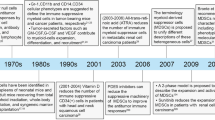
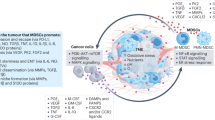
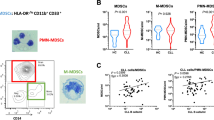
Key Points
-
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells of myeloid origin.
-
MDSCs expand during various pathological conditions, including cancer, inflammation and trauma, and are characterized by the increased production of reactive oxygen and nitrogen species, and by arginase 1 activity.
-
Two subsets of MDSCs have been identified based on their morphology and cell-surface expression of specific molecules. The granulocytic subset has a CD11b+LY6G+LY6Clow phenotype and the monocytic subset has a CD11b+LY6G−LY6Chi phenotype. Although both subsets can suppress T cells, their mechanisms of suppression are different.
-
The expansion of MDSCs is promoted by numerous factors that include prostaglandins, stem-cell factor, macrophage colony-stimulating factor, granulocyte/macrophage colony-stimulating factor, interleukin-6 (IL-6) and vascular endothelial growth factor.
-
The expansion of MDSCs in pathological conditions is associated with their activation. The main factors that cause MDSC activation are interferon-γ, ligands for Toll-like receptors, IL-4, IL-13 and transforming growth factor-β.
-
Immunosuppressive functions of MDSCs require direct cell–cell contact and can result in antigen-specific or antigen-non-specific suppression of T-cell responses.
-
Arginase 1, inducible nitric oxide synthase, reactive oxygen species and peroxynitrite are thought to have a role in MDSC-mediated T-cell suppression.
-
Agents that may be effective for targeting MDSCs for therapy include all-trans retinoic acid, amino-bisphosphonates, inhibitors of cyclooxygenase 2 and phosphodiesterase 5, and some chemotherapeutic drugs.
Abstract
Myeloid-derived suppressor cells (MDSCs) are a heterogeneous population of cells that expand during cancer, inflammation and infection, and that have a remarkable ability to suppress T-cell responses. These cells constitute a unique component of the immune system that regulates immune responses in healthy individuals and in the context of various diseases. In this Review, we discuss the origin, mechanisms of expansion and suppressive functions of MDSCs, as well as the potential to target these cells for therapeutic benefit.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Young, M. R. I., Newby, M. & Wepsic, T. H. Hematopoiesis and suppressor bone marrow cells in mice bearing large metastatic Lewis lung carcinoma tumors. Cancer Res. 47, 100–106 (1987).
Buessow, S. C., Paul, R. D. & Lopez, D. M. Influence of mammary tumor progression on phenotype and function of spleen and in situ lymphocytes in mice. J. Natl Cancer Inst. 73, 249–255 (1984).
Seung, L., Rowley, D., Dubeym, P. & Schreiber, H. Synergy between T-cell immunity and inhibition of paracrine stimulation causes tumor rejection. Proc. Natl Acad. Sci. USA 92, 6254–6258 (1995).
Sinha, P., Clements, V. K., Bunt, S. K., Albelda, S. M. & Ostrand-Rosenberg, S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J. Immunol. 179, 977–983 (2007).
Murdoch, C., Muthana, M., Coffelt, S. B. & Lewis, C. E. The role of myeloid cells in the promotion of tumour angiogenesis. Nature Rev. Cancer 8, 618–631 (2008).
Youn, J. I., Nagaraj, S., Collazo, M. & Gabrilovich, D. I. Subsets of myeloid-derived suppressor cells in tumor-bearing mice. J. Immunol. 181, 5791–5802 (2008).
Bronte, V. et al. Identification of a CD11b+/Gr-1+/CD31+ myeloid progenitor capable of activating or suppressing CD8+ T cells. Blood 96, 3838–3846 (2000).
Kusmartsev, S. & Gabrilovich, D. I. Inhibition of myeloid cell differentiation in cancer: The role of reactive oxygen species. J. Leukoc. Biol. 74, 186–196 (2003).
Li, Q., Pan, P. Y., Gu, P., Xu, D. & Chen, S. H. Role of immature myeloid Gr-1+ cells in the development of antitumor immunity. Cancer Res. 64, 1130–1139 (2004).
