Key Points
-
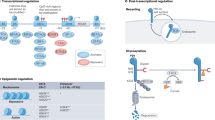
T cell dysfunction ('exhaustion') occurs in chronic infection and cancer
-
Mechanisms of T cell dysfunction are partly disease specific and are partly shared between different diseases
-
T cell dysfunction depends on antigens, co-stimulation, T cell inhibitory cells, metabolic pathways and soluble factors of the tumour microenvironment
-
T cells are regulated by signals of the T cell receptor and co-activating, inhibitory and cytokine receptors
-
T cell inhibition frequently occurs via evolutionarily conserved mechanisms known to function in most inflammatory diseases, assuring efficient downregulation of lymphocyte cytotoxicity to avoid extended tissue damage
-
T cell inhibition can also occur via mechanisms that cancer cells acquired by somatic mutations
Abstract
Recent progress in cancer immunotherapy emphasizes the importance of understanding immune-regulatory pathways in tumours. Dysfunction of antitumour T cells may be due to mechanisms that are evolutionarily conserved or acquired by somatic mutations. The dysfunctional state of T cells has been termed 'exhaustion', on the basis of similarities to dysfunctional T cells in chronic infections. However, despite shared properties, recent studies have identified marked differences between T cell dysfunction in cancer and chronic infection. In this Review, we discuss T cell-intrinsic molecular alterations and metabolic communication in the tumour microenvironment. Identification of the underlying molecular drivers of T cell dysfunction is essential for the continued progress of cancer research and therapy.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kaech, S. M. & Cui, W. Transcriptional control of effector and memory CD8+ T cell differentiation. Nat. Rev. Immunol. 12, 749–761 (2012).
Smyth, M. J., Dunn, G. P. & Schreiber, R. D. Cancer immunosurveillance and immunoediting: the roles of immunity in suppressing tumor development and shaping tumor immunogenicity. Adv. Immunol. 90, 1–50 (2006).
Duan, S. & Thomas, P. G. Balancing immune protection and immune pathology by CD8+ T-Cell responses to influenza infection. Front. Immunol. 7, 25 (2016).
Lee, P. P. et al. Characterization of circulating T cells specific for tumor-associated antigens in melanoma patients. Nat. Med. 5, 677–685 (1999).
Ahmadzadeh, M. et al. Tumor antigen-specific CD8 T cells infiltrating the tumor express high levels of PD-1 and are functionally impaired. Blood 114, 1537–1544 (2009).
Zippelius, A. et al. Effector function of human tumor-specific CD8 T cells in melanoma lesions: a state of local functional tolerance. Cancer Res. 64, 2865–2873 (2004).
Baitsch, L. et al. Exhaustion of tumor-specific CD8+ T cells in metastases from melanoma patients. J. Clin. Invest. 121, 2350–2360 (2011).
Giordano, M. et al. Molecular profiling of CD8 T cells in autochthonous melanoma identifies Maf as driver of exhaustion. EMBO J. 34, 2042–2058 (2015).
Quail, D. F. & Joyce, J. A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 19, 1423–1437 (2013).
Turley, S. J., Cremasco, V. & Astarita, J. L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 15, 669–682 (2015).
Nishikawa, H. & Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Curr. Opin. Immunol. 27, 1–7 (2014).
Roychoudhuri, R. et al. BACH2 represses effector programs to stabilize Treg-mediated immune homeostasis. Nature 498, 506–510 (2013).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
Quezada, S. A. et al. Limited tumor infiltration by activated T effector cells restricts the therapeutic activity of regulatory T cell depletion against established melanoma. J. Exp. Med. 205, 2125–2138 (2008).
Mizukami, Y. et al. CCL17 and CCL22 chemokines within tumor microenvironment are related to accumulation of FOXP3+ regulatory T cells in gastric cancer. Int. J. Cancer 122, 2286–2293 (2008).
Facciabene, A. et al. Tumour hypoxia promotes tolerance and angiogenesis via CCL28 and Treg cells. Nature 475, 226–230 (2011).
Ho, P. C. et al. Immune-based antitumor effects of BRAF inhibitors rely on signaling by CD40L and IFNγ. Cancer Res. 74, 3205–3217 (2014).
Perrot, I. et al. Dendritic cells infiltrating human non-small cell lung cancer are blocked at immature stage. J. Immunol. 178, 2763–2769 (2007).
Engelhardt, J. J. et al. Marginating dendritic cells of the tumor microenvironment cross-present tumor antigens and stably engage tumor-specific T cells. Cancer Cell 21, 402–417 (2012).
