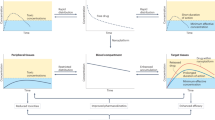
Key Points
-
Biopharmaceutical drugs such as antibodies, peptides and recombinant proteins have high specificity and potency compared to small molecules. These features arise from their macromolecular composition, which provides the structural complexity that is often required for specificity.
-
However, this structural complexity means that biopharmaceutical drugs are large and susceptible to degradation, which makes it challenging to formulate and deliver them. These drugs also have reduced permeation across biological barriers, which complicates their delivery to specific sites or intracellular targets.
-
In this Review we highlight recent advances in formulation and delivery strategies that have facilitated the transformation of product portfolios and development pipelines by this class of compounds. These advances include the use of microsphere-based sustained-release technologies, protein modification methods that make use of polyethylene glycol and other polymers, as well as genetic manipulation of biopharmaceutical drugs such as Fc- and albumin-fusions.
-
We also highlight current and emerging delivery routes that provide alternatives to injection, including transdermal, oral and pulmonary delivery.
-
Current areas of formulation and delivery research show promise for the application of biopharmaceutical drugs to tumour immunotherapy using nanoparticle technology, tissue engineering and enhanced approaches to cell-based therapy.
-
These delivery methods could be used for the targeted delivery of proteins to the brain, which could have implications in the treatment of a wide range of central nervous system disorders. These technologies could potentially increase the effectiveness of conventional approaches that have not yet translated to the clinic, although they have had promising preclinical results.
-
Intracellular delivery of proteins and peptides is a new frontier in delivery research, which could dramatically augment the breadth of targets amenable to biopharmaceutical drug therapy.
Abstract
The formulation and delivery of biopharmaceutical drugs, such as monoclonal antibodies and recombinant proteins, poses substantial challenges owing to their large size and susceptibility to degradation. In this Review we highlight recent advances in formulation and delivery strategies — such as the use of microsphere-based controlled-release technologies, protein modification methods that make use of polyethylene glycol and other polymers, and genetic manipulation of biopharmaceutical drugs — and discuss their advantages and limitations. We also highlight current and emerging delivery routes that provide an alternative to injection, including transdermal, oral and pulmonary delivery routes. In addition, the potential of targeted and intracellular protein delivery is discussed.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
[No authors listed.] Biotech products in big pharma clinical pipelines have grown dramatically. Tufts CSDD Impact Report 15, 1–4 (2013).
Pharmaceutical Research and Manufacturers of America. Medicines in Development — Biologics (2013 report). PhRMA [online], (2013).
Albarran, B., Hoffman, A. S. & Stayton, P. S. Efficient intracellular delivery of a pro-apoptotic peptide with a pH-responsive carrier. React. Funct. Polym. 71, 261–265 (2011).
Langer, R. & Folkman, J. Polymers for the sustained release of proteins and other macromolecules. Nature 263, 797–800 (1976).
Cohen, S., Yoshioka, T., Lucarelli, M., Hwang, L. H. & Langer, R. Controlled delivery systems for proteins based on poly(lactic/glycolic acid) microspheres. Pharm. Res. 8, 713–720 (1991).
Ron, E. et al. Controlled release of polypeptides from polyanhydrides. Proc. Natl Acad. Sci. USA 90, 4176–4180 (1993).
Gaur, S. et al. Preclinical study of the cyclodextrin-polymer conjugate of camptothecin CRLX101 for the treatment of gastric cancer. Nanomedicine 8, 721–730 (2012).
Davis, M. E. & Brewster, M. E. Cyclodextrin-based pharmaceutics: past, present and future. Nature Rev. Drug Discov. 3, 1023–1035 (2004).
Muthu, M. S., Rawat, M. K., Mishra, A. & Singh, S. PLGA nanoparticle formulations of risperidone: preparation and neuropharmacological evaluation. Nanomedicine 5, 323–333 (2009).
Dunbar, J. L. et al. Single- and multiple-dose pharmacokinetics of long-acting injectable naltrexone. Alcohol Clin. Exp. Res. 30, 480–490 (2006).
Periti, P., Mazzei, T. & Mini, E. Clinical pharmacokinetics of depot leuprorelin. Clin. Pharmacokinet. 41, 485–504 (2002).
DeYoung, M. B., MacConell, L., Sarin, V., Trautmann, M. & Herbert, P. Encapsulation of exenatide in poly-(D,L-lactide-co-glycolide) microspheres produced an investigational long-acting once-weekly formulation for type 2 diabetes. Diabetes Technol. Ther. 13, 1145–1154 (2011).
Wei, Y. et al. A novel sustained-release formulation of recombinant human growth hormone and its pharmacokinetic, pharmacodynamic and safety profiles. Mol. Pharm. 9, 2039–2048 (2012).
Champion, J. A. & Mitragotri, S. Role of target geometry in phagocytosis. Proc. Natl Acad. Sci. USA 103, 4930–4934 (2006).
Radomsky, M. L., Whaley, K. J., Cone, R. A. & Saltzman, W. M. Macromolecules released from polymers: diffusion into unstirred fluids. Biomaterials 11, 619–624 (1990).
Bock, N., Dargaville, T. R. & Woodruff, M. A. Controlling microencapsulation and release of micronized proteins using poly(ethylene glycol) and electrospraying. Eur. J. Pharm. Biopharm. 87, 366–377 (2014).
Putney, S. D. & Burke, P. A. Improving protein therapeutics with sustained-release formulations. Nature Biotech. 16, 153–157 (1998).
Kim, H. K. & Park, T. G. Microencapsulation of human growth hormone within biodegradable polyester microspheres: protein aggregation stability and incomplete release mechanism. Biotechnol. Bioeng. 65, 659–667 (1999).
Burke, P. A. et al. Poly(lactide-co-glycolide) microsphere formulations of darbepoetin alfa: spray drying is an alternative to encapsulation by spray-freeze drying. Pharm. Res. 21, 500–506 (2004).
Ding, A. G., Shenderova, A. & Schwendeman, S. P. Prediction of microclimate pH in poly(lactic-co-glycolic acid) films. J. Am. Chem. Soc. 128, 5384–5390 (2006).