Kusmartsev, S., Nefedova, Y., Yoder, D. & Gabrilovich, D. I. Antigen-specific inhibition of CD8+ T cell response by immature myeloid cells in cancer is mediated by reactive oxygen species. J. Immunol. 172, 989–999 (2004).
Ochoa, A. C., Zea, A. H., Hernandez, C. & Rodriguez, P. C. Arginase, prostaglandins, and myeloid-derived suppressor cells in renal cell carcinoma. Clin. Cancer Res. 13, 721s–726s (2007).
Almand, B. et al. Increased production of immature myeloid cells in cancer patients. A mechanism of immunosuppression in cancer. J. Immunol. 166, 678–689 (2001).
Schmielau, J. & Finn, O. J. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of T-cell function in advanced cancer patients. Cancer Res. 61, 4756–4760 (2001).
Hestdal, K. et al. Characterization and regulation of RB6–8C5 antigen expression on murine bone marrow cells. J. Immunol. 147, 22–28 (1991).
Dietlin, T. A. et al. Mycobacteria-induced Gr-1+ subsets from distinct myeloid lineages have opposite effects on T cell expansion. J. Leukoc. Biol. 81, 1205–1212 (2007).
Zhu, B. et al. CD11b+Ly-6Chi suppressive monocytes in experimental autoimmune encephalomyelitis. J. Immunol. 179, 5228–5237 (2007).
Movahedi, K. et al. Identification of discrete tumor-induced myeloid-derived suppressor cell subpopulations with distinct T-cell suppressive activity. Blood 111, 4233–4244 (2008). Together with reference 6, this paper describes functional differences between subsets of MDSCs.
Yang, R. et al. CD80 in immune suppression by mouse ovarian carcinoma-associated Gr-1+CD11b+ myeloid cells. Cancer Res. 66, 6807–6815 (2006).
Huang, B. et al. Gr-1+CD115+ immature myeloid suppressor cells mediate the development of tumor-induced T regulatory cells and T-cell anergy in tumor-bearing host. Cancer Res. 66, 1123–1131 (2006).
Gallina, G. et al. Tumors induce a subset of inflammatory monocytes with immunosuppressive activity on CD8+ T cells. J. Clin. Invest. 116, 2777–2790 (2006).
Mirza, N. et al. All-trans-retinoic acid improves differentiation of myeloid cells and immune response in cancer patients. Cancer Res. 66, 9299–9307 (2006).
Diaz-Montero, C. M. et al. Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol. Immunother. 58, 49–59 (2009).
Goni, O., Alcaide, P. & Fresno, M. Immunosuppression during acute Trypanosoma cruzi infection: involvement of Ly6G (Gr1+)CD11b+ immature myeloid suppressor cells. Int. Immunol. 14, 1125–1134 (2002).
Giordanengo, L. et al. Cruzipain, a major Trypanosoma cruzi antigen, conditions the host immune response in favor of parasite. Eur. J. Immunol. 32, 1003–1011 (2002).
Voisin, M. B., Buzoni-Gatel, D., Bout, D. & Velge-Roussel, F. Both expansion of regulatory GR1+ CD11b+ myeloid cells and anergy of T lymphocytes participate in hyporesponsiveness of the lung-associated immune system during acute toxoplasmosis. Infect. Immun. 72, 5487–5492 (2004).
Delano, M. J. et al. MyD88-dependent expansion of an immature GR-1+CD11b+ population induces T cell suppression and Th2 polarization in sepsis. J. Exp. Med. 204, 1463–1474 (2007). This is the first demonstration of a direct role for TLR signalling in the expansion of MDSCs.
Sunderkotter, C. et al. Subpopulations of mouse blood monocytes differ in maturation stage and inflammatory response. J. Immunol. 172, 4410–4417 (2004).
Terrazas, L. I., Walsh, K. L., Piskorska, D., McGuire, E. & Harn, D. A. Jr. The schistosome oligosaccharide lacto-N-neotetraose expands Gr1+ cells that secrete anti-inflammatory cytokines and inhibit proliferation of naive CD4+ cells: a potential mechanism for immune polarization in helminth infections. J. Immunol. 167, 5294–5303 (2001).
Gomez-Garcia, L. et al. Intact glycans from cestode antigens are involved in innate activation of myeloid suppressor cells. Parasite Immunol. 27, 395–405 (2005).
