alitalo, K. Control of vascular morphogenesis and homeostasis through the angiopoietin-Tie system. Nat. Rev. Mol. Cell. Biol. 10, 165–177 (2009).
Scharpfenecker, M., Fiedler, U., Reiss, Y. & Augustin, H. G. The Tie-2 ligand angiopoietin-2 destabilizes quiescent endothelium through an internal autocrine loop mechanism. J. Cell. Sci. 118, 771–780 (2005).
Venneri, M. A. et al. Identification of proangiogenic TIE2-expressing monocytes (TEMs) in human peripheral blood and cancer. Blood 109, 5276–5285 (2007).
Coffelt, S. B. et al. Angiopoietin-2 regulates gene expression in TIE2-expressing monocytes and augments their inherent proangiogenic functions. Cancer Res. 70, 5270–5280 (2010).
Scholz, A. et al. Angiopoietin-2 promotes myeloid cell infiltration in a β2-integrin-dependent manner. Blood 118, 5050–5059 (2011).
Coffelt, S. B. et al. Angiopoietin 2 stimulates TIE2-expressing monocytes to suppress T cell activation and to promote regulatory T cell expansion. J. Immunol. 186, 4183–4190 (2011).
Kloepper, J. et al. Ang-2/VEGF bispecific antibody reprograms macrophages and resident microglia to anti-tumor phenotype and prolongs glioblastoma survival. Proc. Natl Acad. Sci. USA 113, 4476–4481 (2016).
Peterson, T. E. et al. Dual inhibition of Ang-2 and VEGF receptors normalizes tumor vasculature and prolongs survival in glioblastoma by altering macrophages. Proc. Natl Acad. Sci. USA 113, 4470–4475 (2016).
Schmittnaegel, M. et al. Dual angiopoietin-2 and VEGFA inhibition elicits antitumor immunity that is enhanced by PD-1 checkpoint blockade. Sci. Transl. Med. 9, eeaak9670 (2017).
Scholz, A. et al. Endothelial cell-derived angiopoietin-2 is a therapeutic target in treatment-naive and bevacizumab-resistant glioblastoma. EMBO Mol. Med. 8, 39–57 (2016).
Grenga, I., Kwilas, A. R., Donahue, R. N., Farsaci, B. & Hodge, J. W. Inhibition of the angiopoietin/Tie2 axis induces immunogenic modulation, which sensitizes human tumor cells to immune attack. J. Immunother. Cancer. 3, 52 (2015).
Wu, X. et al. Angiopoietin-2 as a Biomarker and Target for Immune Checkpoint Therapy. Cancer. Immunol. Res. 5, 17–28 (2017).
Kuang, D. M. et al. Activated monocytes in peritumoral stroma of hepatocellular carcinoma foster immune privilege and disease progression through PD-L1. J. Exp. Med. 206, 1327–1337 (2009).
Bussolino, F. et al. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J. Cell Biol. 119, 629–641 (1992).
di Tomaso, E. et al. Glioblastoma recurrence after cediranib therapy in patients: lack of “rebound” revascularization as mode of escape. Cancer Res. 71, 19–28 (2011).
Shojaei, F. et al. HGF/c-Met acts as an alternative angiogenic pathway in sunitinib-resistant tumors. Cancer Res. 70, 10090–10100 (2010).
Ilangumaran, S., Villalobos-Hernandez, A., Bobbala, D. & Ramanathan, S. The hepatocyte growth factor (HGF)-MET receptor tyrosine kinase signaling pathway: diverse roles in modulating immune cell functions. Cytokine 82, 125–139 (2016).
Okunishi, K. et al. A novel role of hepatocyte growth factor as an immune regulator through suppressing dendritic cell function. J. Immunol. 175, 4745–4753 (2005).
Benkhoucha, M. et al. Hepatocyte growth factor inhibits CNS autoimmunity by inducing tolerogenic dendritic cells and CD25+Foxp3+ regulatory T cells. Proc. Natl Acad. Sci. USA 107, 6424–6429 (2010).
