Key Points
-
The past century has witnessed the evolution of the 'magic bullet' from concept to clinical reality. Therapeutic antibodies have been established as 'standard of care' agents for several human cancers.
-
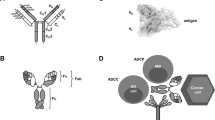
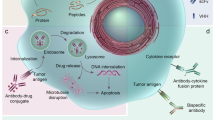
Therapeutic antibodies possess unique and multiple clinically relevant antitumour mechanisms: these include antibody-dependent cellular cytotoxicity, complement-dependent cytotoxicity and the induction of T cell immunity through cross-presentation.
-
Antibodies directed against elements of the tumour microenvironment might synergize with antibodies targeting tumour antigens and provide enhanced therapeutic benefit.
-
Fc receptors for IgG (FcγRs) provide a key link between therapeutic antibodies and the cellular immune system and enable monoclonal antibodies to induce adaptive immune responses. Antibody-induced antitumour adaptive immunity against tumour-specific antigens might already contribute to the patterns of delayed and prolonged antitumour responses seen when antibodies are used alone or in combination with chemotherapy.
-
Monoclonal antibodies can exert synergistic antitumour effects in combination with other immunomodulatory approaches such as chemotherapy, radiotherapy, targeted therapy agents, vaccines or other immunomodulators.
-
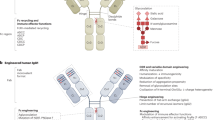
Advances in protein engineering have provided platforms for the development of novel antibody constructs, such as bispecific T cell engager (BiTE) molecules, as well as engineered protein scaffolds, such as designed ankyrin repeat domains (DARPins) and adnectins.
Abstract
Antibodies are important therapeutic agents for cancer. Recently, it has become clear that antibodies possess several clinically relevant mechanisms of action. Many clinically useful antibodies can manipulate tumour-related signalling. In addition, antibodies exhibit various immunomodulatory properties and, by directly activating or inhibiting molecules of the immune system, antibodies can promote the induction of antitumour immune responses. These immunomodulatory properties can form the basis for new cancer treatment strategies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Ehrlich, P. Collected studies on immunity. (New York: J. Wiley & Sons, 1906).
Kohler, G. & Milstein, C. Continuous cultures of fused cells secreting antibody of predefined specificity. Nature 256, 495–497 (1975).
Dunkelberger, J. R. & Song, W. C. Complement and its role in innate and adaptive immune responses. Cell Res. 20, 34–50 (2010).
Zipfel, P. F. & Skerka, C. Complement regulators and inhibitory proteins. Nature Rev. Immunol. 9, 729–740 (2009).
Nimmerjahn, F. & Ravetch, J. V. Fcγ receptors: old friends and new family members. Immunity 24, 19–28 (2006). A comprehensive review of FcγR biology and the importance of FcγR interactions in modulating the immune response.
Roopenian, D. C. & Akilesh, S. FcRn: the neonatal Fc receptor comes of age. Nature Rev. Immunol. 7, 715–725 (2007).
Yeung, Y. A. et al. Engineering human IgG1 affinity to human neonatal Fc receptor: impact of affinity improvement on pharmacokinetics in primates. J. Immunol. 182, 7663–7671 (2009).
Qiao, S. W. et al. Dependence of antibody-mediated presentation of antigen on FcRn. Proc. Natl Acad. Sci. USA 105, 9337–9342 (2008).
Adams, G. P. & Weiner, L. M. Monoclonal antibody therapy of cancer. Nature Biotechnol. 23, 1147–1157 (2005).
Sunada, H., Magun, B. E., Mendelsohn, J. & MacLeod, C. L. Monoclonal antibody against epidermal growth factor receptor is internalized without stimulating receptor phosphorylation. Proc. Natl Acad. Sci. USA 83, 3825–3829 (1986).
Li, S. et al. Structural basis for inhibition of the epidermal growth factor receptor by cetuximab. Cancer Cell 7, 301–311 (2005).