Brys, L. et al. Reactive oxygen species and 12/15-lipoxygenase contribute to the antiproliferative capacity of alternatively activated myeloid cells elicited during helminth infection. J. Immunol. 174, 6095–6104 (2005).
Mencacci, A. et al. CD80+Gr-1+ myeloid cells inhibit development of antifungal Th1 immunity in mice with candidiasis. J. Immunol. 169, 3180–3190 (2002).
Ezernitchi, A. V. et al. TCR ζ down-regulation under chronic inflammation is mediated by myeloid suppressor cells differentially distributed between various lymphatic organs. J. Immunol. 177, 4763–4772 (2006).
Kerr, E. C., Raveney, B. J., Copland, D. A., Dick, A. D. & Nicholson, L. B. Analysis of retinal cellular infiltrate in experimental autoimmune uveoretinitis reveals multiple regulatory cell populations. J. Autoimmun. 31, 354–361 (2008).
Marhaba, R. et al. The importance of myeloid-derived suppressor cells in the regulation of autoimmune effector cells by a chronic contact eczema. J. Immunol. 179, 5071–5081 (2007).
Haile, L. A. et al. Myeloid-derived suppressor cells in inflammatory bowel disease: a new immunoregulatory pathway. Gastroenterology 135, 871–881 (2008).
Makarenkova, V. P., Bansal, V., Matta, B. M., Perez, L. A. & Ochoa, J. B. CD11b+/Gr-1+ myeloid suppressor cells cause T cell dysfunction after traumatic stress. J. Immunol. 176, 2085–2094 (2006).
Bronte, V. et al. Apoptotic death of CD8+ T lymphocytes after immunization: induction of a suppressive population of Mac-1+/Gr-1+ cells. J. Immunol. 161, 5313–5320 (1998).
Cauley, L., Miller, E., Yen, M. & Swain, S. Superantigen-induced CD4 T cell tolerance mediated by myeloid cells and IFN-γ. J. Immunol. 165, 6056–6066 (2000).
Pan, P. Y. et al. Reversion of immune tolerance in advanced malignancy: modulation of myeloid-derived suppressor cell development by blockade of stem-cell factor function. Blood 111, 219–228 (2008).
Sinha, P., Clements, V. K., Fulton, A. M. & Ostrand-Rosenberg, S. Prostaglandin E2 promotes tumor progression by inducing myeloid-derived suppressor cells. Cancer Res. 67, 4507–4513 (2007).
Serafini, P. et al. High-dose GM-CSF-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. 64, 6337–6343 (2004).
Bunt, S. K. et al. Reduced inflammation in the tumor microenvironment delays the accumulation of myeloid-derived suppressor cells and limits tumor progression. Cancer Res. 67, 10019–10026 (2007).
Gabrilovich, D. et al. Vascular endothelial growth factor inhibits the development of dendritic cells and dramatically affects the differentiation of multiple hematopoietic lineages in vivo. Blood 92, 4150–4166 (1998).
Bromberg, J. Stat proteins and oncogenesis. J. Clin. Invest. 109, 1139–1142 (2002).
Nefedova, Y. et al. Regulation of dendritic cell differentiation and antitumor immune response in cancer by pharmacologic-selective inhibition of the janus-activated kinase 2/signal transducers and activators of transcription 3 pathway. Cancer Res. 65, 9525–9535 (2005).
Nefedova, Y. et al. Hyperactivation of STAT3 is involved in abnormal differentiation of dendritic cells in cancer. J. Immunol. 172, 464–474 (2004).
Kortylewski, M. et al. Inhibiting Stat3 signaling in the hematopoietic system elicits multicomponent antitumor immunity. Nature Med. 11, 1314–1321 (2005).
Foell, D., Wittkowski, H., Vogl, T. & Roth, J. S100 proteins expressed in phagocytes: a novel group of damage-associated molecular pattern molecules. J. Leukoc. Biol. 81, 28–37 (2007).
Cheng, P. et al. Inhibition of dendritic cell differentiation and accumulation of myeloid-derived suppressor cells in cancer is regulated by S100A9 protein. J. Exp. Med. 205, 2235–2249 (2008).