Gabrilovich, D. I. & Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat. Rev. Immunol. 9, 162–174 (2009).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Zhao, Q. et al. Activated CD69+ T cells foster immune privilege by regulating IDO expression in tumor-associated macrophages. J. Immunol. 188, 1117–1124 (2012).
Ye, X. Z. et al. Tumor-associated microglia/macrophages enhance the invasion of glioma stem-like cells via TGF-β1 signaling pathway. J. Immunol. 189, 444–453 (2012).
Colegio, O. R. et al. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature 513, 559–563 (2014).
Imtiyaz, H. Z. et al. Hypoxia-inducible factor 2α regulates macrophage function in mouse models of acute and tumor inflammation. J. Clin. Invest. 120, 2699–2714 (2010).
Hanahan, D. & Coussens, L. M. Accessories to the crime: functions of cells recruited to the tumor microenvironment. Cancer Cell 21, 309–322 (2012).
Vonderheide, R. H. & Glennie, M. J. Agonistic CD40 antibodies and cancer therapy. Clin. Cancer Res. 19, 1035–1043 (2013).
Pyonteck, S. M. et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 19, 1264–1272 (2013).
Kalluri, R. & Zeisberg, M. Fibroblasts in cancer. Nat. Rev. Cancer 6, 392–401 (2006).
Marsh, T., Pietras, K. & McAllister, S. S. Fibroblasts as architects of cancer pathogenesis. Biochim. Biophys. Acta 1832, 1070–1078 (2013).
Ko, S. Y. et al. HOXA9 promotes ovarian cancer growth by stimulating cancer-associated fibroblasts. J. Clin. Invest. 122, 3603–3617 (2012).
Erez, N., Truitt, M., Olson, P., Arron, S. T. & Hanahan, D. Cancer-associated fibroblasts are activated in incipient neoplasia to orchestrate tumor-promoting inflammation in an NF-κB-dependent manner. Cancer Cell 17, 135–147 (2010).
Dirat, B. et al. Cancer-associated adipocytes exhibit an activated phenotype and contribute to breast cancer invasion. Cancer Res. 71, 2455–2465 (2011).
Nieman, K. M. et al. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 17, 1498–1503 (2011).
Hanahan, D. & Weinberg, R. A. The hallmarks of cancer. Cell 100, 57–70 (2000).
Carlos, T. M. Leukocyte recruitment at sites of tumor: dissonant orchestration. J. Leukoc. Biol. 70, 171–184 (2001).
Piali, L., Fichtel, A., Terpe, H. J., Imhof, B. A. & Gisler, R. H. Endothelial vascular cell adhesion molecule 1 expression is suppressed by melanoma and carcinoma. J. Exp. Med. 181, 811–816 (1995).
Motz, G. T. et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat. Med. 20, 607–615 (2014).
Mulligan, J. K., Rosenzweig, S. A. & Young, M. R. Tumor secretion of VEGF induces endothelial cells to suppress T cell functions through the production of PGE2. J. Immunother. 33, 126–135 (2010).
Ghesquiere, B., Wong, B. W., Kuchnio, A. & Carmeliet, P. Metabolism of stromal and immune cells in health and disease. Nature 511, 167–176 (2014).
Card, C. M., Yu, S. S. & Swartz, M. A. Emerging roles of lymphatic endothelium in regulating adaptive immunity. J. Clin. Invest. 124, 943–952 (2014).
MacIver, N. J., Michalek, R. D. & Rathmell, J. C. Metabolic regulation of T lymphocytes. Annu. Rev. Immunol. 31, 259–283 (2013).
Ho, P. C. et al. Phosphoenolpyruvate is a metabolic checkpoint of anti-tumor T cell responses. Cell 162, 1217–1228 (2015).
Chang, C. H. et al. Metabolic competition in the tumor microenvironment is a driver of cancer progression. Cell 162, 1229–1241 (2015). This study reveals that glucose competition between cancer and T cells can be detrimental for antitumour T cells. It also shows that PDL1 expression by cancer cells contributes to glucose competition by elevating aerobic glycolysis in cancer cells but dampening it in T cells. This study and reference 43 uncover that deregulated cellular energetics, especially aerobic glycolysis, in cancer cells leads to failure of antitumour T cell responses and that metabolic reprogramming of tumour-specific T cells can increase the therapeutic benefit of adoptive cell therapy.