Kim, R. Cetuximab and panitumumab: are they interchangeable? Lancet Oncol. 10, 1140–1141 (2009).
Van Cutsem, E. et al. Cetuximab and chemotherapy as initial treatment for metastatic colorectal cancer. N. Engl. J. Med. 360, 1408–1417 (2009).
Kuenen, B. et al. A phase I pharmacologic study of necitumumab (IMC-11F8), a fully human IgG1 monoclonal antibody directed against EGFR in patients with advanced solid malignancies. Clin. Cancer Res. 16, 1915–1923 (2010).
Li, D. et al. Therapeutic anti-EGFR antibody 806 generates responses in murine de novo EGFR mutant-dependent lung carcinomas. J. Clin. Invest. 117, 346–352 (2007).
Gan, H. K. et al. Targeting a unique EGFR epitope with monoclonal antibody 806 activates NF-κB and initiates tumor vascular normalization. J. Cell. Mol. Med. 13, 3993–4001 (2009).
Scott, A. M. et al. A phase I clinical trial with monoclonal antibody ch806 targeting transitional state and mutant epidermal growth factor receptors. Proc. Natl Acad. Sci. USA 104, 4071–4076 (2007).
Chen, J. S., Lan, K. & Hung., M. C. Strategies to target HER2/neu overexpression for cancer therapy. Drug Resist. Updat. 6, 129–136 (2003).
Vogel, C. L. et al. Efficacy and safety of trastuzumab as a single agent in first-line treatment of HER2-overexpressing metastatic breast cancer. J. Clin. Oncol. 20, 719–726 (2002).
Hudis, C. A. Trastuzumab — mechanism of action and use in clinical practice. N. Engl. J. Med. 357, 39–51 (2007).
Franklin, M. C. et al. Insights into ErbB signaling from the structure of the ErbB2-pertuzumab complex. Cancer Cell 5, 317–328 (2004).
Scheuer, W. et al. Strongly enhanced antitumor activity of trastuzumab and pertuzumab combination treatment on HER2-positive human xenograft tumor models. Cancer Res. 69, 9330–9336 (2009).
Schoeberl, B. et al. Therapeutically targeting ErbB3: a key node in ligand-induced activation of the ErbB receptor-PI3K axis. Sci. Signal. 2, ra31 (2009).
Hollmen, M., Maatta, J. A., Bald, L., Sliwkowski, M. X. & Elenius, K. Suppression of breast cancer cell growth by a monoclonal antibody targeting cleavable ErbB4 isoforms. Oncogene 28, 1309–1319 (2009).
Ellis, L. M. & Hicklin, D. J. VEGF-targeted therapy: mechanisms of anti-tumour activity. Nature Rev. Cancer 8, 579–591 (2008).
Krupitskaya, Y. & Wakelee, H. A. Ramucirumab, a fully human mAb to the transmembrane signaling tyrosine kinase VEGFR-2 for the potential treatment of cancer. Curr. Opin. Investig. Drugs 10, 597–605 (2009).
Mackey, J. et al. TRIO-012: a multicenter, multinational, randomized, double-blind phase III study of IMC-1121B plus docetaxel versus placebo plus docetaxel in previously untreated patients with HER2-negative, unresectable, locally recurrent or metastatic breast cancer. Clin. Breast Cancer 9, 258–261 (2009).
Wu, Y. et al. Anti-vascular endothelial growth factor receptor-1 antagonist antibody as a therapeutic agent for cancer. Clin. Cancer Res. 12, 6573–6584 (2006).
Hirschi, K. K., Rohovsky, S. A. & D'Amore, P. A. PDGF, TGF-β, and heterotypic cell–cell interactions mediate endothelial cell-induced recruitment of 10T1/2 cells and their differentiation to a smooth muscle fate. J. Cell Biol. 141, 805–814 (1998).
Shen, J. et al. Development of a fully human anti-PDGFRβ antibody that suppresses growth of human tumor xenografts and enhances antitumor activity of an anti-VEGFR2 antibody. Neoplasia 11, 594–604 (2009).