Zhu, G., Mallery, S. R. & Schwendeman, S. P. Stabilization of proteins encapsulated in injectable poly (lactide- co-glycolide). Nature Biotech. 18, 52–57 (2000).
Hrkach, J. et al. Preclinical development and clinical translation of a PSMA-targeted docetaxel nanoparticle with a differentiated pharmacological profile. Sci. Transl. Med. 4, 128ra39 (2012).
Rahman, M. A. et al. Systemic delivery of siRNA nanoparticles targeting RRM2 suppresses head and neck tumor growth. J. Control Release 159, 384–392 (2012).
Simpkins, F. et al. Chemoimmunotherapy using pegylated liposomal doxorubicin and interleukin-18 in recurrent ovarian cancer: a phase I dose-escalation study. Cancer Immunol. Res. 1, 168–178 (2013).
Barenholz, Y. Doxil® — the first FDA-approved nano-drug: lessons learned. J. Control Release 160, 117–134 (2012).
Miele, E., Spinelli, G. P., Miele, E., Tomao, F. & Tomao, S. Albumin-bound formulation of paclitaxel (Abraxane ABI-007) in the treatment of breast cancer. Int. J. Nanomed. 4, 99–105 (2009).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Bertrand, N., Wu, J., Xu, X., Kamaly, N. & Farokhzad, O. C. Cancer nanotechnology: the impact of passive and active targeting in the era of modern cancer biology. Adv. Drug Deliv. Rev. 66, 2–25 (2014).
Park, J. et al. Combination delivery of TGF-β inhibitor and IL-2 by nanoscale liposomal polymeric gels enhances tumour immunotherapy. Nature Mater. 11, 895–905 (2012).
Stephan, M. T., Moon, J. J., Um, S. H., Bershteyn, A. & Irvine, D. J. Therapeutic cell engineering with surface-conjugated synthetic nanoparticles. Nature Med. 16, 1035–1041 (2010).
Chaudhari, K. R. et al. Opsonization, biodistribution, cellular uptake and apoptosis study of PEGylated PBCA nanoparticle as potential drug delivery carrier. Pharm. Res. 29, 53–68 (2012).
Nance, E. A. et al. A dense poly(ethylene glycol) coating improves penetration of large polymeric nanoparticles within brain tissue. Sci. Transl. Med. 4, 149ra119 (2012).
Sykes, E. A., Chen, J., Zheng, G. & Chan, W. C. Investigating the impact of nanoparticle size on active and passive tumor targeting efficiency. ACS Nano. 8, 5696–5706 (2014).
Sugahara, K. N. et al. Coadministration of a tumor-penetrating peptide enhances the efficacy of cancer drugs. Science 328, 1031–1035 (2010).
Zhao, L. et al. Nanoparticle vaccines. Vaccine 32, 327–337 (2014).
Leleux, J. & Roy, K. Micro and nanoparticle-based delivery systems for vaccine immunotherapy: an immunological and materials perspective. Adv. Healthc. Mater. 2, 72–94 (2013).
Bregy, A. et al. The role of Gliadel wafers in the treatment of high-grade gliomas. Expert Rev. Anticancer Ther. 13, 1453–1461 (2013).
Burdick, J. & Prestwich, G. Hyaluronic acid hydrogels for biomedical applications. Adv. Mater. 23, H41–H56 (2011).
Ghalanbor, Z., Korber, M. & Bodmeier, R. Improved lysozyme stability and release properties of poly(lactide-co-glycolide) implants prepared by hot-melt extrusion. Pharm. Res. 27, 371–379 (2010).
Okumu, F. W. et al. Sustained delivery of human growth hormone from a novel gel system: SABER. Biomaterials 23, 4353–4358 (2002).
Pechenov, S., Shenoy, B., Yang, M. X., Basu, S. K. & Margolin, A. L. Injectable controlled release formulations incorporating protein crystals. J. Control Release 96, 149–158 (2004).
Brodbeck, K. J., Pushpala, S. & McHugh, A. J. Sustained release of human growth hormone from PLGA solution depots. Pharm. Res. 16, 1825–1829 (1999).
Ravivarapu, H. B., Moyer, K. L. & Dunn, R. L. Sustained suppression of pituitary-gonadal axis with an injectable, in situ forming implant of leuprolide acetate. J. Pharm. Sci. 89, 732–741 (2000).
Wright, J. C., Sekar, M., Osdol, W., Su, H. C. & Miksztal, A. R. in Long Acting Injections and Implants (eds Wright, J. C. & Burgess, D. J.) 153–166 (Springer, 2012).
Zentner, G. M. et al. Biodegradable block copolymers for delivery of proteins and water-insoluble drugs. J. Control Release 72, 203–215 (2001).
Amiram, M., Luginbuhl, K. M., Li, X., Feinglos, M. N. & Chilkoti, A. A depot-forming glucagon-like peptide-1 fusion protein reduces blood glucose for five days with a single injection. J. Control Release 172, 144–151 (2013).
Chan, Y. P., Meyrueix, R., Kravtzoff, R., Nicolas, F. & Lundstrom, K. Review on Medusa:a polymer-based sustained release technology for protein and peptide drugs. Expert Opin. Drug Deliv. 4, 441–451 (2007).
Oudard, S. et al. Pharmacokinetics (PK) and immunologic responses in a phase I/II study of a sustained release formulation of IL-2 in renal cell carcinoma (RCC) patients. J. Clin. Oncol. Abstr. 24, S2558 (2006).
Roberts, J., Linden, M., Cervin, C. & Tiberg, F. Octreotide fluid crystal provides sustained octreotide bioavailability and similar IGF1 suppression to that of octreotide LAR (Sandostatin LAR): randomized, open-label, Phase I, repeat-dose study in healthy volunteers. Endocrine Abstracts 35, P914 (2014).
Jiskoot, W. et al. Protein instability and immunogenicity: roadblocks to clinical application of injectable protein delivery systems for sustained release. J. Pharm. Sci. 101, 946–954 (2012).