Zhao, E. et al. Cancer mediates effector T cell dysfunction by targeting microRNAs and EZH2 via glycolysis restriction. Nat. Immunol. 17, 95–103 (2016).
Choi, Y. S., Eto, D., Yang, J. A., Lao, C. & Crotty, S. Cutting edge: STAT1 is required for IL-6-mediated Bcl6 induction for early follicular helper cell differentiation. J. Immunol. 190, 3049–3053 (2013).
Yan, Y. et al. IDO upregulates regulatory T cells via tryptophan catabolite and suppresses encephalitogenic T cell responses in experimental autoimmune encephalomyelitis. J. Immunol. 185, 5953–5961 (2010).
Gajewski, T. F. et al. Cancer immunotherapy strategies based on overcoming barriers within the tumor microenvironment. Curr. Opin. Immunol. 25, 268–276 (2013).
Rodriguez, P. C. et al. Arginase I-producing myeloid-derived suppressor cells in renal cell carcinoma are a subpopulation of activated granulocytes. Cancer Res. 69, 1553–1560 (2009).
Tanaka, K. et al. Compensatory glutamine metabolism promotes glioblastoma resistance to mTOR inhibitor treatment. J. Clin. Invest. 125, 1591–1602 (2015).
Fischer, K. et al. Inhibitory effect of tumor cell-derived lactic acid on human T cells. Blood 109, 3812–3819 (2007).
Robey, I. F. et al. Bicarbonate increases tumor pH and inhibits spontaneous metastases. Cancer Res. 69, 2260–2268 (2009).
Estrella, V. et al. Acidity generated by the tumor microenvironment drives local invasion. Cancer Res. 73, 1524–1535 (2013).
Kuroda, E. & Yamashita, U. Mechanisms of enhanced macrophage-mediated prostaglandin E2 production and its suppressive role in TH1 activation in TH2-dominant BALB/c mice. J. Immunol. 170, 757–764 (2003).
Eruslanov, E., Daurkin, I., Ortiz, J., Vieweg, J. & Kusmartsev, S. Pivotal advance: tumor-mediated induction of myeloid-derived suppressor cells and M2-polarized macrophages by altering intracellular PGE2 catabolism in myeloid cells. J. Leukoc. Biol. 88, 839–848 (2010).
Chen, J. H. et al. Prostaglandin E2 and programmed cell death 1 signaling coordinately impair CTL function and survival during chronic viral infection. Nat. Med. 21, 327–334 (2015).
Kurtova, A. V. et al. Blocking PGE2-induced tumour repopulation abrogates bladder cancer chemoresistance. Nature 517, 209–213 (2015).
Deaglio, S. et al. Adenosine generation catalyzed by CD39 and CD73 expressed on regulatory T cells mediates immune suppression. J. Exp. Med. 204, 1257–1265 (2007).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Michalek, R. D. et al. Cutting edge: distinct glycolytic and lipid oxidative metabolic programs are essential for effector and regulatory CD4+ T cell subsets. J. Immunol. 186, 3299–3303 (2011).
Restifo, N. P., Smyth, M. J. & Snyder, A. Acquired resistance to immunotherapy and future challenges. Nat. Rev. Cancer 16, 121–126 (2016).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Khalili, J. S. et al. Oncogenic BRAF(V600E) promotes stromal cell-mediated immunosuppression via induction of interleukin-1 in melanoma. Clin. Cancer Res. 18, 5329–5340 (2012). This paper shows that melanoma cells with the oncogenic mutation BRAF(V600E) produce IL-1, leading to T cell inhibition via PDL1 and cyclo-oxygenase 2 (COX2) upregulation in stromal cells of the TME.
Peng, W. et al. Loss of PTEN promotes resistance to T cell-mediated immunotherapy. Cancer Discov. 6, 202–216 (2016).
Peng, D. et al. Epigenetic silencing of TH1-type chemokines shapes tumour immunity and immunotherapy. Nature 527, 249–253 (2015). This paper describes an immune evasion strategy of ovarian cancer cells, through epigenetic silencing of the T H 1-type cytokines CXCL9 and CXCL10, thereby avoiding the attraction of antitumour T cells in humans and mice.
Rooney, M. S., Shukla, S. A., Wu, C. J., Getz, G. & Hacohen, N. Molecular and genetic properties of tumors associated with local immune cytolytic activity. Cell 160, 48–61 (2015).
Restifo, N. P. et al. Loss of functional beta2-microglobulin in metastatic melanomas from five patients receiving immunotherapy. J. Natl Cancer Inst. 88, 100–108 (1996).