Quezada, S. A., Jarvinen, L. Z., Lind, E. F. & Noelle, R. J. CD40/CD154 interactions at the interface of tolerance and immunity. Annu. Rev. Immunol. 22, 307–328 (2004).
Elgueta, R. et al. Molecular mechanism and function of CD40/CD40L engagement in the immune system. Immunol. Rev. 229, 152–172 (2009).
Khalil, M. & Vonderheide, R. H. Anti-CD40 agonist antibodies: preclinical and clinical experience. Update Cancer Ther. 2, 61–65 (2007).
Oflazoglu, E. et al. Macrophages and Fc-receptor interactions contribute to the antitumour activities of the anti-CD40 antibody SGN-40. Br. J. Cancer 100, 113–117 (2009).
Advani, R. et al. Phase I study of the humanized anti-CD40 monoclonal antibody dacetuzumab in refractory or recurrent non-Hodgkin's lymphoma. J. Clin. Oncol. 27, 4371–4377 (2009).
Leach, D. R., Krummel, M. F. & Allison, J. P. Enhancement of antitumor immunity by CTLA-4 blockade. Science 271, 1734–1736 (1996). The first report that CTLA4 blockade can enhance antitumour immunity and promote tumour clearance.
Shrikant, P., Khoruts, A. & Mescher, M. F. CTLA-4 blockade reverses CD8+ T cell tolerance to tumor by a CD4+ T cell- and IL-2-dependent mechanism. Immunity 11, 483–493 (1999).
Peggs, K. S., Quezada, S. A., Chambers, C. A., Korman, A. J. & Allison, J. P. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J. Exp. Med. 206, 1717–1725 (2009).
Sarnaik, A. A. & Weber, J. S. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer J. 15, 169–173 (2009).
Small, E. J. et al. A pilot trial of CTLA-4 blockade with human anti-CTLA-4 in patients with hormone-refractory prostate cancer. Clin. Cancer Res. 13, 1810–1815 (2007).
Weber, J. Ipilimumab: controversies in its development, utility and autoimmune adverse events. Cancer Immunol. Immunother. 58, 823–830 (2009).
Rabinovich, G. A., Gabrilovich, D. & Sotomayor, E. M. Immunosuppressive strategies that are mediated by tumor cells. Annu. Rev. Immunol. 25, 267–296 (2007).
Grutter, C. et al. A cytokine-neutralizing antibody as a structural mimetic of 2 receptor interactions. Proc. Natl Acad. Sci. USA 105, 20251–20256 (2008).
Petrausch, U. et al. Disruption of TGF-β signaling prevents the generation of tumor-sensitized regulatory T cells and facilitates therapeutic antitumor immunity. J. Immunol. 183, 3682–3689 (2009).
Curiel, T. J. et al. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nature Med. 10, 942–949 (2004).
Onizuka, S. et al. Tumor rejection by in vivo administration of anti-CD25 (interleukin-2 receptor α) monoclonal antibody. Cancer Res. 59, 3128–3133 (1999).
Hong, H. et al. Depletion of CD4+CD25+ regulatory T cells enhances natural killer T cell-mediated anti-tumour immunity in a murine mammary breast cancer model. Clin. Exp. Immunol. 159, 93–99 (2009).
Rech, A. J. & Vonderheide, R. H. Clinical use of anti-CD25 antibody daclizumab to enhance immune responses to tumor antigen vaccination by targeting regulatory T cells. Ann. N. Y Acad. Sci. 1174, 99–106 (2009).
Lazar, G. A. et al. Engineered antibody Fc variants with enhanced effector function. Proc. Natl Acad. Sci. USA 103, 4005–4010 (2006).
Kubota, T. et al. Engineered therapeutic antibodies with improved effector functions. Cancer Sci. 100, 1566–1572 (2009).
