Key Points
-
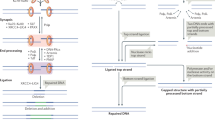
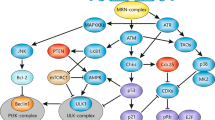
DNA double-stranded break (DSB) damage activates ataxia-telangiectasia mutated (ATM)-dependent cell-cycle checkpoints, leading to cell-cycle arrest and efficient DNA repair. The DNA DSB damage is repaired mainly by the non-homologous end-joining pathway in mammalian cells.
-
Viral infection induces DNA-damage responses to DSBs that arise in infected host cells. These DNA-damage responses are required for the survival of host cells, but they can have either a positive or a negative effect on productive infection, depending on the type of virus.
-
DNA-damage responses activate innate immune responses through several pathways, including the upregulation of expression of NKG2D (natural killer group 2, member D) ligands and interferon-regulatory factors.
-
DNA DSBs are necessary intermediates of V(D)J recombination. They are required for generation of the large repertoire of antigen-specific cells that are the main components of the adaptive arm of the immune system. DNA-damage responses to DSBs have important roles in efficient V(D)J recombination and in prevention of recombination-activating gene (RAG)-protein-induced genetic instability in lymphocytes.
-
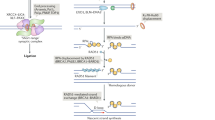
Activation-induced cytidine deaminase (AID)-induced DNA lesions are required for both class-switch recombination and somatic hypermutation. DNA-damage responses to DSBs are important for class-switch recombination but dispensable for somatic hypermutation.
-
Granzyme A and granzyme C are released by cytotoxic T lymphocytes and induce caspase-independent apoptosis of target cells, by activating pathways that introduce DNA DSBs into the genome of these cells.
Abstract
Chromosome breakage is frequently associated with viral infection and cellular transformation, but it is also required for two processes that are crucial for the development and function of adaptive immunity: V(D)J recombination and class-switch recombination. The cellular responses that result from this type of DNA damage, which are mostly activated by the protein kinase ataxia-telangiectasia mutated (ATM), lead to cell-cycle arrest at several checkpoints and efficient DNA repair. This Review focuses on the important roles of these DNA-damage responses in the activation of innate immunity and the targeting of the innate immune response to infected or transformed cells, as well as in the development and function of adaptive immunity.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Savitsky, K. et al. A single ataxia telangiectasia gene with a product similar to PI-3 kinase. Science 268, 1749–1753 (1995).The discovery of the protein kinase ATM is a milestone in the studies of cellular responses to DNA DSB damage.
Shiloh, Y. ATM and related protein kinases: safeguarding genome integrity. Nature Rev. Cancer 3, 155–168 (2003).
Bakkenist, C. J. & Kastan, M. B. DNA damage activates ATM through intermolecular autophosphorylation and dimer dissociation. Nature 421, 499–506 (2003).This paper describes that ATM is activated through autophosphorylation.
Assenmacher, N. & Hopfner, K. P. MRE11/RAD50/NBS1: complex activities. Chromosoma 113, 157–166 (2004).
Celeste, A. et al. Histone H2AX phosphorylation is dispensable for the initial recognition of DNA breaks. Nature Cell Biol. 5, 675–679 (2003).
Falck, J., Coates, J. & Jackson, S. P. Conserved modes of recruitment of ATM, ATR and DNA-PKcs to sites of DNA damage. Nature 434, 605–611 (2005).This study uncovers the mechanism of ATM activation at the biochemical level.
Uziel, T., et al. Requirement of the MRN complex for ATM activation by DNA damage. EMBO J. 22, 5612–5621 (2003).
Carson, C. T. et al. The Mre11 complex is required for ATM activation and the G2/M checkpoint. EMBO J. 22, 6610–6620 (2003).
Mochan, T. A., Venere, M., DiTullio, R. A. Jr & Halazonetis, T. D. 53BP1 and NFBD1/MDC1–Nbs1 function in parallel interacting pathways activating ataxia-telangiectasia mutated (ATM) in response to DNA damage. Cancer Res. 63, 8586–8591 (2003).