Sinha, P. et al. Proinflammatory s100 proteins regulate the accumulation of myeloid-derived suppressor cells. J. Immunol. 181, 4666–4675 (2008). References 49 and 50 describe a new role for S100 proteins in the regulation of MDSC expansion in cancer.
Turovskaya, O. et al. RAGE, carboxylated glycans and S100A8/A9 play essential roles in colitis-associated carcinogenesis. Carcinogenesis 29, 2035–2043 (2008).
Kusmartsev, S. & Gabrilovich, D.I. STAT1 signaling regulates tumor-associated macrophage-mediated T cell deletion. J. Immunol. 174, 4880–4891 (2005).
Kusmartsev, S., Nagaraj, S. & Gabrilovich, D. I. Tumor-associated CD8+ T cell tolerance induced by bone marrow-derived immature myeloid cells. J. Immunol. 175, 4583–4592 (2005).
Bronte, V. et al. IL-4-induced arginase 1 suppresses alloreactive T cells in tumor-bearing mice. J. Immunol. 170, 270–278 (2003).
Rutschman, R. et al. Cutting Edge: Stat6-dependent substrate depletion regulates nitric oxide production. J. Immunol. 166, 2173–2177 (2001).
Sinha, P., Clements, V. K. & Ostrand-Rosenberg, S. Interleukin-13-regulated M2 macrophages in combination with myeloid suppressor cells block immune surveillance against metastasis. Cancer Res. 65, 11743–11751 (2005).
Terabe, M. et al. Transforming growth factor-β production and myeloid cells are an effector mechanism through which CD1d-restricted T cells block cytotoxic T lymphocyte-mediated tumor immunosurveillance: abrogation prevents tumor recurrence. J. Exp. Med. 198, 1741–1752 (2003).
Serafini, P., Mgebroff, S., Noonan, K. & Borrello, I. Myeloid-derived suppressor cells promote cross-tolerance in B-cell lymphoma by expanding regulatory T cells. Cancer Res. 68, 5439–5449 (2008).
Bronte, V. & Zanovello, P. Regulation of immune responses by L-arginine metabolism. Nature Rev. Immunol. 5, 641–654 (2005).
Rodriguez, P. C. & Ochoa, A. C. Arginine regulation by myeloid derived suppressor cells and tolerance in cancer: mechanisms and therapeutic perspectives. Immunol. Rev. 222, 180–191 (2008).
Rodriguez, P. C. et al. Arginase I in myeloid suppressor cells is induced by COX-2 in lung carcinoma. J. Exp. Med. 202, 931–939 (2005). This study shows that prostaglandin E2 has an important role in MDSC-mediated T-2011 cell suppression and suggests a new therapeutic target for the treatment of cancer.
Rodriguez, P. C. et al. Regulation of T cell receptor CD3ζ chain expression by L-arginine. J. Biol. Chem. 277, 21123–21129 (2002).
Rodriguez, P. C., Quiceno, D. G. & Ochoa, A. C. L-arginine availability regulates T-lymphocyte cell-cycle progression. Blood 109, 1568–1573 (2007).
Bingisser, R., Tilbrook, P., Holt, P. & Kees, U. Macrophage-derived nitric oxide regulates T-cell activation via reversible disruption of the Jak3/STAT5 signaling pathway. J. Immunol. 160, 5729–5734 (1998).
Harari, O. & Liao, J. K. Inhibition of MHC II gene transcription by nitric oxide and antioxidants. Curr. Pharm. Des. 10, 893–898 (2004).
Rivoltini, L. et al. Immunity to cancer: attack and escape in T lymphocyte–tumor cell interaction. Immunol. Rev. 188, 97–113 (2002).
Szuster-Ciesielska, A., Hryciuk-Umer, E., Stepulak, A., Kupisz, K. & Kandefer-Szerszen, M. Reactive oxygen species production by blood neutrophils of patients with laryngeal carcinoma and antioxidative enzyme activity in their blood. Acta Oncol. 43, 252–258 (2004).
Waris, G. & Ahsan, H. Reactive oxygen species: role in the development of cancer and various chronic conditions. J. Carcinog. 5, 14 (2006).
Mantovani, G. et al. Antioxidant agents are effective in inducing lymphocyte progression through cell cycle in advanced cancer patients: assessment of the most important laboratory indexes of cachexia and oxidative stress. J. Mol. Med. 81, 664–673 (2003).