Steinhilber, D. et al. Surfactant free preparation of biodegradable dendritic polyglycerol nanogels by inverse nanoprecipitation for encapsulation and release of pharmaceutical biomacromolecules. J. Control Release 169, 289–295 (2013).
Schweizer, D., Schonhammer, K., Jahn, M. & Gopferich, A. Protein-polyanion interactions for the controlled release of monoclonal antibodies. Biomacromolecules 14, 75–83 (2013).
Schweizer, D. et al. Pharmacokinetics, biocompatibility and bioavailability of a controlled release monoclonal antibody formulation. J. Control Release 172, 975–982 (2013).
Hey, T., Knoller, H. & Vorstheim, P. in Therapeutic Proteins: Strategies to Modulate Their Plasma Half-Lives (ed. Kontermann, R.) 117–140 (Wiley-VCH Verlag GmbH, 2012).
Pasut, G. & Veronese, F. M. State of the art in PEGylation: the great versatility achieved after forty years of research. J. Control Release 161, 461–472 (2012).
Constantinou, A. et al. Site-specific polysialylation of an antitumor single-chain Fv fragment. Bioconjug. Chem. 20, 924–931 (2009).
Mero, A., Pasqualin, M., Campisi, M., Renier, D. & Pasut, G. Conjugation of hyaluronan to proteins. Carbohydr. Polym. 92, 2163–2170 (2013).
Peters, J. All About Albumin (Academic Press, 1995).
Bush, M. A. et al. Safety, tolerability, pharmacodynamics and pharmacokinetics of albiglutide, a long-acting glucagon-like peptide-1 mimetic, in healthy subjects. Diabetes Obes. Metab. 11, 498–505 (2009).
Santagostino, E. et al. Safety and pharmacokinetics of a novel recombinant fusion protein linking coagulation factor IX with albumin (rIX-FP) in hemophilia B patients. Blood 120, 2405–2411 (2012).
McDonagh, C. F. et al. Antitumor activity of a novel bispecific antibody that targets the ErbB2/ErbB3 oncogenic unit and inhibits heregulin-induced activation of ErbB3. Mol. Cancer Ther. 11, 582–593 (2012).
Schulte, S. Innovative coagulation factors: albumin fusion technology and recombinant single-chain factor VIII. Thromb. Res. 131, S2–S6 (2013).
Powell, J. S. et al. Phase 3 study of recombinant factor IX Fc fusion protein in hemophilia B. N. Engl. J. Med. 369, 2313–2323 (2013).
Pandey, B. K. et al. Impact of site-specific PEGylation on the conformational stability and folding rate of the Pin WW domain depends strongly on PEG oligomer length. Bioconjug. Chem. 24, 796–802 (2013).
Ranganathan, R., Lu, K. P., Hunter, T. & Noel, J. P. Structural and functional analysis of the mitotic rotamase Pin1 suggests substrate recognition is phosphorylation dependent. Cell 89, 875–886 (1997).
Sherman, M. R., Williams, L. D., Sobczyk, M. A., Michaels, S. J. & Saifer, M. G. Role of the methoxy group in immune responses to mPEG-protein conjugates. Bioconjug. Chem. 23, 485–499 (2012).
Saifer, M. G., Williams, L. D., Sobczyk, M. A., Michaels, S. J. & Sherman, M. R. Selectivity of binding of PEGs and PEG-like oligomers to anti-PEG antibodies induced by methoxyPEG-proteins. Mol. Immunol. 57, 236–246 (2014).
Santi, D. V., Schneider, E. L., Reid, R., Robinson, L. & Ashley, G. W. Predictable and tunable half-life extension of therapeutic agents by controlled chemical release from macromolecular conjugates. Proc. Natl Acad. Sci. USA 109, 6211–6216 (2012).
Tong, J., Luxenhofer, R., Yi, X., Jordan, R. & Kabanov, A. V. Protein modification with amphiphilic block copoly(2-oxazoline)s as a new platform for enhanced cellular delivery. Mol. Pharm. 7, 984–992 (2010).
Egrie, J. C., Dwyer, E., Browne, J. K., Hitz, A. & Lykos, M. A. Darbepoetin alfa has a longer circulating half-life and greater in vivo potency than recombinant human erythropoietin. Exp. Hematol. 31, 290–299 (2003).
Schellenberger, V. et al. A recombinant polypeptide extends the in vivo half-life of peptides and proteins in a tunable manner. Nature Biotech. 27, 1186–1190 (2009).
Cleland, J. L., Shore, C.R. & Kipnes, M. S. A placebo controlled single ascending dose Phase 1 for safety, tolerability, pharmacokinetics and pharmacodynamics of VRS-859 in patients with T2DM (Poster). Diabetologia 54, S318 (2011).
Cleland, J. L. et al. A novel long-acting human growth hormone fusion protein (VRS-317): enhanced in vivo potency and half-life. J. Pharm. Sci. 101, 2744–2754 (2012).
Yuen, K. C. et al. A long-acting human growth hormone with delayed clearance (VRS-317): results of a double-blind, placebo-controlled, single ascending dose study in growth hormone-deficient adults. J. Clin. Endocrinol. Metab. 98, 2595–2603 (2013).
Zalevsky, J. et al. Enhanced antibody half-life improves in vivo activity. Nature Biotech. 28, 157–159 (2010).
Suzuki, T. et al. Importance of neonatal FcR in regulating the serum half-life of therapeutic proteins containing the Fc domain of human IgG1: a comparative study of the affinity of monoclonal antibodies and Fc-fusion proteins to human neonatal FcR. J. Immunol. 184, 1968–1976 (2010).
Yeung, Y. A. et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 182, 7663–7671 (2009).
Andersen, J. T. et al. Structure-based mutagenesis reveals the albumin-binding site of the neonatal Fc receptor. Nature Commun. 3, 610 (2012).
Sleep, D., Cameron, J. & Evans, L. R. Albumin as a versatile platform for drug half-life extension. Biochim. Biophys. Acta 1830, 5526–5534 (2013).