Clynes, R. A., Towers, T. L., Presta, L. G. & Ravetch, J. V. Inhibitory Fc receptors modulate in vivo cytoxicity against tumor targets. Nature Med. 6, 443–446 (2000). This study shows that Fc receptor-dependent mechanisms contribute substantially to the antitumour effects of antibodies, indicating that an optimal antibody against tumours should bind preferentially to activating Fc receptors and minimally to the inhibitory FcγRIIB.
Cartron, G. et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcγRIIIa gene. Blood 99, 754–758 (2002). The first study to show that responsiveness to antibody therapy is associated with polymorphisms in genes encoding FcγRIII in patients. It strongly implicates the importance of Fc–FcγR dependent interactions, such as ADCC, in the antitumour activity of rituximab.
Weng, W. K. & Levy, R. Two immunoglobulin G. fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 21, 3940–3947 (2003).
Musolino, A. et al. Immunoglobulin G. fragment C receptor polymorphisms and clinical efficacy of trastuzumab-based therapy in patients with HER-2/neu-positive metastatic breast cancer. J. Clin. Oncol. 26, 1789–1796 (2008).
Bibeau, F. et al. Impact of FcγRIIa–FcγRIIIa polymorphisms and KRAS mutations on the clinical outcome of patients with metastatic colorectal cancer treated with cetuximab plus irinotecan. J. Clin. Oncol. 27, 1122–1129 (2009).
Horton, H. M. et al. Potent in vitro and in vivo activity of an Fc-engineered anti-CD19 monoclonal antibody against lymphoma and leukemia. Cancer Res. 68, 8049–8057 (2008).
Zalevsky, J. et al. The impact of Fc engineering on an anti-CD19 antibody: increased Fcγ receptor affinity enhances B-cell clearing in nonhuman primates. Blood 113, 3735–3743 (2009).
Lundin, J. et al. Phase II trial of subcutaneous anti-CD52 monoclonal antibody alemtuzumab (Campath-1H) as first-line treatment for patients with B-cell chronic lymphocytic leukemia (B-CLL). Blood 100, 768–773 (2002).
Di Gaetano, N. et al. Complement activation determines the therapeutic activity of rituximab in vivo. J. Immunol. 171, 1581–1587 (2003).
Cragg, M. S. & Glennie, M. J. Antibody specificity controls in vivo effector mechanisms of anti-CD20 reagents. Blood 103, 2738–2743 (2004).
Racila, E. et al. A polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphoma. Clin. Cancer Res. 14, 6697–6703 (2008).
Coiffier, B. et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1–2 study. Blood 111, 1094–1100 (2008).
Wang, S. Y. et al. Depletion of the C3 component of complement enhances the ability of rituximab-coated target cells to activate human NK cells and improves the efficacy of monoclonal antibody therapy in an in vivo model. Blood 4, 5322–5330 (2009).
Cartron, G., Watier, H., Golay, J. & Solal-Celigny, P. From the bench to the bedside: ways to improve rituximab efficacy. Blood 104, 2635–2642 (2004).
Albert, M. L., Sauter, B. & Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class I-restricted CTLs. Nature 392, 86–89 (1998).
Berard, F. et al. Cross-priming of naive CD8 T cells against melanoma antigens using dendritic cells loaded with killed allogeneic melanoma cells. J. Exp. Med. 192, 1535–1544 (2000).
Hoffmann, T. K., Meidenbauer, N., Dworacki, G., Kanaya, H. & Whiteside, T. L. Generation of tumor-specific T-lymphocytes by cross-priming with human dendritic cells ingesting apoptotic tumor cells. Cancer Res. 60, 3542–3549 (2000).
Dhodapkar, K. M., Krasovsky, J., Williamson, B. & Dhodapkar, M. V. Antitumor monoclonal antibodies enhance cross-presentation of cellular antigens and the generation of myeloma-specific killer T cells by dendritic cells. J. Exp. Med. 195, 125–133 (2002). An important study describing cross-presentation as one potential mechanism of action of cancer-specific monoclonal antibodies.