Rutella, S. et al. Hepatocyte growth factor favors monocyte differentiation into regulatory interleukin (IL)-10++IL-12low/neg accessory cells with dendritic-cell features. Blood 108, 218–227 (2006).
Baek, J. H., Birchmeier, C., Zenke, M. & Hieronymus, T. The HGF receptor/Met tyrosine kinase is a key regulator of dendritic cell migration in skin immunity. J. Immunol. 189, 1699–1707 (2012).
Benkhoucha, M., Molnarfi, N., Schneiter, G., Walker, P. R. & Lalive, P. H. The neurotrophic hepatocyte growth factor attenuates CD8+ cytotoxic T-lymphocyte activity. J. Neuroinflamm. 10, 154 (2013).
Komarowska, I. et al. Hepatocyte growth factor receptor c-Met instructs T cell cardiotropism and promotes T cell migration to the heart via autocrine chemokine release. Immunity 42, 1087–1099 (2015).
Skibinski, G., Skibinska, A. & James, K. The role of hepatocyte growth factor and its receptor c-met in interactions between lymphocytes and stromal cells in secondary human lymphoid organs. Immunology 102, 506–514 (2001).
Gordin, M. et al. c-Met and its ligand hepatocyte growth factor/scatter factor regulate mature B cell survival in a pathway induced by CD74. J. Immunol. 185, 2020–2031 (2010).
Balan, M. et al. Novel roles of c-Met in the survival of renal cancer cells through the regulation of HO-1 and PD-L1 expression. J. Biol. Chem. 290, 8110–8120 (2015).
Kwilas, A. R., Ardiani, A., Donahue, R. N., Aftab, D. T. & Hodge, J. W. Dual effects of a targeted small-molecule inhibitor (cabozantinib) on immune-mediated killing of tumor cells and immune tumor microenvironment permissiveness when combined with a cancer vaccine. J. Transl Med. 12, 294 (2014).
Heldin, C. H., Eriksson, U. & Ostman, A. New members of the platelet-derived growth factor family of mitogens. Arch. Biochem. Biophys. 398, 284–290 (2002).
Agrawal, S., Ganguly, S., Hajian, P., Cao, J. N. & Agrawal, A. PDGF upregulates CLEC-2 to induce T regulatory cells. Oncotarget 6, 28621–28632 (2015).
Chen, C. F. et al. Regulation of T cell proliferation by JMJD6 and PDGF-BB during chronic hepatitis B infection. Sci. Rep. 4, 6359 (2014).
Cheng, J. et al. Platelet-derived growth factor-BB accelerates prostate cancer growth by promoting the proliferation of mesenchymal stem cells. J. Cell. Biochem. 114, 1510–1518 (2013).
Han, Z. et al. The role of immunosuppression of mesenchymal stem cells in tissue repair and tumor growth. Cell. Biosci. 2, 8 (2012).
Griffioen, A. W., Damen, C. A., Martinotti, S., Blijham, G. H. & Groenewegen, G. Endothelial intercellular adhesion molecule-1 expression is suppressed in human malignancies: the role of angiogenic factors. Cancer Res. 56, 1111–1117 (1996).
Griffioen, A. W. Anti-angiogenesis: making the tumor vulnerable to the immune system. Cancer Immunol. Immunother. 57, 1553–1558 (2008).
Dirkx, A. E. et al. Tumor angiogenesis modulates leukocyte-vessel wall interactions in vivo by reducing endothelial adhesion molecule expression. Cancer Res. 63, 2322–2329 (2003).
Tromp, S. C. et al. Tumor angiogenesis factors reduce leukocyte adhesion in vivo. Int. Immunol. 12, 671–676 (2000).
Bouzin, C., Brouet, A., De Vriese, J., Dewever, J. & Feron, O. Effects of vascular endothelial growth factor on the lymphocyte-endothelium interactions: identification of caveolin-1 and nitric oxide as control points of endothelial cell anergy. J. Immunol. 178, 1505–1511 (2007).
