Key Points
-
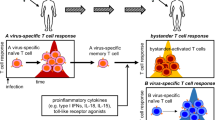
Cytotoxic T lymphocytes (CTLs) and natural killer (NK) cells provide protection against a wide variety of pathogens.
-
Key components of the killing machinery are found in the cytoplasmic granules of CTLs and NK cells.
-
The mechanism of killing involves directed exocytosis of cytolytic effectors, such as perforin and granzymes.
-
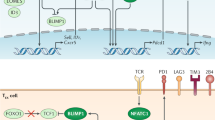
Granzyme B initiates apoptosis through the cleavage and activation of caspases.
-
Granzyme B gains access to the target cell by receptor-mediated endocytosis.
-
Perforin facilitates the uptake and release of granzyme B into the cytoplasm of the target cell.
-
Other substrates for granzyme B indicate that it has an effect on the mitochondrial and caspase-independent pathways to cell death.
-
Other granzymes and cytoplasmic proteins can also act to induce target-cell destruction.
-
Viruses have evolved numerous strategies to inhibit apoptosis and CTL- and NK-cell-mediated killing.
Abstract
Cytotoxic T lymphocytes (CTLs) provide potent defences against virus infection and intracellular pathogens. However, CTLs have a dark side — their lytic machinery can be directed against self-tissues in autoimmune disorders, transplanted cells during graft rejection and host tissues to cause graft-versus-host disease, which is one of the most serious diseases related to CTL function. Although this duplicitous behaviour might seem contradictory, both beneficial and detrimental effects are the result of the same effector proteins. So, an understanding of the mechanisms that are used by CTLs to destroy targets and a knowledge of pathogen immune-evasion strategies will provide vital information for the design of new therapies.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Geiger, B., Rosen, D. & Berke, G. Spatial relationships of microtubule-organizing centers and the contact area of cytotoxic T lymphocytes and target cells. J. Cell Biol. 95, 137–143 (1982).
Kupfer, A. & Dennert, G. Reorientation of the microtubule-organizing center and the Golgi apparatus in cloned cytotoxic lymphocytes triggered by binding to lysable target cells. J. Immunol. 133, 2762–2766 (1984).
Yannelli, J. R., Sullivan, J. A., Mandell, G. L. & Engelhard, V. H. Reorientation and fusion of cytotoxic T-lymphocyte granules after interaction with target cells as determined by high-resolution cinemicrography. J. Immunol. 136, 377–382 (1986).
Zagury, D. Direct analysis of individual killer T cells: susceptibility of target cells to lysis and secretion of hydrolytic enzymes by CTL. Adv. Exp. Med. Biol. 146, 149–169 (1982).
Henkart, P. A. Mechanism of lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 3, 31–58 (1985).An authoritative review of the early work on cell-mediated cytotoxicity.
Helgason, C. D., Prendergast, J. A., Berke, G. & Bleackley, R. C. Peritoneal exudate lymphocyte and mixed lymphocyte culture hybridomas are cytolytic in the absence of cytotoxic cell proteinases and perforin. Eur. J. Immunol. 22, 3187–3190 (1992).
Ostergaard, H. L., Kane, K. P., Mescher, M. F. & Clark, W. R. Cytotoxic T-lymphocyte-mediated lysis without release of serine esterase. Nature 330, 71–72 (1987).
Rouvier, E., Luciani, M. F. & Golstein, P. Fas involvement in Ca2+-independent T-cell-mediated cytotoxicity. J. Exp. Med. 177, 195–200 (1993).
Santamaria, P. Effector lymphocytes in autoimmunity. Curr. Opin. Immunol. 13, 663–669 (2001).
Kagi, D. et al. Reduced incidence and delayed onset of diabetes in perforin-deficient nonobese diabetic mice. J. Exp. Med. 186, 989–997 (1997).
Kreuwel, H. T. et al. Comparing the relative role of perforin/granzyme versus Fas/Fas-ligand cytotoxic pathways in CD8+ T-cell-mediated insulin-dependent diabetes mellitus. J. Immunol. 163, 4335–4341 (1999).
Itoh, N. et al. Requirement of Fas for the development of autoimmune diabetes in nonobese diabetic mice. J. Exp. Med. 186, 613–618 (1997).
Su, X. et al. Significant role for Fas in the pathogenesis of autoimmune diabetes. J. Immunol. 164, 2523–2532 (2000).
Amrani, A. et al. Perforin-independent β-cell destruction by diabetogenic CD8+ T lymphocytes in transgenic nonobese diabetic mice. J. Clin. Invest. 103, 1201–1209 (1999).
Nagata, S. & Golstein, P. The Fas death factor. Science 267, 1449–1456 (1995).A comprehensive summary of the discovery and function of the FAS protein.
Krammer, P. H. CD95's deadly mission in the immune system. Nature 407, 789–795 (2000).
Peter, M. E. & Krammer, P. H. Mechanisms of CD95 (APO-1/Fas)-mediated apoptosis. Curr. Opin. Immunol. 10, 545–551 (1998).
Kagi, D. et al. Cytotoxicity mediated by T cells and natural killer cells is greatly impaired in perforin-deficient mice. Nature 369, 31–37 (1994).
Kojima, H. et al. Two distinct pathways of specific killing revealed by perforin-mutant cytotoxic T lymphocytes. Immunity 1, 357–364 (1994).
Lowin, B., Beermann, F., Schmidt, A. & Tschopp, J. A null mutation in the perforin gene impairs cytolytic T-lymphocyte- and natural-killer-cell-mediated cytotoxicity. Proc. Natl Acad. Sci. USA 91, 11571–11575 (1994).