Dhodapkar, K. M. et al. Selective blockade of inhibitory Fcγ receptor enables human dendritic cell maturation with IL-12p70 production and immunity to antibody-coated tumor cells. Proc. Natl Acad. Sci. USA 102, 2910–2915 (2005).
Haynes, N. M., van der Most, R. G., Lake, R. A. & Smyth, M. J. Immunogenic anti-cancer chemotherapy as an emerging concept. Curr. Opin. Immunol. 20, 545–557 (2008).
Hurwitz, H. et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N. Engl. J. Med. 350, 2335–2342 (2004). An important clinical trial that led to the approval of combined bevacizumab with chemotherapy for the treatment of metastatic colorectal cancer.
Slamon, D. J. et al. Use of chemotherapy plus a monoclonal antibody against HER2 for metastatic breast cancer that overexpresses HER2. N. Engl. J. Med. 344, 783–792 (2001). A Phase III clinical trial that was important for the approval of combined trastuzumab with chemotherapy to treat patients with HER2-overexpressing metastatic breast cancer.
Taylor, C. et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin. Cancer Res. 13, 5133–5143 (2007).
Coiffier, B. et al. CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N. Engl. J. Med. 346, 235–242 (2002).
Forstpointner, R. et al. The addition of rituximab to a combination of fludarabine, cyclophosphamide, mitoxantrone (FCM) significantly increases the response rate and prolongs survival as compared with FCM alone in patients with relapsed and refractory follicular and mantle cell lymphomas: results of a prospective randomized study of the German Low-Grade Lymphoma Study Group. Blood 104, 3064–3071 (2004).
McBride, W. H. et al. A sense of danger from radiation. Radiat. Res. 162, 1–19 (2004).
Bonner, J. A. et al. Radiotherapy plus cetuximab for locoregionally advanced head and neck cancer: 5-year survival data from a phase 3 randomised trial, and relation between cetuximab-induced rash and survival. Lancet Oncol. 11, 21–28 (2009).
Willett, C. G. et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: a multidisciplinary phase II study. J. Clin. Oncol. 27, 3020–3026 (2009).
Gulley, J. L. et al. Combining a recombinant cancer vaccine with standard definitive radiotherapy in patients with localized prostate cancer. Clin. Cancer Res. 11, 3353–3362 (2005).
Roses, R. E., Xu, M., Koski, G. K. & Czerniecki, B. J. Radiation therapy and Toll-like receptor signaling: implications for the treatment of cancer. Oncogene 27, 200–207 (2008).
Khan, K. D. et al. A phase 2 study of rituximab in combination with recombinant interleukin-2 for rituximab-refractory indolent non-Hodgkin's lymphoma. Clin. Cancer Res. 12, 7046–7053 (2006).
Alpaugh, R. K. et al. Phase IB trial for malignant melanoma using R24 monoclonal antibody, interleukin-2/α-interferon. Med. Oncol. 15, 191–198 (1998).
van der Kolk, L. E., Grillo-Lopez, A. J., Baars, J. W. & van Oers, M. H. Treatment of relapsed B-cell non-Hodgkin's lymphoma with a combination of chimeric anti-CD20 monoclonal antibodies (rituximab) and G-CSF: final report on safety and efficacy. Leukemia 17, 1658–1664 (2003).
Gilman, A. L. et al. Phase I study of ch14.18 with granulocyte-macrophage colony-stimulating factor and interleukin-2 in children with neuroblastoma after autologous bone marrow transplantation or stem-cell rescue: a report from the Children's Oncology Group. J. Clin. Oncol. 27, 85–91 (2009).
Huang, X. et al. Combined therapy of local and metastatic 4T1 breast tumor in mice using SU6668, an inhibitor of angiogenic receptor tyrosine kinases, and the immunostimulator B7.2–IgG fusion protein. Cancer Res. 62, 5727–5735 (2002).
Matar, P. et al. Combined epidermal growth factor receptor targeting with the tyrosine kinase inhibitor gefitinib (ZD1839) and the monoclonal antibody cetuximab (IMC-C225): superiority over single-agent receptor targeting. Clin. Cancer Res. 10, 6487–6501 (2004).