Difilippantonio, S. et al. Role of Nbs1 in the activation of the Atm kinase revealed in humanized mouse models. Nature Cell Biol. 7, 675–685 (2005).
Lee, J. H. & Paull, T. T. Direct activation of the ATM protein kinase by the Mre11/Rad50/Nbs1 complex. Science 304, 93–96 (2004).
You, Z., Chahwan, C., Bailis, J., Hunter, T. & Russell, P. ATM activation and its recruitment to damaged DNA require binding to the C terminus of Nbs1. Mol. Cell. Biol. 25, 5363–5379 (2005).
Rogakou, E. P., Pilch, D. R., Orr, A. H., Ivanova, V. S. & Bonner, W. M. DNA double-stranded breaks induce histone H2AX phosphorylation on serine 139. J. Biol. Chem. 273, 5858–5868 (1998).
Fernandez-Capetillo, O. et al. DNA damage-induced G2–M checkpoint activation by histone H2AX and 53BP1. Nature Cell Biol. 4, 993–997 (2002).
Kang, J. et al. Functional interaction of H2AX, NBS1, and p53 in ATM-dependent DNA damage responses and tumor suppression, Mol. Cell. Biol. 25, 661–670 (2005).
Paull, T. T. et al. A critical role for histone H2AX in recruitment of repair factors to nuclear foci after DNA damage. Curr. Biol. 10, 886–895 (2000).
Kobayashi, J. et al. NBS1 localizes to γ-H2AX foci through interaction with the FHA/BRCT domain. Curr. Biol. 12, 1846–1851 (2002).
Ward, I. M., Minn, K., Jorda, K. G. & Chen, J. Accumulation of checkpoint protein 53BP1 at DNA breaks involves its binding to phosphorylated histone H2AX. J. Biol. Chem. 278, 19579–19582 (2003).
Kurz, E. U. & Lees-Miller, S. P. DNA damage-induced activation of ATM and ATM-dependent signaling pathways. DNA Repair (Amst.) 3, 889–900 (2004).
Zhao, S. et al. Functional link between ataxia-telangiectasia and Nijmegen breakage syndrome gene products. Nature 405, 473–477 (2000).
Lim, D. S. et al. ATM phosphorylates p95/nbs1 in an S-phase checkpoint pathway. Nature 404, 613–617 (2000).
Gatei, M. et al. ATM-dependent phosphorylation of nibrin in response to radiation exposure. Nature Genet. 25, 115–119 (2000).
Zhu, J., Petersen, S., Tessarollo, L. & Nussenzweig, A. Targeted disruption of the Nijmegen breakage syndrome gene NBS1 leads to early embryonic lethality in mice. Curr. Biol. 11, 105–109 (2001).
Kang, J., Bronson, R. T. & Xu, Y. Targeted disruption of NBS1 reveals its roles in mouse development and DNA repair. EMBO J. 21, 1447–1455 (2002).
Gottlieb, T. M. & Jackson, S. P. The DNA-dependent protein kinase: requirement for DNA ends and association with Ku antigen. Cell 72, 131–142 (1993).
Suwa, A. et al. DNA-dependent protein kinase (Ku protein–p350 complex) assembles on double-stranded DNA. Proc. Natl Acad. Sci. USA 91, 6904–6908 (1994).
Rooney, S., Chaudhuri, J. & Alt, F. W. The role of the non-homologous end-joining pathway in lymphocyte development. Immunol. Rev. 200, 115–131 (2004).
Meek, K., Gupta, S., Ramsden, D. A. & Lees-Miller, S. P. The DNA-dependent protein kinase: the director at the end. Immunol. Rev. 200, 132–141 (2004).
Kurimasa, A. et al. Requirement for the kinase activity of human DNA-dependent protein kinase catalytic subunit in DNA strand break rejoining. Mol. Cell. Biol. 19, 3877–3884 (1999).