Kermode, M. Unsafe injections in low-income country health settings: need for injection safety promotion to prevent the spread of blood-borne viruses. Health Promot. Int. 19, 95–103 (2004).
Hirose, M., Beverly, E. A. & Weinger, K. Quality of life and technology: impact on children and families with diabetes. Curr. Diab. Rep. 12, 711–720 (2012).
Ricotti, L., Assaf, T., Dario, P. & Menciassi, A. Wearable and implantable pancreas substitutes. J. Artif. Organs 16, 9–22 (2013).
Papargyri, P. et al. An observational 7-year study of continuous subcutaneous insulin infusion for the treatment of type 1 diabetes mellitus. Endocrinol. Nutr. 61, 141–146 (2013).
Schaepelynck, P. et al. Advances in pump technology: insulin patch pumps, combined pumps and glucose sensors, and implanted pumps. Diabetes Metab. 37, S85–S93 (2011).
Richards Grayson, A. C. et al. Multi-pulse drug delivery from a resorbable polymeric microchip device. Nature Mater. 2, 767–772 (2003).
Farra, R. et al. First-in-human testing of a wirelessly controlled drug delivery microchip. Sci. Transl. Med. 4, 122ra21 (2012).
Zisser, H. et al. Clinical update on optimal prandial insulin dosing using a refined run-to-run control algorithm. J. Diabetes Sci. Technol. 3, 487–491 (2009).
Engwerda, E. E., Abbink, E. J., Tack, C. J. & de Galan, B. E. Improved pharmacokinetic and pharmacodynamic profile of rapid-acting insulin using needle-free jet injection technology. Diabetes Care 34, 1804–1808 (2011).
Baxter, J. & Mitragotri, S. Needle-free liquid jet injections: mechanisms and applications. Expert Rev. Med. Devices 3, 565–574 (2006).
Jackson, L. A. et al. Safety and immunogenicity of varying dosages of trivalent inactivated influenza vaccine administered by needle-free jet injectors. Vaccine 19, 4703–4709 (2001).
Arora, A. et al. Needle-free delivery of macromolecules across the skin by nanoliter-volume pulsed microjets. Proc. Natl Acad. Sci. USA 104, 4255–4260 (2007).
Stachowiak, J. C., Li, T. H., Arora, A., Mitragotri, S. & Fletcher, D. A. Dynamic control of needle-free jet injection. J. Control Release 135, 104–112 (2009).
Taberner, A., Hogan, N. C. & Hunter, I. W. Needle-free jet injection using real-time controlled linear Lorentz-force actuators. Med. Eng. Phys. 34, 1228–1235 (2012).
Edwards, D. A. et al. Large porous particles for pulmonary drug delivery. Science 276, 1868–1871 (1997).
Peichl, P. et al. Salmon calcitonin nasal spray treatment for postmenopausal women after hip fracture with total hip arthroplasty. J. Bone Miner. Metab. 23, 243–252 (2005).
Whitehead, K., Shen, Z. & Mitragotri, S. Oral delivery of macromolecules using intestinal patches: applications for insulin delivery. J. Control Release 98, 37–45 (2004).
Mitragotri, S., Blankschtein, D. & Langer, R. Ultrasound-mediated transdermal protein delivery. Science 269, 850–853 (1995).
Alba, N., Naik, A., Guy, R. H. & Kalia, Y. N. Effect of charge and molecular weight on transdermal peptide delivery by iontophoresis. Pharm. Res. 22, 2069–2078 (2005).
Lennernas, H. Intestinal permeability and its relevance for absorption and elimination. Xenobiotica 37, 1015–1051 (2007).
Wertz, P. W. Current understanding of skin biology pertinent to skin penetration: skin biochemistry. Skin Pharmacol. Physiol. 26, 217–226 (2013).
Rosenstock, J. et al. Safety and efficacy of inhaled human insulin (exubera) during discontinuation and readministration of therapy in adults with type 2 diabetes: a 3-year randomized controlled trial. Diabetes Technol. Ther. 11, 697–705 (2009).
Zisser, H. et al. Technosphere insulin effectively controls postprandial glycemia in patients with type 2 diabetes mellitus. Diabetes Technol. Ther. 14, 997–1001 (2012).
Prausnitz, M. R. & Langer, R. Transdermal drug delivery. Nature Biotech. 26, 1261–1268 (2008).
Chen, Y. et al. Transdermal protein delivery by a coadministered peptide identified via phage display. Nature Biotech. 24, 455–460 (2006).
Hsu, T. & Mitragotri, S. Delivery of siRNA and other macromolecules into skin and cells using a peptide enhancer. Proc. Natl Acad. Sci. USA 108, 15816–15821 (2011).
Rothbard, J. B. et al. Conjugation of arginine oligomers to cyclosporin A facilitates topical delivery and inhibition of inflammation. Nature Med. 6, 1253–1257 (2000).
Chen, M., Gupta, V., Anselmo, A. C., Muraski, J. A. & Mitragotri, S. Topical delivery of hyaluronic acid into skin using SPACE-peptide carriers. J. Control Release 173, 67–74 (2014).
Karande, P. et al. Transcutaneous immunization using common chemicals. J. Control Release 138, 134–140 (2009).
Mitragotri, S. Engineering approaches to transdermal drug delivery: a tribute to contributions of Prof. Robert Langer. Skin Pharmacol. Physiol. 26, 263–276 (2013).
Park, E. J., Dodds, J. & Smith, N. B. Dose comparison of ultrasonic transdermal insulin delivery to subcutaneous insulin injection. Int. J. Nanomed. 3, 335–341 (2008).
Rastogi, R., Anand, S., Dinda, A. K. & Koul, V. Investigation on the synergistic effect of a combination of chemical enhancers and modulated iontophoresis for transdermal delivery of insulin. Drug Dev. Ind. Pharm. 36, 993–1004 (2010).
Medi, B. M. & Singh, J. Electronically facilitated transdermal delivery of human parathyroid hormone (1-34). Int. J. Pharm. 263, 25–33 (2003).