Ramalingam, S. et al. Dual inhibition of the epidermal growth factor receptor with cetuximab, an IgG1 monoclonal antibody, and gefitinib, a tyrosine kinase inhibitor, in patients with refractory non-small cell lung cancer (NSCLC): a phase I study. J. Thorac. Oncol. 3, 258–264 (2008).
Scaltriti, M. et al. Lapatinib, a HER2 tyrosine kinase inhibitor, induces stabilization and accumulation of HER2 and potentiates trastuzumab-dependent cell cytotoxicity. Oncogene 28, 803–814 (2009).
Blackwell, K. L. et al. Randomized study of Lapatinib alone or in combination with trastuzumab in women with ErbB2-positive, trastuzumab-refractory metastatic breast cancer. J. Clin. Oncol. 28, 1124–1130 (2010).
van Elsas, A., Hurwitz, A. A. & Allison, J. P. Combination immunotherapy of B16 melanoma using anti-cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) and granulocyte/macrophage colony-stimulating factor (GM-CSF)-producing vaccines induces rejection of subcutaneous and metastatic tumors accompanied by autoimmune depigmentation. J. Exp. Med. 190, 355–366 (1999).
Takaku, S. et al. Blockade of TGF-β enhances tumor vaccine efficacy mediated by CD8+T cells. Int. J. Cancer 126, 1666–1674 (2009).
Terabe, M. et al. Synergistic enhancement of CD8+ T cell-mediated tumor vaccine efficacy by an anti-transforming growth factor-β monoclonal antibody. Clin. Cancer Res. 15, 6560–6569 (2009).
Disis, M. L. et al. Concurrent trastuzumab and HER2/neu-specific vaccination in patients with metastatic breast cancer. J. Clin. Oncol. 27, 4685–4692 (2009).
Wolf, E., Hofmeister, R., Kufer, P., Schlereth, B. & Baeuerle, P. A. BiTEs: bispecific antibody constructs with unique anti-tumor activity. Drug Discov. Today 10, 1237–1244 (2005).
Brischwein, K. et al. MT110: a novel bispecific single-chain antibody construct with high efficacy in eradicating established tumors. Mol. Immunol. 43, 1129–1143 (2006).
Gebauer, M. & Skerra, A. Engineered protein scaffolds as next-generation antibody therapeutics. Curr. Opin. Chem. Biol. 13, 245–255 (2009).
Mamluk, R. et al. Anti-tumor effect of CT-322 as an adnectin inhibitor of vascular endothelial growth factor receptor-2. mAbs 2, 199–208 (2010).
Jahrsdorfer, B. & Weiner, G. J. Immunostimulatory CpG oligodeoxynucleotides and antibody therapy of cancer. Semin. Oncol. 30, 476–482 (2003).
Metelitsa, L. S. et al. Antidisialoganglioside/granulocyte macrophage-colony-stimulating factor fusion protein facilitates neutrophil antibody-dependent cellular cytotoxicity and depends on FcγRII (CD32) and Mac-1 (CD11b/CD18) for enhanced effector cell adhesion and azurophil granule exocytosis. Blood 99, 4166–4173 (2002).
Pages, F. et al. Immune infiltration in human tumors: a prognostic factor that should not be ignored. Oncogene 29, 1093–1102 (2009).
Tassi, E. et al. Non-redundant role for IL-12 and IL-27 in modulating Th2 polarization of carcinoembryonic antigen specific CD4 T cells from pancreatic cancer patients. PLoS ONE 4, e7234 (2009).
Martin-Orozco, N. et al. T helper 17 cells promote cytotoxic T cell activation in tumor immunity. Immunity 31, 787–798 (2009).
Langowski, J. L. et al. IL-23 promotes tumour incidence and growth. Nature 442, 461–465 (2006).
Zou, W. Regulatory T cells, tumour immunity and immunotherapy. Nature Rev. Immunol. 6, 295–307 (2006).
McLaughlin, P. et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 16, 2825–2833 (1998).