Hellwig, S. M. et al. Endothelial CD34 is suppressed in human malignancies: role of angiogenic factors. Cancer Lett. 120, 203–211 (1997).
Melder, R. J. et al. During angiogenesis, vascular endothelial growth factor and basic fibroblast growth factor regulate natural killer cell adhesion to tumor endothelium. Nat. Med. 2, 992–997 (1996).
Dirkx, A. E. et al. Anti-angiogenesis therapy can overcome endothelial cell anergy and promote leukocyte-endothelium interactions and infiltration in tumors. FASEB J. 20, 621–630 (2006).
Fiedler, U. et al. Angiopoietin-2 sensitizes endothelial cells to TNF-α and has a crucial role in the induction of inflammation. Nat. Med. 12, 235–239 (2006).
Srivastava, K. et al. Postsurgical adjuvant tumor therapy by combining anti-angiopoietin-2 and metronomic chemotherapy limits metastatic growth. Cancer. Cell. 26, 880–895 (2014).
Shetty, S. et al. Common lymphatic endothelial and vascular endothelial receptor-1 mediates the transmigration of regulatory T cells across human hepatic sinusoidal endothelium. J. Immunol. 186, 4147–4155 (2011).
Karikoski, M. et al. Clever-1/stabilin-1 controls cancer growth and metastasis. Clin. Cancer Res. 20, 6452–6464 (2014).
Huang, H. et al. VEGF suppresses T-lymphocyte infiltration in the tumor microenvironment through inhibition of NF-κB-induced endothelial activation. FASEB J. 29, 227–238 (2015).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Buckanovich, R. J. et al. Endothelin B receptor mediates the endothelial barrier to T cell homing to tumors and disables immune therapy. Nat. Med. 14, 28–36 (2008).
Huang, X. et al. Lymphoma endothelium preferentially expresses Tim-3 and facilitates the progression of lymphoma by mediating immune evasion. J. Exp. Med. 207, 505–520 (2010).
Pittet, C. L., Newcombe, J., Prat, A. & Arbour, N. Human brain endothelial cells endeavor to immunoregulate CD8 T cells via PD-1 ligand expression in multiple sclerosis. J. Neuroinflamm. 8, 155 (2011).
Eppihimer, M. J. et al. Expression and regulation of the PD-L1 immunoinhibitory molecule on microvascular endothelial cells. Microcirculation 9, 133–145 (2002).
Mazanet, M. M. & Hughes, C. C. B7-H1 is expressed by human endothelial cells and suppresses T cell cytokine synthesis. J. Immunol. 169, 3581–3588 (2002).
Rodig, N. et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur. J. Immunol. 33, 3117–3126 (2003).
Allen, E. et al. Combined antiangiogenic and anti-PD-L1 therapy stimulates tumor immunity through HEV formation. Sci. Transl. Med. 9, eeaak9679 (2017).
Jin, Y. et al. A novel function for programmed death ligand-1 regulation of angiogenesis. Am. J. Pathol. 178, 1922–1929 (2011).
Dieterich, L. C. et al. Tumor-associated lymphatic vessels upregulate PDL1 to inhibit t-cell activation. Front. Immunol. 8, 66 (2017).
Siemann, D. W. The unique characteristics of tumor vasculature and preclinical evidence for its selective disruption by tumor-vascular disrupting agents. Cancer Treat. Rev. 37, 63–74 (2011).
Jain, R. K., Martin, J. D. & Stylianopoulos, T. The role of mechanical forces in tumor growth and therapy. Annu. Rev. Biomed. Eng. 16, 321–346 (2014).
Hobson, B. & Denekamp, J. Endothelial proliferation in tumours and normal tissues: continuous labelling studies. Br. J. Cancer 49, 405–413 (1984).
Baluk, P., Hashizume, H. & McDonald, D. M. Cellular abnormalities of blood vessels as targets in cancer. Curr. Opin. Genet. Dev. 15, 102–111 (2005).
Masiero, M. et al. A core human primary tumor angiogenesis signature identifies the endothelial orphan receptor ELTD1 as a key regulator of angiogenesis. Cancer. Cell. 24, 229–241 (2013).