Kim do, K., Choi, S. W. & Kwak, Y. H. The effect of SonoPrep® on EMLA® cream application for pain relief prior to intravenous cannulation. Eur. J. Pediatr. 171, 985–988 (2012).
Song, J. M. et al. Microneedle delivery of H5N1 influenza virus-like particles to the skin induces long-lasting B- and T-cell responses in mice. Clin. Vaccine Immunol. 17, 1381–1389 (2010).
Weldon, W. C. et al. Microneedle vaccination with stabilized recombinant influenza virus hemagglutinin induces improved protective immunity. Clin. Vaccine Immunol. 18, 647–654 (2011).
Morishita, M. & Peppas, N. A. Is the oral route possible for peptide and protein drug delivery? Drug Discov. Today 11, 905–910 (2006).
Niu, M. et al. Hypoglycemic activity and oral bioavailability of insulin-loaded liposomes containing bile salts in rats: the effect of cholate type, particle size and administered dose. Eur. J. Pharm. Biopharm. 81, 265–272 (2012).
Motlekar, N. A., Srivenugopal, K. S., Wachtel, M. S. & Youan, B. B. Oral delivery of low-molecular-weight heparin using sodium caprate as absorption enhancer reaches therapeutic levels. J. Drug Target 13, 573–583 (2005).
Yang, T., Arnold, J. J. & Ahsan, F. Tetradecylmaltoside (TDM) enhances in vitro and in vivo intestinal absorption of enoxaparin, a low molecular weight heparin. J. Drug Target 13, 29–38 (2005).
Whitehead, K., Karr, N. & Mitragotri, S. Discovery of synergistic permeation enhancers for oral drug delivery. J. Control Release 128, 128–133 (2008).
Gupta, V., Hwang, B. H., Doshi, N. & Mitragotri, S. A permeation enhancer for increasing transport of therapeutic macromolecules across the intestine. J. Control Release 172, 541–549 (2013).
Wood, K. M., Stone, G. M. & Peppas, N. A. Wheat germ agglutinin functionalized complexation hydrogels for oral insulin delivery. Biomacromolecules 9, 1293–1298 (2008).
Ahn, S. et al. Oral delivery of an anti-diabetic peptide drug via conjugation and complexation with low molecular weight chitosan. J. Control Release 170, 226–232 (2013).
Dunnhaupt, S. et al. In vivo evaluation of an oral drug delivery system for peptides based on S-protected thiolated chitosan. J. Control Release 160, 477–485 (2012).
Morishita, M. et al. Novel oral insulin delivery systems based on complexation polymer hydrogels: single and multiple administration studies in type 1 and 2 diabetic rats. J. Control Release 110, 587–594 (2006).
Eiamtrakarn, S. et al. Gastrointestinal mucoadhesive patch system (GI-MAPS) for oral administration of G-CSF, a model protein. Biomaterials 23, 145–152 (2002).
Gupta, V. et al. Mucoadhesive intestinal devices for oral delivery of salmon calcitonin. J. Control Release 172, 753–762 (2013).
Pridgen, E. M. et al. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci. Transl. Med. 5, 213ra167 (2013).
Maher, S., Leonard, T. W., Jacobsen, J. & Brayden, D. J. Safety and efficacy of sodium caprate in promoting oral drug absorption: from in vitro to the clinic. Adv. Drug Deliv. Rev. 61, 1427–1449 (2009).
Raoof, A. A. et al. Effect of sodium caprate on the intestinal absorption of two modified antisense oligonucleotides in pigs. Eur. J. Pharm. Sci. 17, 131–138 (2002).
Karsdal, M. A. et al. The effect of oral salmon calcitonin delivered with 5-CNAC on bone and cartilage degradation in osteoarthritic patients: a 14-day randomized study. Osteoarthritis Cartilage 18, 150–159 (2010).
Mousa, S. A. et al. Pharmacokinetics and pharmacodynamics of oral heparin solid dosage form in healthy human subjects. J. Clin. Pharmacol. 47, 1508–1520 (2007).
Tillman, L. G., Geary, R. S. & Hardee, G. E. Oral delivery of antisense oligonucleotides in man. J. Pharm. Sci. 97, 225–236 (2008).
Harrison, C. Deal watch: Chiasma and Roche partner in oral peptide drug delivery. Nature Rev. Drug Discov. 12, 255 (2013).
Eldor, R., Arbit, E., Corcos, A. & Kidron, M. Glucose-reducing effect of the ORMD-0801 oral insulin preparation in patients with uncontrolled type 1 diabetes: a pilot study. PLoS ONE 8, e59524 (2013).
Kidron, M., Shushlav, Y., Ovadia, O. & Arbit, E. A novel glucagon-like peptide-1 analog delivered orally reduces postprandial glucose excursion in a porcine model. In: Ninth Annual Diabetes Technology Meeting, 5–7 November 2009, San Francisco. J. Diabetes Sci. Technol. 4, Abstract A71 (2010).
Welling, S. H. et al. The role of citric acid in oral peptide and protein formulations: relationship between calcium chelation and proteolysis inhibition. Eur. J. Pharm. Biopharm. 86, 544–551 (2014).
Binkley, N. et al. A phase 3 trial of the efficacy and safety of oral recombinant calcitonin: the Oral Calcitonin in Postmenopausal Osteoporosis (ORACAL) trial. J. Bone Miner. Res. 27, 1821–1829 (2012).
Pozzilli, P., Raskin, P. & Parkin, C. G. Review of clinical trials: update on oral insulin spray formulation. Diabetes Obes. Metab. 12, 91–96 (2010).
Senel, S., Rathbone, M. J., Cansiz, M. & Pather, I. Recent developments in buccal and sublingual delivery systems. Expert Opin. Drug Deliv. 9, 615–628 (2012).
Lee, Y. C., Simamora, P., Pinsuwan, S. & Yalkowsky, S. H. Review on the systemic delivery of insulin via the ocular route. Int. J. Pharm. 233, 1–18 (2002).