Karre, K., Ljunggren, H. G., Piontek, G. & Kiessling, R. Selective rejection of H–2-deficient lymphoma variants suggests alternative immune defence strategy. Nature 319, 675–678 (1986).
Raulet, D. H. Missing self recognition and self tolerance of natural killer (NK) cells. Semin. Immunol. 18, 145–150 (2006).
Bessoles, S. et al. Adaptations of natural killer cells to self-MHC class I. Front. Immunol. 5, 349 (2014).
Winograd, R. et al. Induction of T-cell immunity overcomes complete resistance to PD1 and CTLA4 blockade and improves survival in pancreatic carcinoma. Cancer Immunol. Res. 3, 399–411 (2015).
Green, M. R. et al. Integrative analysis reveals selective 9p24.1 amplification, increased PD1 ligand expression, and further induction via JAK2 in nodular sclerosing Hodgkin lymphoma and primary mediastinal large B-cell lymphoma. Blood 116, 3268–3277 (2010).
Kataoka, K. et al. Aberrant PDL1 expression through 3′-UTR disruption in multiple cancers. Nature 534, 402–406 (2016).
Cancer Genome Atlas Network. Genomic classification of cutaneous melanoma. Cell 161, 1681–1696 (2015).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat. Rev. Cancer 12, 252–264 (2012).
Taube, J. M. et al. Colocalization of inflammatory response with B7-H1 expression in human melanocytic lesions supports an adaptive resistance mechanism of immune escape. Sci. Transl Med. 4, 127ra37 (2012). This study shows that, in the TME of human melanoma, PDL1 is expressed in microanatomical regions with rich TIL infiltration, suggesting that PDL1 upregulation is due to local immune responses.
Ribas, A. Adaptive immune resistance: how cancer protects from immune attack. Cancer Discov. 5, 915–919 (2015).
Woo, S. R., Corrales, L. & Gajewski, T. F. Innate immune recognition of cancer. Annu. Rev. Immunol. 33, 445–474 (2015).
Yang, X. et al. Targeting the tumor microenvironment with interferon-β bridges innate and adaptive immune responses. Cancer Cell 25, 37–48 (2014).
Baitsch, L., Fuertes-Marraco, S. A., Legat, A., Meyer, C. & Speiser, D. E. The three main stumbling blocks for anticancer T cells. Trends Immunol. 33, 364–372 (2012).
Nguyen, L. T. & Ohashi, P. S. Clinical blockade of PD1 and LAG3 — potential mechanisms of action. Nat. Rev. Immunol. 15, 45–56 (2015).
Spranger, S. et al. Up-regulation of PD-L1, IDO, and Tregs in the melanoma tumor microenvironment is driven by CD8+ T cells. Sci. Transl Med. 5, 200ra116 (2013). This study shows that CD8+ T cells drive immune-inhibitory mechanisms in human and mouse tumours, indicating that immunosuppression can result from natural conserved immune regulation rather than from properties of cancer cells acquired by mutation and Darwinian selection.
Mahoney, K. M. & Atkins, M. B. Prognostic and predictive markers for the new immunotherapies. Oncology 28 (Suppl. 3), 39–48 (2014).
Barber, D. L. et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature 439, 682–687 (2006). This paper shows that exhausted T cells in chronic LCMV infection express high levels of PD1 and that the blockade of PD1 enhances CD8+ T cell responses and antiviral immunity.
Zajac, A. J. et al. Viral immune evasion due to persistence of activated T cells without effector function. J. Exp. Med. 188, 2205–2213 (1998). This study describes immune responses in mice chronically infected with LCMV. It shows that CD8+ T cells are either deleted or lose effector functions, reducing their capacity to eradicate the virus.
Kaech, S. M. & Wherry, E. J. Heterogeneity and cell-fate decisions in effector and memory CD8+ T cell differentiation during viral infection. Immunity 27, 393–405 (2007).
Speiser, D. E. et al. T cell differentiation in chronic infection and cancer: functional adaptation or exhaustion? Nat. Rev. Immunol. 14, 768–774 (2014).
Wherry, E. J. & Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 15, 486–499 (2015).
Okamura, T. et al. CD4+CD25-LAG3+ regulatory T cells controlled by the transcription factor EGR2. Proc. Natl Acad. Sci. USA 106, 13974–13979 (2009).