Blunt, T. et al. Defective DNA-dependent protein kinase activity is linked to V(D)J recombination and DNA repair defects associated with the murine scid mutation. Cell 80, 813–823 (1995).
Ma, Y., Schwarz, K. & Lieber, M. R. The Artemis: DNA-PKcs endonuclease cleaves DNA loops, flaps, and gaps. DNA Repair (Amst.) 4, 845–851 (2005).
Mahajan, K. N., Nick McElhinny, S. A., Mitchell, B. S. & Ramsden, D. A. Association of DNA polymerase μ (pol μ) with Ku and ligase IV: role for pol μ in end-joining double-strand break repair. Mol. Cell. Biol. 22, 5194–5202 (2002).
Bertocci, B., De Smet, A., Berek, C., Weill, J. C. & Reynaud, C. A. Immunoglobulin κ light chain gene rearrangement is impaired in mice deficient for DNA polymerase μ. Immunity 19, 203–211 (2003).
Dai, Y. et al. Nonhomologous end joining and V(D)J recombination require an additional factor. Proc. Natl Acad. Sci. USA 100, 2462–2467 (2003).
Skalka, A. M. & Katz, R. A. Retroviral DNA integration and the DNA damage response. Cell Death Differ. 12, 971–978 (2005).
Daniel, R., Katz, R. A. & Skalka, A. M. A role for DNA-PK in retroviral DNA integration. Science 284, 644–647 (1999).This study provided the first indication of the importance of DNA DSB repair in productive retroviral infection.
Daniel, R. et al. Wortmannin potentiates integrase-mediated killing of lymphocytes and reduces the efficiency of stable transduction by retroviruses. Mol. Cell. Biol. 21, 1164–1172 (2001).
Daniel, R. et al. Histone H2AX is phosphorylated at sites of retroviral DNA integration but is dispensable for postintegration repair. J. Biol. Chem. 279, 45810–45814 (2004).
Lau, A. et al. Suppression of HIV-1 infection by a small molecule inhibitor of the ATM kinase. Nature Cell Biol. 7, 493–500 (2005).
Daniel, R. et al. Evidence that the retroviral DNA integration process triggers an ATR-dependent DNA damage response. Proc. Natl Acad. Sci. USA 100, 4778–4783 (2003).
Cuconati, A., Mukherjee, C., Perez, D. & White, E. DNA damage response and MCL-1 destruction initiate apoptosis in adenovirus-infected cells. Genes Dev. 17, 2922–2932 (2003).
Stracker, T. H., Carson, C. T. & Weitzman, M. D. Adenovirus oncoproteins inactivate the Mre11–Rad50–NBS1 DNA repair complex. Nature 418, 348–352 (2002).This paper, together with reference 47, provides compelling evidence for the involvement of DNA-DSB-damage responses in adenoviral infection.
Weiden, M. D. & Ginsberg, H. S. Deletion of the E4 region of the genome produces adenovirus DNA concatemers. Proc. Natl Acad. Sci. USA 91, 153–157 (1994).
Tosi, M. F. Innate immune responses to infection. J. Allergy Clin. Immunol. 116, 241–249 (2005).
Raulet, D. H. Roles of the NKG2D immunoreceptor and its ligands. Nature Rev. Immunol. 3, 781–790 (2003).
Smyth, M. J. et al. NKG2D function protects the host from tumor initiation. J. Exp. Med. 202, 583–588 (2005).
Gasser, S., Orsulic, S., Brown, E. J. & Raulet, D. H. The DNA damage pathway regulates innate immune system ligands of the NKG2D receptor. Nature 436, 1186–1190 (2005).
DiTullio, R. A. Jr et al. 53BP1 functions in an ATM-dependent checkpoint pathway that is constitutively activated in human cancer. Nature Cell Biol. 4, 998–1002 (2002).
Taniguchi, T., Ogasawara, K., Takaoka, A. & Tanaka, N. IRF family of transcription factors as regulators of host defense. Annu. Rev. Immunol. 19, 623–655 (2001).