Agostinelli, E. & Seiler, N. Non-irradiation-derived reactive oxygen species (ROS) and cancer: therapeutic implications. Amino Acids 31, 341–355 (2006).
Sauer, H., Wartenberg, M. & Hescheler, J. Reactive oxygen species as intracellular messengers during cell growth and differentiation. Cell. Physiol. Biochem. 11, 173–186 (2001).
Vickers, S. M., MacMillan-Crow, L. A., Green, M., Ellis, C. & Thompson, J. A. Association of increased immunostaining for inducible nitric oxide synthase and nitrotyrosine with fibroblast growth factor transformation in pancreatic cancer. Arch. Surg. 134, 245–251 (1999).
Cobbs, C. S. et al. Inactivation of wild-type p53 protein function by reactive oxygen and nitrogen species in malignant glioma cells. Cancer Res. 63, 8670–8673 (2003).
Bentz, B. G., Haines, G. K. 3rd & Radosevich, J. A. Increased protein nitrosylation in head and neck squamous cell carcinogenesis. Head Neck 22, 64–70 (2000).
Dairou, J., Dupret, J. M. & Rodrigues-Lima, F. Impairment of the activity of the xenobiotic-metabolizing enzymes arylamine N-acetyltransferases 1 and 2 (NAT1/NAT2) by peroxynitrite in mouse skeletal muscle cells. FEBS Lett. 579, 4719–4723 (2005).
Ekmekcioglu, S. et al. Inducible nitric oxide synthase and nitrotyrosine in human metastatic melanoma tumors correlate with poor survival. Clin. Cancer Res. 6, 4768–4775 (2000).
Kinnula, V. L. et al. Ultrastructural and chromosomal studies on manganese superoxide dismutase in malignant mesothelioma. Am. J. Respir. Cell Mol. Biol. 31, 147–153 (2004).
Nakamura, Y. et al. Nitric oxide in breast cancer: induction of vascular endothelial growth factor-C and correlation with metastasis and poor prognosis. Clin. Cancer Res. 12, 1201–1207 (2006).
Bronte, V. et al. Boosting antitumor responses of T lymphocytes infiltrating human prostate cancers. J. Exp. Med. 201, 1257–1268 (2005). This paper shows that peroxynitrite could be involved in T-2011 cell suppression in tumour tissues.
Nagaraj, S. et al. Altered recognition of antigen is a novel mechanism of CD8+ T cell tolerance in cancer. Nature Med. 13, 828–835 (2007). This paper describes the mechanism of MDSC-mediated CD8+T-2011 cell tolerance that involves post-translational modification of the TCR by peroxynitrite.
Dugast, A. S. et al. Myeloid-derived suppressor cells accumulate in kidney allograft tolerance and specifically suppress effector T cell expansion. J. Immunol. 180, 7898–7906 (2008).
Kusmartsev, S., Li, Y. & Chen, S.-H. Gr-1+ myeloid cells derived from tumor-bearing mice inhibit primary T cell activation induced through CD3/CD28 costimulation. J. Immunol. 165, 779–785 (2000).
Watanabe, S. et al. Tumor-induced CD11b+Gr-1+ myeloid cells suppress T cell sensitization in tumor-draining lymph nodes. J. Immunol. 181, 3291–3300 (2008).
Stoll, S., Delon, J., Brotz, T. M. & Germain, R. N. Dynamic imaging of T cell–dendritic cell interactions in lymph nodes. Science 296, 1873–1876 (2002).
Miller, M. J., Safrina, O., Parker, I. & Cahalan, M. D. Imaging the single cell dynamics of CD4+ T cell activation by dendritic cells in lymph nodes. J. Exp. Med. 200, 847–856 (2004).
Gabrilovich, D. I., Velders, M., Sotomayor, E. & Kast, W. M. Mechanism of immune dysfunction in cancer mediated by immature Gr-1+ myeloid cells. J. Immunol. 166, 5398–5406 (2001).
Willimsky, G. et al. Immunogenicity of premalignant lesions is the primary cause of general cytotoxic T lymphocyte unresponsiveness. J. Exp. Med. 205, 1687–1700 (2008).