Cunningham, D. et al. Cetuximab monotherapy and cetuximab plus irinotecan in irinotecan-refractory metastatic colorectal cancer. N. Engl. J. Med. 351, 337–345 (2004).
Miller, K. et al. Paclitaxel plus bevacizumab versus paclitaxel alone for metastatic breast cancer. N. Engl. J. Med. 357, 2666–2676 (2007).
Sandler, A. et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N. Engl. J. Med. 355, 2542–2550 (2006).
Weiner, L. M. et al. Dose and schedule study of panitumumab monotherapy in patients with advanced solid malignancies. Clin. Cancer Res. 14, 502–508 (2008).
Wierda, W. G. et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J. Clin. Oncol. 1 Mar 2010 (doi:10.1200/JCO.2009.25.3187).
Sievers, E. L. et al. Selective ablation of acute myeloid leukemia using antibody-targeted chemotherapy: a phase I study of an anti-CD33 calicheamicin immunoconjugate. Blood 93, 3678–3684 (1999).
Witzig, T. E. et al. Randomized controlled trial of yttrium-90-labeled ibritumomab tiuxetan radioimmunotherapy versus rituximab immunotherapy for patients with relapsed or refractory low-grade, follicular, or transformed B-cell non-Hodgkin's lymphoma. J. Clin. Oncol. 20, 2453–2463 (2002).
Kaminski, M. S. et al. Radioimmunotherapy with iodine 131I tositumomab for relapsed or refractory B-cell non-Hodgkin lymphoma: updated results and long-term follow-up of the University of Michigan experience. Blood 96, 1259–1266 (2000).
Gough, M. J. et al. OX40 agonist therapy enhances CD8 infiltration and decreases immune suppression in the tumor. Cancer Res. 68, 5206–5215 (2008).
Berger, R. et al. Phase I safety and pharmacokinetic study of CT-011, a humanized antibody interacting with PD-1, in patients with advanced hematologic malignancies. Clin. Cancer Res. 14, 3044–3051 (2008).
Molckovsky, A. & Siu, L. L. First-in-class, first-in-human phase I results of targeted agents: Highlights of the 2008 American Society of Clinical Oncology meeting. J. Hematol. Oncol. 1, 20–29 (2008).
Murillo, O. et al. Therapeutic antitumor efficacy of anti-CD137 agonistic monoclonal antibody in mouse models of myeloma. Clin. Cancer Res. 14, 6895–6906 (2008).
Rudge, G., Barrett, S. P., Scott, B. & van Driel, I. R. Infiltration of a mesothelioma by IFN-γ-producing cells and tumor rejection after depletion of regulatory T cells. J. Immunol. 178, 4089–4096 (2007).
Shahied, L. S. et al. Bispecific minibodies targeting HER2/neu and CD16 exhibit improved tumor lysis when placed in a divalent tumor antigen binding format. J. Biol. Chem. 279, 53907–53914 (2004).
Acknowledgements
L.M.W. and S.W. are supported by the US National Institutes of Health (grants CA51008, CA50633 and CA121033).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Related links
Related links
FURTHER INFORMATION
Glossary
- Monoclonal antibodies
-
Antibodies containing uniform variable regions and thus specific for a single epitope. Originally, monoclonal antibodies were derived from a single B lymphocyte clone. Genetic manipulation now allows genes from multiple sources of B lymphocytes (for example, mouse and human) to be combined.
- Chimeric antibody
-
An antibody encoded by genes from more than one species, usually with antigen-binding regions from mouse genes and constant regions from human genes. The aim of this process is to prevent a mouse-specific antibody response in humans treated with the antibody.
- Humanized antibody
-
A genetically engineered mouse antibody in which the protein sequence has been modified to increase the similarity of the antibody to human antibodies. This is to prevent a mouse-specific antibody response in humans treated with the antibody.
- Human epidermal growth factor receptor 2
-
(HER2; also known as ERBB2). A type I membrane glycoprotein that is a member of the ERBB family of tyrosine kinase receptors. This tumour-associated antigen is overexpressed in 10–40% of breast cancer and other carcinomas.