Pamment, J., Ramsay, E., Kelleher, M., Dornan, D. & Ball, K. L. Regulation of the IRF-1 tumour modifier during the response to genotoxic stress involves an ATM-dependent signalling pathway. Oncogene 21, 7776–7785 (2002).
Tamura, T. et al. An IRF-1-dependent pathway of DNA damage-induced apoptosis in mitogen-activated T lymphocytes. Nature 376, 596–599 (1995).
Barnes, B., Lubyova, B. & Pitha, P. M. On the role of IRF in host defense. J. Interferon Cytokine Res. 22, 59–71 (2002).
Kim, T. et al. Activation of interferon regulatory factor 3 in response to DNA-damaging agents. J. Biol. Chem. 274, 30686–30689 (1999).
Karpova, A. Y., Trost, M., Murray, J. M., Cantley, L. C. & Howley, P. M. Interferon regulatory factor-3 is an in vivo target of DNA-PK. Proc. Natl Acad. Sci. USA 99, 2818–2823 (2002).
Bassing, C. H., Swat, W. & Alt, F. W. The mechanism and regulation of chromosomal V(D)J recombination. Cell 109, S45–S55 (2002).
Schatz, D. G. V(D)J recombination. Immunol. Rev. 200, 5–11 (2004).
Brandt, V. L. & Roth, D. B. V(D)J recombination: how to tame a transposase. Immunol. Rev. 200, 249–260 (2004).
Gellert, M. V(D)J recombination: RAG proteins, repair factors, and regulation. Annu. Rev. Biochem. 71, 101–132 (2002).
Lee, G. S., Neiditch, M. B., Salus, S. S. & Roth, D. B. RAG proteins shepherd double-strand breaks to a specific pathway, suppressing error-prone repair, but RAG nicking initiates homologous recombination. Cell 117, 171–184 (2004).This paper describes the roles of full-length RAG proteins in suppression of error-prone DNA repair.
Chen, H. T. et al. Response to RAG-mediated VDJ cleavage by NBS1 and γ-H2AX. Science 290, 1962–1965 (2000).
Perkins, E. J. et al. Sensing of intermediates in V(D)J recombination by ATM. Genes Dev. 16, 159–164 (2002).
Barlow, C. et al. Atm-deficient mice: a paradigm of ataxia telangiectasia. Cell 86, 159–171 (1996).
Xu, Y. et al. Targeted disruption of ATM leads to growth retardation, chromosomal fragmentation during meiosis, immune defects, and thymic lymphoma. Genes Dev. 10, 2411–2422 (1996).References 62 and 63 provide a comprehensive analysis of two strains of ATM-deficient mice.
Elson, A. et al. Pleiotropic defects in ataxia-telangiectasia protein-deficient mice. Proc. Natl Acad. Sci. USA 93, 13084–13089 (1996).
Chao, C., Yang, E. M. & Xu, Y. Rescue of defective T cell development and function in Atm−/− mice by a functional TCR αβ transgene. J. Immunol. 164, 345–349 (2000).
Xu, Y. ATM in lymphoid development and tumorigenesis. Adv. Immunol. 72, 179–189 (1999).
Moshous, D. et al. Artemis, a novel DNA double-strand break repair/V(D)J recombination protein, is mutated in human severe combined immune deficiency. Cell 105, 177–186 (2001).
Zhang, X. et al. Artemis is a phosphorylation target of ATM and ATR and is involved in the G2/M DNA damage checkpoint response. Mol. Cell. Biol. 24, 9207–9220 (2004).
Riballo, E. et al. A pathway of double-strand break rejoining dependent upon ATM, Artemis, and proteins locating to γ-H2AX foci. Mol. Cell 16, 715–724 (2004).
Bassing, C. H. & Alt, F. W. The cellular response to general and programmed DNA double strand breaks. DNA Repair (Amst.) 3, 781–796 (2004).