Monu, N. & Frey, A. B. Suppression of proximal T cell receptor signaling and lytic function in CD8+ tumor-infiltrating T cells. Cancer Res. 67, 11447–11454 (2007).
Fricke, I. et al. Vascular endothelial growth factor-trap overcomes defects in dendritic cell differentiation but does not improve antigen-specific immune responses. Clin. Cancer Res. 13, 4840–4848 (2007).
Antonia, S. J. et al. Combination of p53 cancer vaccine with chemotherapy in patients with extensive stage small cell lung cancer. Clin. Cancer Res. 12, 878–887 (2006).
Mantovani, A., Sozzani, S., Locati, M., Allavena, P. & Sica, A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 23, 549–555 (2002).
Muller, A. J. & Prendergast, G. C. Indoleamine 2, 3-dioxygenase in immune suppression and cancer. Curr. Cancer Drug Targets 7, 31–40 (2007).
Hengesbach, L. & Hoag, K. Physiological concentrations of retinoic acid favor myeloid dendritic cell development over granulocyte development in cultures of bone marrow cells from mice. J. Nutr. 134, 2653–2659 (2004).
Kuwata, T. et al. Vitamin A deficiency in mice causes a systemic expansion of myeloid cells. Blood 95, 3349–3356 (2000).
Walkley, C., Yuan, Y., Chandraratna, R. & McArthur, G. Retinoic acid receptor antagonism in vivo expands the numbers of precursor cells during granulopoiesis. Leukemia 16, 1763–1772 (2002).
Kusmartsev, S. et al. All-trans-retinoic acid eliminates immature myeloid cells from tumor-bearing mice and improves the effect of vaccination. Cancer Res. 63, 4441–4449 (2003).
Nefedova, Y. et al. Mechanism of all-trans retinoic acid effect on tumor-associated myeloid-derived suppressor cells. Cancer Res. 67, 11021–11028 (2007).
Lathers, D., Clark, J., Achille, N. & Young, M. Phase 1B study to improve immune responses in head and neck cancer patients using escalating doses of 25-hydroxyvitamin D3. Cancer Immunol. Immunother. 53, 422–430 (2004).
Fricke, I. et al. Treatment of cancer patients with VEGF-Trap overcomes defects in DC differentiation but is insufficient to improve antigen-specific immune responses. Clin. Cancer Res. 13, 4840–4848 (2007).
Kusmartsev, S. et al. Oxidative stress regulates expression of VEGFR1 in myeloid cells: link to tumor-induced immune suppression in renal cell carcinoma. J. Immunol. 181, 346–353 (2008).
Melani, C., Sangaletti, S., Barazzetta, F. M., Werb, Z. & Colombo, M. P. Amino-biphosphonate-mediated MMP-9 inhibition breaks the tumor-bone marrow axis responsible for myeloid-derived suppressor cell expansion and macrophage infiltration in tumor stroma. Cancer Res. 67, 11438–11446 (2007).
Talmadge, J. E. et al. Chemoprevention by cyclooxygenase-2 inhibition reduces immature myeloid suppressor cell expansion. Int. Immunopharmacol. 7, 140–151 (2007).
Zea, A. H. et al. Arginase-producing myeloid suppressor cells in renal cell carcinoma patients: a mechanism of tumor evasion. Cancer Res. 65, 3044–3048 (2005).
Serafini, P. et al. Phosphodiesterase-5 inhibition augments endogenous antitumor immunity by reducing myeloid-derived suppressor cell function. J. Exp. Med. 203, 2691–2702 (2006).
De Santo, C. et al. Nitroaspirin corrects immune dysfunction in tumor-bearing hosts and promotes tumor eradication by cancer vaccination. Proc. Natl Acad. Sci. USA 102, 4185–4190 (2005).
Suzuki, E., Kapoor, V., Jassar, A. S., Kaiser, L. R. & Albelda, S. M. Gemcitabine selectively eliminates splenic Gr-1+/CD11b+ myeloid suppressor cells in tumor-bearing animals and enhances antitumor immune activity. Clin. Cancer Res. 11, 6713–6721 (2005). References 21 and 104–106 show different therapeutic options of eliminating MDSCs or their activity for the treatment of cancer.