Davis, J. L., Gilger, B. C. & Robinson, M. R. Novel approaches to ocular drug delivery. Curr. Opin. Mol. Ther. 6, 195–205 (2004).
Illum, L. Nasal drug delivery — possibilities, problems and solutions. J. Control Release 87, 187–198 (2003).
Urtti, A. in Proteins and Peptides: Pharmacokinetic, Pharmacodynamic, and Metabolic Outcomes 1st edn (eds Mrsny, R. & Daugherty, A.) 163–172 (Informa, 2010).
Zolot, R. S., Basu, S. & Million, R. P. Antibody–drug conjugates. Nature Rev. Drug Discov. 12, 259–260 (2013).
Zheng, D. et al. Topical delivery of siRNA-based spherical nucleic acid nanoparticle conjugates for gene regulation. Proc. Natl Acad. Sci. USA 109, 11975–11980 (2012).
Akinc, A. et al. Development of lipidoid-siRNA formulations for systemic delivery to the liver. Mol. Ther. 17, 872–879 (2009).
Alvarez-Erviti, L. et al. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature Biotech. 29, 341–345 (2011).
Davis, M. E. et al. Evidence of RNAi in humans from systemically administered siRNA via targeted nanoparticles. Nature 464, 1067–1070 (2010).
Wu, S. Y., Lopez-Berestein, G, Calin, G. A. & Sood, A. K. RNAi therapies: drugging the undruggable. Sci. Transl. Med. 6, 240ps247 (2014).
Bard, F. et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nature Med. 6, 916–919 (2000).
Thompson, E. J. & Keir, G. Laboratory investigation of cerebrospinal fluid proteins. Ann. Clin. Biochem. 27, 425–435 (1990).
Atwal, J. K. et al. A therapeutic antibody targeting BACE1 inhibits amyloid-β production in vivo. Sci. Transl. Med. 3, 84ra43 (2011).
Masliah, E. et al. Passive immunization reduces behavioral and neuropathological deficits in an alpha-synuclein transgenic model of Lewy body disease. PLoS ONE 6, e19338 (2011).
Chai, X. et al. Passive immunization with anti-Tau antibodies in two transgenic models: reduction of Tau pathology and delay of disease progression. J. Biol. Chem. 286, 34457–34467 (2011).
Yanamandra, K. et al. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron 80, 402–414 (2013).
Karran, E. & Hardy, J. Antiamyloid therapy for Alzheimer's disease — are we on the right road? N. Engl. J. Med. 370, 377–378 (2014).
Jonason, A. et al. P573: Development of anti-SEMA4D monoclonal antibody for the treatment of multiple sclerosis. Mult. Scler. 19, S240–S241 (2013).
Calias, P. et al. CNS penetration of intrathecal-lumbar idursulfase in the monkey, dog and mouse: implications for neurological outcomes of lysosomal storage disorder. PLoS ONE 7, e30341 (2012).
Liu, Y. et al. Ultrasound-enhanced drug transport and distribution in the brain. AAPS PharmSciTech 11, 1005–1017 (2010).
Cooke, M. J., Wang, Y., Morshead, C. M. & Shoichet, M. S. Controlled epi-cortical delivery of epidermal growth factor for the stimulation of endogenous neural stem cell proliferation in stroke-injured brain. Biomaterials 32, 5688–5697 (2011).
Sirianni, R. W., Olausson, P., Chiu, A. S., Taylor, J. R. & Saltzman, W. M. The behavioral and biochemical effects of BDNF containing polymers implanted in the hippocampus of rats. Brain Res. 1321, 40–50 (2010).
Boado, R. J., Lu, J. Z., Hui, E. K., Sumbria, R. K. & Pardridge, W. M. Pharmacokinetics and brain uptake in the rhesus monkey of a fusion protein of arylsulfatase a and a monoclonal antibody against the human insulin receptor. Biotechnol. Bioeng. 110, 1456–1465 (2013).
Yu, Y. J. et al. Boosting brain uptake of a therapeutic antibody by reducing its affinity for a transcytosis target. Sci. Transl. Med. 3, 84ra44 (2011).
Couch, J. A. et al. Addressing safety liabilities of TfR bispecific antibodies that cross the blood-brain barrier. Sci. Transl. Med. 5, 183ra57 (2013).
Niewoehner, J. et al. Increased brain penetration and potency of a therapeutic antibody using a monovalent molecular shuttle. Neuron 81, 49–60 (2014).
Lachowicz, J. E. et al. ANG4043, a brain-penetrant anti-HER2 mAb increases survival in mice bearing intracranial BT-474 breast tumor cells. Cancer Res. 73 (Suppl. 24), P6-11-05 (2013).
Price, T. O. et al. Transport across the blood-brain barrier of pluronic leptin. J. Pharmacol. Exp. Ther. 333, 253–263 (2010).
Kang, C. E., Tator, C. H. & Shoichet, M. S. Poly(ethylene glycol) modification enhances penetration of fibroblast growth factor 2 to injured spinal cord tissue from an intrathecal delivery system. J. Control Release 144, 25–31 (2010).
Tong, J. et al. Conjugates of superoxide dismutase 1 with amphiphilic poly(2-oxazoline) block copolymers for enhanced brain delivery: synthesis, characterization and evaluation in vitro and in vivo. Mol. Pharm. 10, 360–377 (2013).
Walensky, L. D. et al. A stapled BID BH3 helix directly binds and activates BAX. Mol. Cell 24, 199–210 (2006).
LaBelle, J. L. et al. A stapled BIM peptide overcomes apoptotic resistance in hematologic cancers. J. Clin. Invest. 122, 2018–2031 (2012).
Moellering, R. E. et al. Direct inhibition of the NOTCH transcription factor complex. Nature 462, 182–188 (2009).
Chang, Y. S. et al. Stapled α-helical peptide drug development: a potent dual inhibitor of MDM2 and MDMX for p53-dependent cancer therapy. Proc. Natl Acad. Sci. USA 110, E3445–E3454 (2013).