Schlissel, M., Constantinescu, A., Morrow, T., Baxter, M. & Peng, A. Double-strand signal sequence breaks in V(D)J recombination are blunt, 5′-phosphorylated, RAG-dependent, and cell cycle regulated. Genes Dev. 7, 2520–2532 (1993).This study provides evidence that V(D)J recombination is restricted to the G1 phase and G0 phase of the cell cycle.
Lin, W. C. & Desiderio, S. Regulation of V(D)J recombination activator protein RAG-2 by phosphorylation. Science 260, 953–959 (1993).This paper describes a mechanism for the restriction of V(D)J recombination to the G1 phase and G0 phase of the cell cycle, through the destabilization of V(D)J recombinase in S phase.
Li, Z., Dordai, D. I., Lee, J. & Desiderio, S. A conserved degradation signal regulates RAG-2 accumulation during cell division and links V(D)J recombination to the cell cycle. Immunity 5, 575–589 (1996).
Pagano, M. Control of DNA synthesis and mitosis by the Skp2–p27–Cdk1/2 axis. Mol. Cell 14, 414–416 (2004).
Zhu, C. et al. Unrepaired DNA breaks in p53-deficient cells lead to oncogenic gene amplification subsequent to translocations. Cell 109, 811–821 (2002).
Difilippantonio, M. J. et al. Evidence for replicative repair of DNA double-strand breaks leading to oncogenic translocation and gene amplification. J. Exp. Med. 196, 469–480 (2002).
Kim, D. R., Dai, Y., Mundy, C. L., Yang, W. & Oettinger, M. A. Mutations of acidic residues in RAG1 define the active site of the V(D)J recombinase. Genes Dev. 13, 3070–3080 (1999).
Landree, M. A., Wibbenmeyer, J. A. & Roth, D. B. Mutational analysis of RAG1 and RAG2 identifies three catalytic amino acids in RAG1 critical for both cleavage steps of V(D)J recombination. Genes Dev. 13, 3059–3069 (1999).
Fugmann, S. D., Villey, I. J., Ptaszek, L. M. & Schatz, D. G. Identification of two catalytic residues in RAG1 that define a single active site within the RAG1/RAG2 protein complex. Mol. Cell 5, 97–107 (2000).
Agrawal, A., Eastman, Q. M. & Schatz, D. G. Transposition mediated by RAG1 and RAG2 and its implications for the evolution of the immune system. Nature 394, 744–751 (1998).
Hiom, K., Melek, M. & Gellert, M. DNA transposition by the RAG1 and RAG2 proteins: a possible source of oncogenic translocations. Cell 94, 463–470 (1998).
Raghavan, S. C., Swanson, P. C., Wu, X., Hsieh, C. L. & Lieber, M. R. A non-B-DNA structure at the Bcl-2 major breakpoint region is cleaved by the RAG complex. Nature 428, 88–93 (2004).
Raghavan, S. C., Swanson, P. C., Ma, Y. & Lieber, M. R. Double-strand break formation by the RAG complex at the Bcl-2 major breakpoint region and at other non-B DNA structures in vitro. Mol. Cell. Biol. 25, 5904–5919 (2005).
Wagner, S. D. & Neuberger, M. S. Somatic hypermutation of immunoglobulin genes. Annu. Rev. Immunol. 14, 441–457 (1996).
Revy, P. et al. Activation-induced cytidine deaminase (AID) deficiency causes the autosomal recessive form of the hyper-IgM syndrome (HIGM2). Cell 102, 565–575 (2000).
Muramatsu, M. et al. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell 102, 553–563 (2000).This paper describes the groundbreaking discovery that AID is required for both CSR and SHM.
Petersen-Mahrt, S. K., Harris, R. S. & Neuberger, M. S. AID mutates E. coli suggesting a DNA deamination mechanism for antibody diversification. Nature 418, 99–103 (2002).This study provided the first indication that AID can function as a DNA cytidine deaminase.
Chaudhuri, J. et al. Transcription-targeted DNA deamination by the AID antibody diversification enzyme. Nature 422, 726–730 (2003).