Ko, H. J. et al. A combination of chemoimmunotherapies can efficiently break self-tolerance and induce antitumor immunity in a tolerogenic murine tumor model. Cancer Res. 67, 7477–7486 (2007).
Ishida, T. et al. Dendritic cells transduced with wild type p53 gene elicit potent antitumor immune responses. Clinic. Exper. Immunol. 117, 244–251 (1999).
Ishida, T., Oyama, T., Carbone, D. & Gabrilovich, D. I. Defective function of Langerhans cells in tumor-bearing animals is the result of defective maturation from hematopoietic progenitors. J. Immunol. 161, 4842–4851 (1998).
Shojaei, F. & Ferrara, N. Refractoriness to antivascular endothelial growth factor treatment: role of myeloid cells. Cancer Res. 68, 5501–5504 (2008).
van Cruijsen, H. et al. Defective differentiation of myeloid and plasmacytoid dendritic cells in advanced cancer patients is not normalized by tyrosine kinase inhibition of the vascular endothelial growth factor receptor. Clin. Dev. Immunol. 2007, 17315 (2007).
Shojaei, F. et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nature Biotechnol. 25, 911–920 (2007).
Bronte, V. et al. Unopposed production of granulocyte–macrophage colony-stimulating factor by tumors inhibits CD8+ T cell responses by dysregulating antigen-presenting cellmaturation. J. Immunol. 162, 5728–5737 (1999).
Young, M., Wright, M. & Young, M. Antibodies to colony-stimulating factors block Lewis lung carcinoma cell stimulation of immune-suppressive bone marrow cells. Cancer Immunol. Immunother. 33, 146–152 (1991).
Young, M. R. & Lathers, D. M. Myeloid progenitor cells mediate immune suppression in patients with head and neck cancers. Int. J. Immunopharmacol. 21, 241–252 (1999).
Fu, Y., Watson, G., Jimenez, J., Wang, Y. & Lopez, D. Expansion of immunoregulatory macrophages by granulocyte–macrophage colony-stimulating factor derived from a murine mammary tumor. Cancer Res. 50, 227–234 (1990).
Daud, A. I. et al. Phenotypic and functional analysis of dendritic cells and clinical outcome in patients with high-risk melanoma treated with adjuvant granulocyte macrophage colony-stimulating factor. J. Clin. Oncol. 26, 3235–3241 (2008).
Filipazzi, P. et al. Identification of a new subset of myeloid suppressor cells in peripheral blood of melanoma patients with modulation by a granulocyte–macrophage colony-stimulation factor-based antitumor vaccine. J. Clin. Oncol. 25, 2546–2553 (2007).
Sawanobori, Y. et al. Chemokine-mediated rapid turnover of myeloid-derived suppressor cells in tumor-bearing mice. Blood 111, 5457–5466 (2008).
Menetrier-Caux, C. et al. Inhibition of the differentiation of dendritic cells from CD34+ progenitors by tumor cells: role of interleukin-6 and macrophage-colony-stimulating factor. Blood 92, 4778–4791 (1998).
Birkle, S., Zeng, G., Gao, L., Yu, R. K. & Aubry, J. Role of tumor-associated gangliosides in cancer progression. Biochimie 85, 455–463 (2003).
Shurin, G. V. et al. Neuroblastoma-derived gangliosides inhibit dendritic cell generation and function. Cancer Res. 61, 363–369 (2001).
Biswas, S. K. et al. A distinct and unique transcriptional program expressed by tumor-associated macrophages (defective NF-κB and enhanced IRF-3/STAT1 activation). Blood 107, 2112–2122 (2006).
Mazzoni, A. et al. Myeloid suppressor lines inhibit T cell responses by an NO-dependent mechanism. J. Immunol. 168, 689–695 (2002).
Huang, B. et al. CCL2/CCR2 pathway mediates recruitment of myeloid suppressor cells to cancers. Cancer Lett. 252, 86–92 (2007).
Markiewski, M. M. et al. Modulation of the antitumor immune response by complement. Nature Immunol. 9, 1225–1235 (2008).
Yang, L. et al. Abrogation of TGFβ signaling in mammary carcinomas recruits Gr-1+CD11b+ myeloid cells that promote metastasis. Cancer Cell 13, 23–35 (2008).