- Complement-dependent cytotoxicity
-
(CDC). A mechanism of killing cells in which antibody, bound to the target cell surface, fixes complement, which results in assembly of the membrane attack complex that punches holes in the target cell membrane resulting in subsequent cell lysis.
- Immunoreceptor tyrosine-based activation motif
-
(ITAM). A sequence that is present in the cytoplasmic domains of the invariant chains of various cell-surface immune receptors. Following phosphorylation of their tyrosine residues, ITAMs function as docking sites for SRC homology 2 (SH2)-domain-containing tyrosine kinases and adaptor molecules, thereby facilitating intracellular-signalling cascades.
- Immunoreceptor tyrosine-based inhibitory motif
-
(ITIM). A motif that is present in the cytoplasmic domain of several inhibitory receptors. After ligand binding, ITIMs are tyrosine phosphorylated and recruit inhibitory phosphatases.
- Antibody-dependent cell-mediated cytotoxicity
-
(ADCC). A mechanism by which NK cells kill other cells; for example, virus-infected target cells that are coated with antibodies. The Fc portions of the coating antibodies interact with Fc receptors that are expressed by NK cells, thereby initiating signalling cascades that result in the release of cytotoxic granules (containing perforin and granzyme B), which induce apoptosis of the antibody-coated cell.
- Cross-presentation
-
The ability of certain antigen-presenting cells to load peptides that are derived from exogenous antigens onto MHC class I molecules and present these at the cell surface. This property is atypical, as most cells exclusively present peptides derived from endogenous proteins on MHC class I molecules.
- Angiogenesis
-
The process of developing new blood vessels. Angiogenesis is important in the normal development of the embryo and fetus. It is also important for tumour growth.
- FOLFIRI chemotherapy
-
A chemotherapy regimen for the treatment of colorectal cancer, made up of the drug folinic acid (Leucovorin), fluorouracil (5-FU) and irinotecan (Camptosar).
- Heregulin
-
A family of ligands known to bind the HER3 and HER4 receptors, also capable of inducing phosphorylation of HER2.
- Regulatory T (TReg) cell
-
A type of CD4+ T cell that is characterized by its expression of forkhead box P3 (FOXP3) and high levels of CD25. TReg cells can suppress many types of immune responses.
- Tumour-associated antigens
-
Antigens that are expressed by tumour cells. These belong to three main categories: tissue-differentiation antigens (which are also expressed by non-malignant cells), mutated or aberrantly expressed molecules and cancer testis antigens, which are normally expressed only by spermatocytes and occasionally in the placenta.
- Bispecific antibody
-
Antibodies engineered to express two distinct antigen binding sites. They are most often used therapeutically to physically cross-link a tumour cell and an immune effector cell.
- Co-stimulation
-
Receptor-mediated signals required in addition to antigen-receptor engagement to achieve complete lymphocyte activation.
Rights and permissions
About this article
Cite this article
Weiner, L., Surana, R. & Wang, S. Monoclonal antibodies: versatile platforms for cancer immunotherapy. Nat Rev Immunol 10, 317–327 (2010). https://doi.org/10.1038/nri2744
Issue Date:
DOI: https://doi.org/10.1038/nri2744
This article is cited by
-
Radiolabeled chemotherapeutics as a novel strategy for targeted cancer therapy: current insights and future perspectives
Journal of Radioanalytical and Nuclear Chemistry (2024)
-
Review of cancer therapies for the perioperative physician
Perioperative Medicine (2023)
-
Phase I study evaluating the Fc-optimized FLT3 antibody FLYSYN in AML patients with measurable residual disease
Journal of Hematology & Oncology (2023)
-
Polymeric biomaterial-inspired cell surface modulation for the development of novel anticancer therapeutics
Biomaterials Research (2023)
-
Responsive biomaterials: optimizing control of cancer immunotherapy
Nature Reviews Materials (2023)