Seaman, S. et al. Genes that distinguish physiological and pathological angiogenesis. Cancer. Cell. 11, 539–554 (2007).
Palazon, A., Aragones, J., Morales-Kastresana, A., de Landazuri, M. O. & Melero, I. Molecular pathways: hypoxia response in immune cells fighting or promoting cancer. Clin. Cancer Res. 18, 1207–1213 (2012).
Facciabene, A. et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475, 226–230 (2011).
Movahedi, K. et al. Different tumor microenvironments contain functionally distinct subsets of macrophages derived from Ly6C(high) monocytes. Cancer Res. 70, 5728–5739 (2010).
Jain, R. K. Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307, 58–62 (2005).
Huang, Y., Goel, S., Duda, D. G., Fukumura, D. & Jain, R. K. Vascular normalization as an emerging strategy to enhance cancer immunotherapy. Cancer Res. 73, 2943–2948 (2013).
Aarjans, M. et al. Bevacizumab-induced normalization of blood vessels in tumors hampers antibody uptake. Cancer Res. 73, 3347–3355 (2013).
Heskamp, S. et al. Bevacizumab reduces tumor targeting of antiepidermal growth factor and anti-insulin-like growth factor 1 receptor antibodies. Int. J. Cancer. 133, 307–314 (2013).
Hamzah, J. et al. Vascular normalization in Rgs5-deficient tumours promotes immune destruction. Nature 453, 410–414 (2008).
Huang, Y. et al. Vascular normalizing doses of antiangiogenic treatment reprogram the immunosuppressive tumor microenvironment and enhance immunotherapy. Proc. Natl Acad. Sci. USA 109, 17561–17566 (2012).
Nasarre, P. et al. Host-derived angiopoietin-2 affects early stages of tumor development and vessel maturation but is dispensable for later stages of tumor growth. Cancer Res. 69, 1324–1333 (2009).
Falcon, B. L. et al. Contrasting actions of selective inhibitors of angiopoietin-1 and angiopoietin-2 on the normalization of tumor blood vessels. Am. J. Pathol. 175, 2159–2170 (2009).
Park, J. S. et al. Normalization of tumor vessels by Tie2 activation and Ang2 inhibition enhances drug delivery and produces a favorable tumor microenvironment. Cancer. Cell. 30, 953–967 (2016).
Schmittnaegel, M. & De Palma, M. Reprogramming tumor blood vessels for enhancing immunotherapy. Trends Cancer. 3, 809–812 (2017).
Zhao, Y. et al. Targeting vascular endothelial-cadherin in tumor-associated blood vessels promotes T-cell-mediated immunotherapy. Cancer Res. 77, 4434–4447 (2017).
Scharping, N. E., Menk, A. V., Whetstone, R. D., Zeng, X. & Delgoffe, G. M. Efficacy of PD-1 blockade is potentiated by metformin-induced reduction of tumor hypoxia. Cancer. Immunol. Res. 5, 9–16 (2017).
Martinet, L. et al. Human solid tumors contain high endothelial venules: association with T− and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. 71, 5678–5687 (2011).
Dieu-Nosjean, M. C. et al. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 271, 260–275 (2016).
Johansson-Percival, A. et al. De novo induction of intratumoral lymphoid structures and vessel normalization enhances immunotherapy in resistant tumors. Nat. Immunol. 18, 1207–1217 (2017).
Fricke, I. et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer Res. 13, 4840–4848 (2007).
Du Four, S. et al. Combined VEGFR and CTLA-4 blockade increases the antigen-presenting function of intratumoral DCs and reduces the suppressive capacity of intratumoral MDSCs. Am. J. Cancer. Res. 6, 2514–2531 (2016).
Yuan, J. et al. Pretreatment serum VEGF is associated with clinical response and overall survival in advanced melanoma patients treated with ipilimumab. Cancer. Immunol. Res. 2, 127–132 (2014).