Schietinger, A., Delrow, J. J., Basom, R. S., Blattman, J. N. & Greenberg, P. D. Rescued tolerant CD8 T cells are preprogrammed to reestablish the tolerant state. Science 335, 723–727 (2012).
Smith, T. R., Verdeil, G., Marquardt, K. & Sherman, L. A. Contribution of TCR signaling strength to CD8+ T cell peripheral tolerance mechanisms. J. Immunol. 193, 3409–3416 (2014).
Thomas, D. A. & Massague, J. TGFβ directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell 8, 369–380 (2005).
Delisle, J. S. et al. The TGFβ-SMAD3 pathway inhibits CD28-dependent cell growth and proliferation of CD4 T cells. Genes Immun. 14, 115–126 (2013).
Stephen, T. L. et al. Transforming growth factor β-mediated suppression of antitumor T cells requires FOXP1 transcription factor expression. Immunity 41, 427–439 (2014). This study establishes that FOXP1 cooperates with SMAD2 and SMAD3 to mediate TGFβ-driven suppression in tumour-infiltrated lymphocytes.
Quigley, M. et al. Transcriptional analysis of HIV-specific CD8+ T cells shows that PD1 inhibits T cell function by upregulating BATF. Nat. Med. 16, 1147–1151 (2010).
Fuertes Marraco, S. A., Neubert, N. J., Verdeil, G. & Speiser, D. E. Inhibitory receptors beyond T cell exhaustion. Front. Immunol. 6, 310 (2015).
Odorizzi, P. M., Pauken, K. E., Paley, M. A., Sharpe, A. & Wherry, E. J. Genetic absence of PD1 promotes accumulation of terminally differentiated exhausted CD8+ T cells. J. Exp. Med. 212, 1125–1137 (2015).
Walker, L. S. & Sansom, D. M. The emerging role of CTLA4 as a cell-extrinsic regulator of T cell responses. Nat. Rev. Immunol. 11, 852–863 (2011).
Kuo, C. T. & Leiden, J. M. Transcriptional regulation of T lymphocyte development and function. Annu. Rev. Immunol. 17, 149–187 (1999).
Martinez, G. J. et al. The transcription factor NFAT promotes exhaustion of activated CD8+ T cells. Immunity 42, 265–278 (2015). This study shows that NFAT, in the absence of AP-1, induces a panel of genes associated with T cell exhaustion.
Xiao, G., Deng, A., Liu, H., Ge, G. & Liu, X. Activator protein 1 suppresses antitumor T-cell function via the induction of programmed death 1. Proc. Natl Acad. Sci. USA 109, 15419–15424 (2012).
Baitsch, L. et al. Extended co-expression of inhibitory receptors by human CD8 T-cells depending on differentiation, antigen-specificity and anatomical localization. PLoS ONE 7, e30852 (2012).
Coornaert, B. et al. T cell antigen receptor stimulation induces MALT1 paracaspase–mediated cleavage of the NF-κB inhibitor A20. Nat. Immunol. 9, 263–271 (2008).
Giordano, M. et al. The tumor necrosis factor alpha-induced protein 3 (TNFAIP3, A20) imposes a brake on antitumor activity of CD8 T cells. Proc. Natl Acad. Sci. USA 111, 11115–11120 (2014).
Intlekofer, A. M. et al. Effector and memory CD8+ T cell fate coupled by T-bet and eomesodermin. Nat. Immunol. 6, 1236–1244 (2005).
Kurachi, M. et al. The transcription factor BATF operates as an essential differentiation checkpoint in early effector CD8+ T cells. Nat. Immunol. 15, 373–383 (2014).
Sekiya, T. et al. NR4A receptors are essential for thymic regulatory T cell development and immune homeostasis. Nat. Immunol. 14, 230–237 (2013).
Fassett, M. S., Jiang, W., D'Alise, A. M., Mathis, D. & Benoist, C. Nuclear receptor NR4A1 modulates both regulatory T-cell (Treg) differentiation and clonal deletion. Proc. Natl Acad. Sci. USA 109, 3891–3896 (2012).
Raveney, B. J., Oki, S. & Yamamura, T. Nuclear receptor NR4A2 orchestrates TH17 cell-mediated autoimmune inflammation via IL-21 signalling. PLoS ONE 8, e56595 (2013).
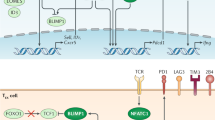
Shin, H. et al. A role for the transcriptional repressor BLIMP1 in CD8+ T cell exhaustion during chronic viral infection. Immunity 31, 309–320 (2009).