Young, M. R. I., Wright, M. A., Matthews, J. P., Malik, I. & Pandit, R. Suppression of T cell proliferation by tumor-induced granulocyte-macrophage progenitor cells producing transforming growth factor-beta and nitric oxide. J. Immunol. 156, 1916–1921 (1996).
Beck, C., Schreiber, K., Schreiber, H. & Rowley, D. A. C-kit+ FcR+ myelocytes are increased in cancer and prevent the proliferation of fully cytolytic T cells in the presence of immune serum. Eur. J. Immunol. 33, 19–28 (2003).
Bunt, S. K., Sinha, P., Clements, V. K., Leips, J. & Ostrand-Rosenberg, S. Inflammation induces myeloid-derived suppressor cells that facilitate tumor progression. J. Immunol. 176, 284–290 (2006).
Song, X. et al. CD11b+/Gr-1+ immature myeloid cells mediate suppression of T cells in mice bearing tumors of IL-1β-secreting cells. J. Immunol. 175, 8200–8208 (2005).
Yang, L. et al. Expansion of myeloid immune suppressor Gr+CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6, 409–421 (2004).
Drevets, D. A. et al. The Ly-6Chigh monocyte subpopulation transports Listeria monocytogenes into the brain during systemic infection of mice. J. Immunol. 172, 4418–4424 (2004).
Acknowledgements
We thank the members of the Gabrilovich laboratory for their contributions. We apologize to those colleagues whose data we could not cite owing to space limitations.
Author information
Authors and Affiliations
Corresponding author
Related links
FURTHER INFORMATION
Glossary
- Sepsis
-
A systemic response to severe infection or tissue damage that leads to a hyperactive and unbalanced network of pro-inflammatory mediators. Vascular permeability, cardiac function and metabolic balance are affected, resulting in tissue necrosis, multi-organ failure and death.
- Complete Freund's adjuvant
-
An oil that contains an emulsifying agent and killed mycobacteria, which increase the immune response to an immunogen. For administration, a water-in-oil emulsion is made with a solution that contains the immunogen of interest.
- Experimental autoimmune encephalomyelitis
-
(EAE). An animal model of the human autoimmune disease multiple sclerosis. EAE is induced in experimental animals by immunization with myelin or peptides that are derived from myelin. The animals develop a paralytic disease with inflammation and demyelination in the brain and spinal cord.
- Myelopoiesis
-
The process of differentiation of common myeloid progenitor cells to polymorphonuclear leukocytes and monocytes.
- Tumour immunosurveillance
-
The process of recognition of tumour antigens and elimination of the tumours by the immune system.
- T-cell anergy
-
A state of T-cell unresponsiveness to stimulation with antigen. It can be induced by stimulation with a large amount of specific antigen in the absence of the engagement of co-stimulatory molecules.
- Regulatory T (TReg) cells
-
A specialized type of CD4+ T cell that can suppress the responses of other immune cells. These cells provide a crucial mechanism for the maintenance of peripheral self tolerance and are characterized by the expression of CD25 and the transcription factor forkhead box P3.
- Tumour-associated macrophage
-
A cell that differentiates from circulating blood monocytes and myeloid-derived suppressor cells that have infiltrated tumours. These cells can have positive or negative effects on tumorigenesis.
Rights and permissions
About this article
Cite this article
Gabrilovich, D., Nagaraj, S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 9, 162–174 (2009). https://doi.org/10.1038/nri2506
Issue Date:
DOI: https://doi.org/10.1038/nri2506
This article is cited by
-
Tumor microenvironment and immune system preservation in early-stage breast cancer: routes for early recurrence after mastectomy and treatment for lobular and ductal forms of disease
BMC Immunology (2024)
-
Involvement of CX3CR1+ cells appearing in the abdominal cavity in the immunosuppressive environment immediately after gastric cancer surgery
World Journal of Surgical Oncology (2024)
-
Unraveling the complexity of STAT3 in cancer: molecular understanding and drug discovery
Journal of Experimental & Clinical Cancer Research (2024)
-
Nasopharyngeal carcinoma: current views on the tumor microenvironment's impact on drug resistance and clinical outcomes
Molecular Cancer (2024)
-
Tumor-derived extracellular vesicles: how they mediate glioma immunosuppression
Molecular Biology Reports (2024)