Wu, X. et al. Combined anti-VEGF and anti-CTLA-4 therapy elicits humoral immunity to galectin-1 which is associated with favorable clinical outcomes. Cancer. Immunol. Res. 5, 446–454 (2017).
Wu, X. et al. VEGF neutralization plus CTLA-4 blockade alters soluble and cellular factors associated with enhancing lymphocyte infiltration and humoral recognition in melanoma. Cancer. Immunol. Res. 4, 858–868 (2016).
Rini, B. I. et al. Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer 117, 758–767 (2011).
Yasuda, S. et al. Simultaneous blockade of programmed death 1 and vascular endothelial growth factor receptor 2 (VEGFR2) induces synergistic anti-tumour effect in vivo. Clin. Exp. Immunol. 172, 500–506 (2013).
Ghosh, S., Paul, A. & Sen, E. Tumor necrosis factor α-induced hypoxia-inducible factor 1α-β-catenin axis regulates major histocompatibility complex class I gene activation through chromatin remodeling. Mol. Cell. Biol. 33, 2718–2731 (2013).
McDermott, D. F. et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). J. Clin. Oncol. 35, 431–431 (2017).
Huang, H. et al. Specifically targeting angiopoietin-2 inhibits angiogenesis, Tie2-expressing monocyte infiltration, and tumor growth. Clin. Cancer Res. 17, 1001–1011 (2011).
Wu, F. T. et al. Efficacy of Cotargeting angiopoietin-2 and the VEGF pathway in the adjuvant postsurgical setting for early breast, colorectal, and renal cancers. Cancer Res. 76, 6988–7000 (2016).
Gabrilovich, D. I., Ishida, T., Nadaf, S., Ohm, J. E. & Carbone, D. P. Antibodies to vascular endothelial growth factor enhance the efficacy of cancer immunotherapy by improving endogenous dendritic cell function. Clin. Cancer Res. 5, 2963–2970 (1999).
Bose, A. et al. Sunitinib facilitates the activation and recruitment of therapeutic anti-tumor immunity in concert with specific vaccination. Int. J. Cancer 129, 2158–2170 (2011).
Jaini, R., Rayman, P., Cohen, P. A., Finke, J. H. & Tuohy, V. K. Combination of sunitinib with anti-tumor vaccination inhibits T cell priming and requires careful scheduling to achieve productive immunotherapy. Int. J. Cancer 134, 1695–1705 (2014).
Rini, B. I. et al. IMA901, a multipeptide cancer vaccine, plus sunitinib versus sunitinib alone, as first-line therapy for advanced or metastatic renal cell carcinoma (IMPRINT): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol. 17, 1599–1611 (2016).
Huo, M. et al. Tumor-targeted delivery of sunitinib base enhances vaccine therapy for advanced melanoma by remodeling the tumor microenvironment. J. Control. Release 245, 81–94 (2017).
Manning, E. A. et al. A vascular endothelial growth factor receptor-2 inhibitor enhances antitumor immunity through an immune-based mechanism. Clin. Cancer Res. 13, 3951–3959 (2007).
Facciponte, J. G. et al. Tumor endothelial marker 1-specific DNA vaccination targets tumor vasculature. J. Clin. Invest. 124, 1497–1511 (2014).
Shrimali, R. K. et al. Antiangiogenic agents can increase lymphocyte infiltration into tumor and enhance the effectiveness of adoptive immunotherapy of cancer. Cancer Res. 70, 6171–6180 (2010).
Avella, D. M. et al. Regression of established hepatocellular carcinoma is induced by chemoimmunotherapy in an orthotopic murine model. Hepatology 55, 141–152 (2012).
Shi, S. et al. Combining antiangiogenic therapy with adoptive cell immunotherapy exerts better antitumor effects in non-small cell lung cancer models. PLOS One 8, e65757 (2013).
Schoenfeld, J. et al. Active immunotherapy induces antibody responses that target tumor angiogenesis. Cancer Res. 70, 10150–10160 (2010).