Pham, P., Bransteitter, R., Petruska, J. & Goodman, M. F. Processive AID-catalysed cytosine deamination on single-stranded DNA simulates somatic hypermutation. Nature 424, 103–107 (2003).
Bransteitter, R., Pham, P., Scharff, M. D. & Goodman, M. F. Activation-induced cytidine deaminase deaminates deoxycytidine on single-stranded DNA but requires the action of RNase. Proc. Natl Acad. Sci. USA 100, 4102–4107 (2003).
Ramiro, A. R., Stavropoulos, P., Jankovic, M. & Nussenzweig, M. C. Transcription enhances AID-mediated cytidine deamination by exposing single-stranded DNA on the nontemplate strand. Nature Immunol. 4, 452–456 (2003).
Petersen, S. et al. AID is required to initiate Nbs1/γ-H2AX focus formation and mutations at sites of class switching. Nature 414, 660–665 (2001).This study provides compelling evidence that AID induces DNA DSB damage in switch regions during CSR.
Wuerffel, R. A., Du, J., Thompson, R. J. & Kenter, A. L. Ig Sγ3 DNA-specific double strand breaks are induced in mitogen-activated B cells and are implicated in switch recombination. J. Immunol. 159, 4139–4144 (1997).
Catalan, N. et al. The block in immunoglobulin class switch recombination caused by activation-induced cytidine deaminase deficiency occurs prior to the generation of DNA double strand breaks in switch μ region. J. Immunol. 171, 2504–2509 (2003).
Chaudhuri, J. & Alt, F. W. Class-switch recombination: interplay of transcription, DNA deamination and DNA repair. Nature Rev. Immunol. 4, 541–552 (2004).
Reina-San-Martin, B., Chen, H. T., Nussenzweig, A. & Nussenzweig, M. C. ATM is required for efficient recombination between immunoglobulin switch regions. J. Exp. Med. 200, 1103–1110 (2004).
Lumsden, J. M. et al. Immunoglobulin class switch recombination is impaired in Atm-deficient mice. J. Exp. Med. 200, 1111–1121 (2004).
Wang, B., Matsuoka, S., Carpenter, P. B. & Elledge, S. J. 53BP1, a mediator of the DNA damage checkpoint. Science 298, 1435–1438 (2002).
Ward, I. M. et al. 53BP1 is required for class switch recombination. J. Cell Biol. 165, 459–464 (2004).
Manis, J. P. et al. 53BP1 links DNA damage-response pathways to immunoglobulin heavy chain class-switch recombination. Nature Immunol. 5, 481–487 (2004).
Reina-San-Martin, B. et al. H2AX is required for recombination between immunoglobulin switch regions but not for intra-switch region recombination or somatic hypermutation. J. Exp. Med. 197, 1767–1778 (2003).
Reina-San-Martin, B., Nussenzweig, M. C., Nussenzweig, A. & Difilippantonio, S. Genomic instability, endoreduplication, and diminished Ig class-switch recombination in B cells lacking Nbs1. Proc. Natl Acad. Sci. USA 102, 1590–1595 (2005).
Kracker, S. et al. Nibrin functions in Ig class-switch recombination. Proc. Natl Acad. Sci. USA 102, 1584–1589 (2005).
Peters, A. & Storb, U. Somatic hypermutation of immunoglobulin genes is linked to transcription initiation. Immunity 4, 57–65 (1996).
Sale, J. E. & Neuberger, M. S. TdT-accessible breaks are scattered over the immunoglobulin V domain in a constitutively hypermutating B cell line. Immunity 9, 859–869 (1998).
Papavasiliou, F. N. & Schatz, D. G. Cell-cycle-regulated DNA double-stranded breaks in somatic hypermutation of immunoglobulin genes. Nature 408, 216–221 (2000).
Bross, L. et al. DNA double-strand breaks in immunoglobulin genes undergoing somatic hypermutation. Immunity 13, 589–597 (2000).