Gaj, T., Guo, J., Kato, Y., Sirk, S. J. & Barbas, C. F. Targeted gene knockout by direct delivery of zinc-finger nuclease proteins. Nature Methods 9, 805–807 (2012).
Cronican, J. J. et al. Potent delivery of functional proteins into mammalian cells in vitro and in vivo using a supercharged protein. ACS Chem. Biol. 5, 747–752 (2010).
Cronican, J. J. et al. A class of human proteins that deliver functional proteins into mammalian cells in vitro and in vivo. Chem. Biol. 18, 833–838 (2011).
Chen, Z. et al. Receptor-mediated delivery of engineered nucleases for genome modification. Nucleic Acids Res. 41, e182 (2013).
Duvall, C. L., Convertine, A. J., Benoit, D. S., Hoffman, A. S. & Stayton, P. S. Intracellular delivery of a proapoptotic peptide via conjugation to a RAFT synthesized endosomolytic polymer. Mol. Pharm. 7, 468–476 (2010).
Foster, S., Duvall, C. L., Crownover, E. F., Hoffman, A. S. & Stayton, P. S. Intracellular delivery of a protein antigen with an endosomal-releasing polymer enhances CD8 T-cell production and prophylactic vaccine efficacy. Bioconjug Chem. 21, 2205–2212 (2010).
Cleland, J. L., Powell, M. F. & Shire, S. J. The development of stable protein formulations: a close look at protein aggregation, deamidation, and oxidation. Crit. Rev. Ther. Drug Carrier Syst. 10, 307–377 (1993).
Daugherty, A. L. & Mrsny, R. J. Formulation and delivery issues for monoclonal antibody therapeutics. Adv. Drug Deliv. Rev. 58, 686–706 (2006).
Rajagopal, K. Wood, J., Tran, B., Patapoff, T. W. & Nivaggioli, T. Trehalose limits BSA aggregation in spray-dried formulations at high temperatures: implications in preparing polymer implants for long-term protein delivery. J. Pharm. Sci. 102, 2655–2666 (2013).
Allison, S. D., Chang, B., Randolph, T. W. & Carpenter, J. F. Hydrogen bonding between sugar and protein is responsible for inhibition of dehydration-induced protein unfolding. Arch. Biochem. Biophys. 365, 289–298 (1999).
Sasahara, K., McPhie, P. & Minton, A. P. Effect of dextran on protein stability and conformation attributed to macromolecular crowding. J. Mol. Biol. 326, 1227–1237 (2003).
Kerwin, B. A. Polysorbates 20 and 80 used in the formulation of protein biotherapeutics: structure and degradation pathways. J. Pharm. Sci. 97, 2924–2935 (2008).
Schwendeman, S. P. et al. Stabilization of tetanus and diphtheria toxoids against moisture-induced aggregation. Proc. Natl Acad. Sci. USA 92, 11234–11238 (1995).
Yadav, S., Shire, S. & Kalonia, D. Factors affecting the viscosity in high concentration solutions of different monoclonal antibodies. J. Pharm. Sci. 99, 4812–4829 (2010).
Du, W. & Klibanov, A. M. Hydrophobic salts markedly diminish viscosity of concentrated protein solutions. Biotechnol. Bioeng. 108, 632–636 (2011).
Liu, J., Nguyen, M., Andya, J. & Shire, S. Reversible self-association increases the viscosity of a concentrated monoclonal antibody in aqueous solution. J. Pharm. Sci. 94, 1928–1940 (2005).
Inoue, N., Takai, E., Arakawa, T. & Shiraki, K. Arginine and lysine reduce the high viscosity of serum albumin solutions for pharmaceutical injection. J. Biosci. Bioeng. 117, 539–543 (2014).
Yearley, E. J. et al. Observation of small cluster formation in concentrated monoclonal antibody solutions and its implications to solution viscosity. Biophys. J. 106, 1763–1770 (2014).
Jiang, P. et al. Effective targeting of the tumor microenvironment for cancer therapy. Anticancer Res. 32, 1203–1212 (2012).
Domansky, K. et al. Perfused multiwell plate for 3D liver tissue engineering. Lab. Chip 10, 51–58 (2010).
Sasaki, S. et al. In vivo evaluation of a novel scaffold for artificial corneas prepared by using ultrahigh hydrostatic pressure to decellularize porcine corneas. Mol. Vis. 15, 2022–2028 (2009).
Teng, Y. D. et al. Functional recovery following traumatic spinal cord injury mediated by a unique polymer scaffold seeded with neural stem cells. Proc. Natl Acad. Sci. USA 99, 3024–3029 (2002).
Wagner, I. et al. A dynamic multi-organ-chip for long-term cultivation and substance testing proven by 3D human liver and skin tissue co-culture. Lab. Chip 13, 3538–3547 (2013).
Orlando, G. et al. Production and implantation of renal extracellular matrix scaffolds from porcine kidneys as a platform for renal bioengineering investigations. Ann. Surg. 256, 363–370 (2012).
Mountziaris, P. M. et al. Effect of temporally patterned TNF-α delivery on in vitro osteogenic differentiation of mesenchymal stem cells cultured on biodegradable polymer scaffolds. J. Biomater. Sci. Polym. Ed. 24, 1794–1813 (2013).
Huang, Y. C., Simmons, C., Kaigler, D., Rice, K. G. & Mooney, D. J. Bone regeneration in a rat cranial defect with delivery of PEI-condensed plasmid DNA encoding for bone morphogenetic protein-4 (BMP-4). Gene Ther. 12, 418–426 (2005).
Cao, L. & Mooney, D. J. Spatiotemporal control over growth factor signaling for therapeutic neovascularization. Adv. Drug Deliv. Rev. 59, 1340–1350 (2007).
Silva, E. A. & Mooney, D. J. Spatiotemporal control of vascular endothelial growth factor delivery from injectable hydrogels enhances angiogenesis. J. Thromb. Haemost. 5, 590–598 (2007).
Brunger, J. M. et al. Scaffold-mediated lentiviral transduction for functional tissue engineering of cartilage. Proc. Natl Acad. Sci. USA 111, E798–806 (2014).