Shaffer, A. L. et al. BLIMP1 orchestrates plasma cell differentiation by extinguishing the mature B cell gene expression program. Immunity 17, 51–62 (2002).
Shaffer, A. L. et al. BCL-6 represses genes that function in lymphocyte differentiation, inflammation, and cell cycle control. Immunity 13, 199–212 (2000).
Harker, J. A., Lewis, G. M., Mack, L. & Zuniga, E. I. Late interleukin-6 escalates T follicular helper cell responses and controls a chronic viral infection. Science 334, 825–829 (2011).
Heinemann, C. et al. IL-27 and IL-12 oppose pro-inflammatory IL-23 in CD4+ T cells by inducing Blimp1. Nat. Commun. 5, 3770 (2014).
Gonin, J. et al. Expression of IL-27 by tumor cells in invasive cutaneous and metastatic melanomas. PLoS ONE 8, e75694 (2013).
Hisada, M. et al. Potent antitumor activity of interleukin-27. Cancer Res. 64, 1152–1156 (2004).
Oniki, S. et al. Interleukin-23 and interleukin-27 exert quite different antitumor and vaccine effects on poorly immunogenic melanoma. Cancer Res. 66, 6395–6404 (2006).
Yu, H., Kortylewski, M. & Pardoll, D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nat. Rev. Immunol. 7, 41–51 (2007).
Waugh, K. A., Leach, S. M. & Slansky, J. E. Targeting transcriptional regulators of CD8+ T cell dysfunction to boost anti-tumor immunity. Vaccines 3, 771–802 (2015).
Kujawski, M. et al. Targeting STAT3 in adoptively transferred T cells promotes their in vivo expansion and antitumor effects. Cancer Res. 70, 9599–9610 (2010).
Yue, C. et al. STAT3 in CD8+ T cells inhibits their tumor accumulation by downregulating CXCR3/CXCL10 axis. Cancer Immunol. Res. 3, 864–870 (2015).
Triplett, T. A. et al. STAT3 signaling is required for optimal regression of large established tumors in mice treated with anti-OX40 and TGFβ receptor blockade. Cancer Immunol. Res. 3, 526–535 (2015).
Siveen, K. S. et al. Targeting the STAT3 signaling pathway in cancer: role of synthetic and natural inhibitors. Biochim. Biophys. Acta 1845, 136–154 (2014).
Sznol, M. & Chen, L. Antagonist antibodies to PD1 and B7-H1 (PDL1) in the treatment of advanced human cancer. Clin. Cancer Res. 19, 1021–1034 (2013).
Schadendorf, D. et al. Pooled analysis of long-term survival data from phase II and phase III trials of ipilimumab in unresectable or metastatic melanoma. J. Clin. Oncol. 33, 1889–1894 (2015). This paper shows the extraordinary long-term therapeutic effects of CTLA4-specific antibody in melanoma patients, with a plateau in the survival curve beginning at 2–3 years after treatment.
Robert, C. et al. Pembrolizumab versus ipilimumab in advanced melanoma. N. Engl. J. Med. 372, 2521–2532 (2015).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N. Engl. J. Med. 372, 2018–2028 (2015).
Sanchez-Paulete, A. R. et al. Cancer immunotherapy with immunomodulatory anti-CD137 and anti-PD1 monoclonal antibodies requires BATF3-dependent dendritic cells. Cancer Discov. 6, 71–79 (2016).
Lesokhin, A. M., Callahan, M. K., Postow, M. A. & Wolchok, J. D. On being less tolerant: enhanced cancer immunosurveillance enabled by targeting checkpoints and agonists of T cell activation. Sci. Transl Med. 7, 280sr1 (2015).
Khalil, D. N., Smith, E. L., Brentjens, R. J. & Wolchok, J. D. The future of cancer treatment: immunomodulation, CARs and combination immunotherapy. Nat. Rev. Clin. Oncol. 13, 273–290 (2016).
Joyce, J. A. & Fearon, D. T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 348, 74–80 (2015).
Zhu, Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014).
June, C. H., Riddell, S. R. & Schumacher, T. N. Adoptive cellular therapy: a race to the finish line. Sci. Transl Med. 7, 280ps7 (2015).
Klebanoff, C. A. et al. Determinants of successful CD8+ T-cell adoptive immunotherapy for large established tumors in mice. Clin. Cancer Res. 17, 5343–5352 (2011).
Gattinoni, L. et al. A human memory T cell subset with stem cell-like properties. Nat. Med. 17, 1290–1297 (2011).