Zan, H., Wu, X., Komori, A., Holloman, W. K. & Casali, P. AID-dependent generation of resected double-strand DNA breaks and recruitment of Rad52/Rad51 in somatic hypermutation. Immunity 18, 727–738 (2003).
Bross, L., Muramatsu, M., Kinoshita, K., Honjo, T. & Jacobs, H. DNA double-strand breaks: prior to but not sufficient in targeting hypermutation. J. Exp. Med. 195, 1187–1192 (2002).
Papavasiliou, F. N. & Schatz, D. G. The activation-induced deaminase functions in a postcleavage step of the somatic hypermutation process. J. Exp. Med. 195, 1193–1198 (2002).
Bemark, M. et al. Somatic hypermutation in the absence of DNA-dependent protein kinase catalytic subunit (DNA-PKcs) or recombination-activating gene (RAG)1 activity. J. Exp. Med. 192, 1509–1514 (2000).
Zan, H. et al. The translesion DNA polymerase ζ plays a major role in Ig and bcl-6 somatic hypermutation. Immunity 14, 643–653 (2001).
Pan-Hammarstrom, Q. et al. ATM is not required in somatic hypermutation of VH, but is involved in the introduction of mutations in the switch μ region. J. Immunol. 170, 3707–3716 (2003).
Yabuki, M., Fujii, M. M. & Maizels, N. The MRE11–RAD50–NBS1 complex accelerates somatic hypermutation and gene conversion of immunoglobulin variable regions. Nature Immunol. 6, 730–736 (2005).
Catalfamo, M. & Henkart, P. A. Perforin and the granule exocytosis cytotoxicity pathway. Curr. Opin. Immunol. 15, 522–527 (2003).
Lieberman, J. The ABCs of granule-mediated cytotoxicity: new weapons in the arsenal. Nature Rev. Immunol. 3, 361–370 (2003).
Fan, Z., Beresford, P. J., Oh, D. Y., Zhang, D. & Lieberman, J. Tumor suppressor NM23-H1 is a granzyme A-activated DNase during CTL-mediated apoptosis, and the nucleosome assembly protein SET is its inhibitor. Cell 112, 659–672 (2003).This paper describes how granzyme A induces apoptosis by introducing DNA DSBs into the genome of target cells.
Beresford, P. J. et al. Granzyme A activates an endoplasmic reticulum-associated caspase-independent nuclease to induce single-stranded DNA nicks. J. Biol. Chem. 276, 43285–43293 (2001).
Johnson, H., Scorrano, L., Korsmeyer, S. J. & Ley, T. J. Cell death induced by granzyme C. Blood 101, 3093–3101 (2003).
Acknowledgements
The author thanks C. Bassing and F. Alt for helpful discussion and critical reading of the manuscript. This work was supported by a grant from the National Institutes of Health (USA).
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The author declares no competing financial interests.
Glossary
- Chromosomal translocation
-
Transfer of a segment of a chromosome to a different chromosome, often leading to an abnormal fusion gene or overexpression of one of the genes that is adjacent to the breakpoint.
- Cell-cycle-checkpoint proteins
-
Proteins that regulate the progression of the cell cycle at three checkpoints: G1–S (the boundary between gap1 phase and synthesis phase), intra-S phase and G2–M (the boundary between gap 2 phase and mitosis phase).
- Homologous recombination
-
Genetic recombination that occurs between strands of DNA with long stretches of homology.
- Non-homologous end joining
-
A pathway that rejoins DNA strand breaks without relying on there being considerable homology between the strands. The main known pathway uses the Ku end-binding complex and is regulated by DNA-dependent protein kinase. The pathway is often used in mammalian cells to repair strand breaks caused by agents that damage DNA, and some of the same enzymes are used during the strand-joining steps of V(D)J recombination.
- Ataxia-telangiectasia
-
A human autosomal recessive disease that is characterized by growth retardation, cerebellar degeneration, oculocutaneous telangiectasia, endocrine dysfunction, gonadal abnormalities, immunodeficiency, high cancer risk and hypersensitivity to ionizing radiation.