Ravichandran, R., Sundarrajan, S., Venugopal, J. R., Mukherjee, S. & Ramakrishna, S. Advances in polymeric systems for tissue engineering and biomedical applications. Macromol. Biosci. 12, 286–311 (2012).
Acknowledgements
The research of S.M. is supported by the US National Institutes of Health (NIH) grant R01DK097379. The research of R.L. is supported by the NIHR37-EB000244 grant (MIT #6928649). The authors thank M. Citron for helpful discussions during the preparation of this Review.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
R.L. and S.M. are shareholders of, R.L., S.M. and P.A.B. are consultants to, and R.L. and S.M. are recipients of research grants from several drug delivery, pharmaceuticals and biotechnology companies, including those whose technologies and products are discussed in this article. The authors are inventors on several patents in the field of drug delivery/formulations that are owned by their current or former employers. The views presented here should not be considered as endorsements of any specific product or company.
Related links
Supplementary information
Supplementary information S1 (table)
Product examples for half-life extension / decreased dosing frequency Products based on depot formulations (PDF 565 kb)
Glossary
- Solvent evaporation
-
A process for microencapsulating drugs or other substances whereby an oil-in-water emulsion is formed, followed by the removal of the organic solvent by its evaporation from the emulsion mixture, resulting in the solidification of the oil phase to form microspheres.
- Atomization
-
A process for microencapsulating drugs or other substances whereby a polymer solution containing the drugs is broken up into droplets, followed by the removal of the polymer solvent by evaporation or other means, resulting in the formation of solid microspheres.
- Burst release
-
The quick release of drugs (usually within minutes to 24 hours) that are encapsulated in microspheres; the drug is associated with the microsphere surface and so is not completely protected from release by the microsphere structure.
- Core-shell nanoparticles
-
Microspheres or precipitates containing a core of one polymer that is surrounded by the shell of another polymer.
- Particulate formulations
-
Formulations comprising microspheres prepared from a polymer or other materials to encapsulate and release proteins.
- Implantable depots
-
Formulations that are too large in volume to be injected, and are instead administered by other means (for example, by insertion through a surgical incision).
- Injectable monoliths
-
A type of depot formulation that is fabricated as a contiguous solid mass, such as a cylinder, and can be administered by positive displacement from a syringe needle without the use of a suspending vehicle.
- Biosimilars
-
A biopharmaceutical drug that is demonstrated to be similar to, or interchangeable with, a licensed biological product, based on the absence of clinically meaningful differences in safety, purity and potency.
- New molecular entity
-
A drug product containing an active moiety or moieties that have not been previously approved by a regulatory authority, either as a single ingredient or as part of a combination product.
- Hydrodynamic radius
-
The effective hydrated radius of a biopharmaceutical drug, which dictates its rate of diffusion in solution and tissues.
- FcRn recycling
-
A process that is mediated by the neonatal Fc receptor (FcRn), which involves the transcytosis of maternal immunoglobulin G (IgG) across the placental membrane. This process is responsible for the long circulating half-lives of IgG and serum albumin throughout life, through a mechanism of protective vesicular trafficking.
- Cmax
-
The maximum plasma or serum concentration of a drug following administration.
- Living polymerization
-
A technique for synthesizing polymers where chain termination and transfer reactions are absent, and the rate of chain initiation substantially exceeds that of chain propagation. The resulting polymer chains have very similar lengths compared to traditional polymerization techniques.
- K d
-
The dissociation constant; a type of equilibrium constant that characterizes the propensity of a complex to separate reversibly into its constituents.
- Implantable pumps
-
Small devices that can be placed within the body and used to deliver a drug. The pumps carry a drug reservoir (which, in some cases, can be refilled through a port, thus avoiding the need for surgical intervention), a control mechanism to regulate delivery, and the delivery catheter.
- Insulin patch pumps
-
A wearable infusion pump that is attached to the skin and delivers insulin into the subcutaneous space.
- Liquid jet injections
-
A type of injection that enables the delivery of drugs into the skin and subdermal tissues — without using needles — by accelerating a stream of drug solution to high velocities.
- Absorption enhancers
-
Chemicals that increase the absorption of drugs across biological barriers such as the skin, intestinal epithelium or cell membrane.
- Chitosans
-
Linear polysaccharides of randomly arranged glucosamine and acetyl glycosamine.
- Therapeutic index
-
A measure of the safety of a particular drug, typically represented by the ratio of the dose causing overt toxicity to the dose providing a therapeutic effect. A drug with a large therapeutic index can be administered with low risk of eliciting a toxic effect.
- Tau
-
A highly soluble microtubule-associated protein found in neurons. Misfolded tau is associated with a variety of neurodegenerative disorders, including Alzheimer's disease, in which interneuronal hyperphosphorylated tau tangles are a common pathological feature.
- Stapled peptides
-
A class of α-helical peptides incorporating α-methylation and hydrocarbon-based macrocyclic bridging features for increased hydrophobicity and conformational stabilization of the helix, resulting in improved membrane permeation.
Rights and permissions
About this article
Cite this article
Mitragotri, S., Burke, P. & Langer, R. Overcoming the challenges in administering biopharmaceuticals: formulation and delivery strategies. Nat Rev Drug Discov 13, 655–672 (2014). https://doi.org/10.1038/nrd4363
Published:
Issue Date:
DOI: https://doi.org/10.1038/nrd4363
This article is cited by
-
Exosome-sheathed ROS-responsive nanogel to improve targeted therapy in perimenopausal depression
Journal of Nanobiotechnology (2023)
-
Immobilized nanoparticles-mediated enzyme therapy; promising way into clinical development
Discover Nano (2023)
-
Process development and quality attributes for the freeze-drying process in pharmaceuticals, biopharmaceuticals and nanomedicine delivery: a state-of-the-art review
Future Journal of Pharmaceutical Sciences (2023)
-
Deep in situ microscopy for real-time analysis of mammalian cell populations in bioreactors
Scientific Reports (2023)
-
Neural modulation with photothermally active nanomaterials
Nature Reviews Bioengineering (2023)