Klebanoff, C. A. et al. Memory T cell-driven differentiation of naive cells impairs adoptive immunotherapy. J. Clin. Invest. 126, 318–334 (2016).
Sukumar, M. et al. Inhibiting glycolytic metabolism enhances CD8+ T cell memory and antitumor function. J. Clin. Invest. 123, 4479–4488 (2013).
Crompton, J. G. et al. AKT inhibition enhances expansion of potent tumor-specific lymphocytes with memory cell characteristics. Cancer Res. 75, 296–305 (2015).
Sukumar, M. et al. Mitochondrial membrane potential identifies cells with enhanced stemness for cellular therapy. Cell. Metab. 23, 63–76 (2016).
Intlekofer, A. M. et al. Anomalous type 17 response to viral infection by CD8+ T cells lacking T-bet and eomesodermin. Science 321, 408–411 (2008).
Grange, M. et al. Activated STAT5 promotes long-lived cytotoxic CD8+ T cells that induce regression of autochthonous melanoma. Cancer Res. 72, 76–87 (2012).
Grange, M. et al. Active STAT5 regulates T-bet and eomesodermin expression in CD8 T cells and imprints a T-bet-dependent Tc1 program with repressed IL-6/TGFβ1 signaling. J. Immunol. 191, 3712–3724 (2013).
Dow, L. E. et al. Inducible in vivo genome editing with CRISPR-Cas9. Nat. Biotechnol. 33, 390–394 (2015).
Pauken, K. E. & Wherry, E. J. SnapShot: T cell exhaustion. Cell 163, 1038–1038.e1 (2015).
Hugo, W. et al. Non-genomic and immune evolution of melanoma acquiring MAPKi resistance. Cell 162, 1271–1285 (2015).
Smyth, M. J., Ngiow, S. F., Ribas, A. & Teng, M. W. Combination cancer immunotherapies tailored to the tumour microenvironment. Nat. Rev. Clin. Oncol. 13, 143–158 (2016).
Fridman, W. H., Pages, F., Sautes-Fridman, C. & Galon, J. The immune contexture in human tumours: impact on clinical outcome. Nat. Rev. Cancer 12, 298–306 (2012). This paper summarizes 124 published articles, revealing the broad principle that intratumoral infiltration of memory CD8+ T cells and CD4+ T H 1 cells is associated with favourable prognosis in the majority of solid human cancers.
Michalek, R. D. et al. Estrogen-related receptor-α is a metabolic regulator of effector T-cell activation and differentiation. Proc. Natl Acad. Sci. USA 108, 18348–18353 (2011).
Wang, R. et al. The transcription factor MYC controls metabolic reprogramming upon T lymphocyte activation. Immunity 35, 871–882 (2011).
Sinclair, L. V. et al. Control of amino-acid transport by antigen receptors coordinates the metabolic reprogramming essential for T cell differentiation. Nat. Immunol. 14, 500–508 (2013).
van der Windt, G. J. et al. Mitochondrial respiratory capacity is a critical regulator of CD8+ T cell memory development. Immunity 36, 68–78 (2012).
O'Sullivan, D. et al. Memory CD8+ T cells use cell-intrinsic lipolysis to support the metabolic programming necessary for development. Immunity 41, 75–88 (2014).
Cui, G. et al. IL-7-Induced glycerol transport and TAG synthesis promotes memory CD8+ T cell longevity. Cell 161, 750–761 (2015).
van der Windt, G. J. et al. CD8 memory T cells have a bioenergetic advantage that underlies their rapid recall ability. Proc. Natl Acad. Sci. USA 110, 14336–14341 (2013).
Blagih, J. et al. The energy sensor AMPK regulates T cell metabolic adaptation and effector responses in vivo. Immunity 42, 41–54 (2015).
Siska, P. J. & Rathmell, J. C. T cell metabolic fitness in antitumor immunity. Trends Immunol. 36, 257–264 (2015).
Chang, C. H. & Pearce, E. L. Emerging concepts of T cell metabolism as a target of immunotherapy. Nat. Immunol. 17, 364–368 (2016).
Doering, T. A. et al. Network analysis reveals centrally connected genes and pathways involved in CD8+ T cell exhaustion versus memory. Immunity 37, 1130–1144 (2012).
Mueller, S. N., Gebhardt, T., Carbone, F. R. & Heath, W. R. Memory T cell subsets, migration patterns, and tissue residence. Annu. Rev. Immunol. 31, 137–161 (2013).