Qin, Z. & Blankenstein, T. CD4+ T cell—mediated tumor rejection involves inhibition of angiogenesis that is dependent on IFNγ receptor expression by nonhematopoietic cells. Immunity 12, 677–686 (2000).
Qin, Z. et al. A critical requirement of interferon gamma-mediated angiostasis for tumor rejection by CD8+ T cells. Cancer Res. 63, 4095–4100 (2003).
Deng, J. et al. IFNgamma-responsiveness of endothelial cells leads to efficient angiostasis in tumours involving down-regulation of Dll4. J. Pathol. 233, 170–182 (2014).
Lu, Y. et al. Responsiveness of stromal fibroblasts to IFN-γ blocks tumor growth via angiostasis. J. Immunol. 183, 6413–6421 (2009).
Kammertoens, T. et al. Tumour ischaemia by interferon-gamma resembles physiological blood vessel regression. Nature 545, 98–102 (2017).
Tian, L. et al. Mutual regulation of tumour vessel normalization and immunostimulatory reprogramming. Nature 544, 250–254 (2017).
Mendel, D. B. et al. In vivo antitumor activity of SU11248, a novel tyrosine kinase inhibitor targeting vascular endothelial growth factor and platelet-derived growth factor receptors: determination of a pharmacokinetic/pharmacodynamic relationship. Clin. Cancer Res. 9, 327–337 (2003).
Xin, H. et al. Sunitinib inhibition of Stat3 induces renal cell carcinoma tumor cell apoptosis and reduces immunosuppressive cells. Cancer Res. 69, 2506–2513 (2009).
Ozao-Choy, J. et al. The novel role of tyrosine kinase inhibitor in the reversal of immune suppression and modulation of tumor microenvironment for immune-based cancer therapies. Cancer Res. 69, 2514–2522 (2009).
Liu, X. D. et al. Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer. Immunol. Res. 3, 1017–1029 (2015).
Koyama, S. et al. Adaptive resistance to therapeutic PD-1 blockade is associated with upregulation of alternative immune checkpoints. Nat. Commun. 7, 10501 (2016).
Acknowledgements
R.S.K.'s research on anti-angiogenic drugs and/or immunotherapies has been supported over the past 2 years by grants from the Canadian Breast Cancer Foundation (CBCF), the Canadian Institutes of Health Research (CIHR), and Worldwide Cancer Research (WCR).
Author information
Authors and Affiliations
Contributions
K.A.K. and R.S.K. both contributed to researching data for the article, discussions of content, writing the manuscript, and editing and reviewing the manuscript before publication.
Corresponding authors
Ethics declarations
Competing interests
R.S.K. has sponsored research agreements with Boehringer Ingelheim, Neovacs, and Genentech. R.S.K. has received honoraria recently from Boehringer Ingelheim, NED Biosystems, Merck Pharmaceuticals, Apobiologix, and Onkaido and consultant fees for Neovacs. K.A.K. declares no competing interests.
Related links
ADDITIONAL INFORMATION
Rights and permissions
About this article
Cite this article
Khan, K., Kerbel, R. Improving immunotherapy outcomes with anti-angiogenic treatments and vice versa. Nat Rev Clin Oncol 15, 310–324 (2018). https://doi.org/10.1038/nrclinonc.2018.9
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrclinonc.2018.9
This article is cited by
-
Antiangiogenic–immune-checkpoint inhibitor combinations: lessons from phase III clinical trials
Nature Reviews Clinical Oncology (2024)
-
TET2-mediated tumor cGAS triggers endothelial STING activation to regulate vasculature remodeling and anti-tumor immunity in liver cancer
Nature Communications (2024)
-
Co-targeting CD47 and VEGF elicited potent anti-tumor effects in gastric cancer
Cancer Immunology, Immunotherapy (2024)
-
Updated systematic review and network meta-analysis of first-line treatments for metastatic renal cell carcinoma with extended follow-up data
Cancer Immunology, Immunotherapy (2024)
-
The role of HIF in angiogenesis, lymphangiogenesis, and tumor microenvironment in urological cancers
Molecular Biology Reports (2024)