- Meiotic recombination
-
The exchange of DNA strands between homologous chromosomes during meiosis.
- Nijmegen breakage syndrome
-
A human autosomal recessive disease that is characterized by multisystem defects, including microcephaly, growth retardation, immunodeficiency and increased cancer risk.
- Hypomorphic mutation
-
A type of mutation in which either the altered gene product has decreased activity or the wild-type gene product has reduced expression.
- Microhomology-mediated NHEJ
-
An error-prone non-homologous end joining (NHEJ) mechanism that involves pairing of the overhanging ends, gap filling or deletion, and ligation of the ends.
- Endonuclease
-
An enzyme that catalyses the hydrolysis of bonds between nucleotides in a DNA molecule.
- Reverse transcribed
-
Reverse-transcriptase-mediated synthesis of DNA using RNA as the template.
- Adenoviruses
-
Double-stranded DNA viruses that encode ∼30 viral proteins. The viral DNA is replicated and transcribed in the nucleus of host cells.
- E3 ubiquitin ligase
-
An enzyme that is required to attach the molecular tag ubiquitin to proteins. Depending on the position and number of ubiquitin molecules that are attached, ubiquitin can target proteins for degradation by the proteasome, sort them into specific subcellular compartments or modify their biological activity.
- DNA transposition
-
The movement of DNA segments from one part of the genome to another.
- Switch region
-
The DNA sequence that precedes each constant (C) gene segment in the immunoglobulin heavy-chain locus. It is involved in DNA rearrangement during class-switch recombination, leading to the expression of a different class of immunoglobulin.
- RNA-editing enzyme
-
An enzyme that is involved in alteration of the nucleotide sequence of an RNA molecule.
- DNA cytidine deaminase
-
An enzyme that catalyses the deamination of deoxycytidine residues into deoxyuridine residues.
- Ligation-mediated PCR
-
(LM-PCR). A method that involves the ligation of unphosphorylated adaptors to broken DNA, followed by PCR. It can detect DNA double-stranded breaks in defined regions of the genome. It has been used extensively to analyse recombination-activating gene (RAG)-protein-dependent breaks during V(D)J recombination. More recently, it has been used to examine breaks in variable (V) gene segments and switch regions during somatic hypermutation and class-switch recombination, respectively.
- Base-excision-repair pathway
-
A DNA-repair pathway that removes single bases from DNA, such as deoxyuridine residues that arise from deamination of deoxycytidine residues. Repair is initiated by a DNA glycosylase that is specialized for a particular class of damage.
- Mismatch-repair pathway
-
A DNA-repair pathway that recognizes and corrects mismatched base pairs (typically those that arise from errors in chromosomal DNA replication).
- Nucleoside diphosphate kinase
-
A house-keeping enzyme that is required for the synthesis of nucleoside triphosphates other than ATP.
Rights and permissions
About this article
Cite this article
Xu, Y. DNA damage: a trigger of innate immunity but a requirement for adaptive immune homeostasis. Nat Rev Immunol 6, 261–270 (2006). https://doi.org/10.1038/nri1804
Published:
Issue Date:
DOI: https://doi.org/10.1038/nri1804
This article is cited by
-
The Role of p53 Expression in Patients with RAS/BRAF Wild-Type Metastatic Colorectal Cancer Receiving Irinotecan and Cetuximab as Later Line Treatment
Targeted Oncology (2021)
-
State-of-the-art strategies for targeting the DNA damage response in cancer
Nature Reviews Clinical Oncology (2019)
-
DNA damage-induced phosphatase Wip1 in regulation of hematopoiesis, immune system and inflammation
Cell Death Discovery (2017)
-
New perspective on targeting the tumor suppressor p53 pathway in the tumor microenvironment to enhance the efficacy of immunotherapy
Journal for Immunotherapy of Cancer (2015)
-
The gain of function of p53 cancer mutant in promoting mammary tumorigenesis
Oncogene (2013)