Park, C. O. & Kupper, T. S. The emerging role of resident memory T cells in protective immunity and inflammatory disease. Nat. Med. 21, 688–697 (2015).
Fan, X. & Rudensky, A. Y. Hallmarks of tissue-resident lymphocytes. Cell 164, 1198–1211 (2016).
Herbst, R. S. et al. Predictive correlates of response to the anti-PD-L1 antibody MPDL3280A in cancer patients. Nature 515, 563–567 (2014).
Speiser, D. E. & Flatz, L. Cancer immunotherapy drives implementation science in oncology. Hum. Vaccin. Immunother. 10, 3107–3110 (2014).
Acknowledgements
The authors acknowledge the vast and invaluable work that could not be cited here because of space limitations. The authors thank M. de Palma and T. Murray for revising the manuscript, and G. Coukos, M. Delorenzi, M. Gilliet, O. Michielin, Ch. Münz, N. Neubert, P. Romero, L. Tillé and D. Zehn and their group members for collaboration. The authors acknowledge all members of their teams and their collaborators and patients for their dedication, contributions and discussions. This work was supported in part by funds from Ludwig Cancer Research, the Cancer Research Institute, the Wilhelm Sander Foundation, the Swiss Cancer Research (3507-09-2014), the Swiss National Science Foundation (CRSII3_141879, 320030_152856), Novartis Foundation for medical-biological Research, Melanoma Research Alliance, Harry J. Lloyd Charitable Trust, SwissTransMed (KIP 18) and a grant from Alfred and Annemarie von Sick.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Type 1 immune responses
-
Viral or intracellular bacterial infections trigger innate immune responses accompanied by the activation of cytotoxic CD8+ T cells and CD4+ T helper 1 cells. Similar T cell populations are believed to mediate the antitumour lymphocyte effects.
- Dysfunctional T cells
-
T cells in chronic infection show reduced proliferation and cytokine production because of repetitive antigen stimulation and dysbalanced co-stimulatory and inhibitory signals. T cells in cancer may show similar functional alterations in the tumour microenvironment because of similar and/or different molecular signals.
- Professional antigen-presenting cells
-
(APCs). Priming and strong boosting of T cells requires professional APCs, particularly dendritic cells, which achieve this goal via the expression of co-stimulatory membrane ligands (for example, CD80 and CD86) and the secretion of cytokines (for example, interleukin-12 and interferons).
- Tumour-infiltrating T lymphocytes
-
(TILs). Intratumoural T cells. In mice and humans, the main immune cell populations that can destroy cancers ('antitumour T cells') are cytotoxic CD8+ T cells and CD4+ T helper 1 (TH1) cells.
- M2-like macrophages
-
A macrophage subset that is stimulated by interleukin-4 (IL-4) or IL-13 and that expresses arginase 1, the mannose receptor CD206 and the IL-4 receptor α-chain.
- M1-like macrophages
-
A macrophage subset that is activated by Toll-like receptor ligands (such as lipopolysaccharide) and interferon-γ. M1-like macrophages express pro-inflammatory cytokines and inducible nitric oxide synthase, among other things.
- Anergic T cells
-
T cells primed through T cell receptor (TCR) stimulation without any co-stimulation become unresponsive to further stimulation with antigen; they stop proliferating and producing cytokines.
- Senescent T cells
-
T cells that have accomplished excessive numbers of divisions reach a point of irreversible cell cycle arrest.
Rights and permissions
About this article
Cite this article
Speiser, D., Ho, PC. & Verdeil, G. Regulatory circuits of T cell function in cancer. Nat Rev Immunol 16, 599–611 (2016). https://doi.org/10.1038/nri.2016.80
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri.2016.80
This article is cited by
-
Age-Related Features of the Response of Cancer Stem Cells and T Cells in Experimental Lung Cancer
Bulletin of Experimental Biology and Medicine (2024)
-
Focus on T cell exhaustion: new advances in traditional Chinese medicine in infection and cancer
Chinese Medicine (2023)
-
Identification and in vitro and in vivo validation of the key role of GSDME in pyroptosis-related genes signature in hepatocellular carcinoma
BMC Cancer (2023)
-
A pilot study on pyroptosis related genes in peripheral blood mononuclear cells of non-small cell lung cancer patients
BMC Pulmonary Medicine (2023)
-
Increased levels of PD1 and glycolysis in CD4+ T cells are positively associated with lymph node metastasis in OSCC
BMC Oral Health (2023)