Kagi, D. et al. Fas and perforin pathways as major mechanisms of T-cell-mediated cytotoxicity. Science 265, 528–530 (1994).
Smyth, M. J. et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192, 755–760 (2000).
van den Broek, M. E. et al. Decreased tumor surveillance in perforin-deficient mice. J. Exp. Med. 184, 1781–1790 (1996).
Dennert, G. & Podack, E. R. Cytolysis by H-2-specific T killer cells. Assembly of tubular complexes on target membranes. J. Exp. Med. 157, 1483–1495 (1983).
Dourmashkin, R. R., Deteix, P., Simone, C. B. & Henkart, P. Electron microscopic demonstration of lesions in target-cell membranes associated with antibody-dependent cellular cytotoxicity. Clin. Exp. Immunol. 42, 554–560 (1980).
Podack, E. R. & Dennert, G. Assembly of two types of tubules with putative cytolytic function by cloned natural killer cells. Nature 302, 442–445 (1983).
Shresta, S., Graubert, T. A., Thomas, D. A., Raptis, S. Z. & Ley, T. J. Granzyme A initiates an alternative pathway for granule-mediated apoptosis. Immunity 10, 595–605 (1999).This study indicates synergy between granzymes A and B in vivo and shows that granzyme-deficient CTLs are less effective at mediating graft-versus-host disease in vivo.
Stinchcombe, J. C., Bossi, G., Booth, S. & Griffiths, G. M. The immunological synapse of CTL contains a secretory domain and membrane bridges. Immunity 15, 751–761 (2001).
Edwards, K. M., Kam, C. M., Powers, J. C. & Trapani, J. A. The human cytotoxic T cell granule serine protease granzyme H has chymotrypsin-like (chymase) activity and is taken up into cytoplasmic vesicles reminiscent of granzyme-B-containing endosomes. J. Biol. Chem. 274, 30468–30473 (1999).
Froelich, C. J. et al. New paradigm for lymphocyte granule-mediated cytotoxicity. Target cells bind and internalize granzyme B, but an endosomolytic agent is necessary for cytosolic delivery and subsequent apoptosis. J. Biol. Chem. 271, 29073–29079 (1996).The first demonstration that granzyme B can bind to a receptor on the surface of target cells.
Jans, D. A. et al. Nuclear targeting of the serine protease granzyme A (fragmentin-1). J. Cell. Sci. 111, 2645–2654 (1998).
Pinkoski, M. J. et al. Entry and trafficking of granzyme B in target cells during granzyme-B–perforin-mediated apoptosis. Blood 92, 1044–1054 (1998).This study describes the uptake of granzyme B into endosomes and its subsequent release by perforin.
Shi, L. et al. Granzyme B (GraB) autonomously crosses the cell membrane and perforin initiates apoptosis and GraB nuclear localization. J. Exp. Med. 185, 855–866 (1997).
Browne, K. A. et al. Cytosolic delivery of granzyme B by bacterial toxins: evidence that endosomal disruption, in addition to transmembrane pore formation, is an important function of perforin. Mol. Cell. Biol. 19, 8604–8615 (1999).The idea that granzyme B gains entry into target cells by a transmembrane perforin channel is challenged by this study.
Motyka, B. et al. Mannose-6-phosphate/insulin-like growth factor II receptor is a death receptor for granzyme B during cytotoxic T-cell-induced apoptosis. Cell 103, 491–500 (2000).This study reveals that the receptor for granzyme B is the mannose-6-phosphate/insulin-like growth factor receptor.
Murphy, M. E. et al. Comparative molecular model building of two serine proteinases from cytotoxic T lymphocytes. Proteins 4, 190–204 (1988).
Harris, J. L., Peterson, E. P., Hudig, D., Thornberry, N. A. & Craik, C. S. Definition and redesign of the extended substrate specificity of granzyme B. J. Biol. Chem. 273, 27364–27373 (1998).
Thornberry, N. A. et al. A combinatorial approach defines specificities of members of the caspase family and granzyme B. Functional relationships established for key mediators of apoptosis. J. Biol. Chem. 272, 17907–17911 (1997).
Estebanez-Perpina, E. et al. Crystal structure of the caspase activator human granzyme B, a proteinase highly specific for an Asp-P1 residue. Biol. Chem. 381, 1203–1214 (2000).
Rotonda, J. et al. The three-dimensional structure of human granzyme B compared to caspase-3, key mediators of cell death with cleavage specificity for aspartic acid in P1. Chem. Biol. 8, 357–368 (2001).
Waugh, S. M., Harris, J. L., Fletterick, R. & Craik, C. S. The structure of the pro-apoptotic protease granzyme B reveals the molecular determinants of its specificity. Nature Struct. Biol. 7, 762–765 (2000).The first three-dimensional structure of granzyme B to be published.
Darmon, A. J., Nicholson, D. W. & Bleackley, R. C. Activation of the apoptotic protease CPP32 by cytotoxic T-cell-derived granzyme B. Nature 377, 446–448 (1995).The discovery that granzyme B activates apoptosis by means of the cleavage and activation of caspases.
Atkinson, E. A. et al. Cytotoxic T-lymphocyte-assisted suicide. Caspase-3 activation is primarily the result of the direct action of granzyme B. J. Biol. Chem. 273, 21261–21266 (1998).
Medema, J. P. et al. Cleavage of FLICE (caspase-8) by granzyme B during cytotoxic T-lymphocyte-induced apoptosis. Eur. J. Immunol. 27, 3492–3498 (1997).
Yang, X. et al. Granzyme B mimics apical caspases. Description of a unified pathway for trans-activation of executioner caspase-3 and -7. J. Biol. Chem. 273, 34278–34283 (1998).
Andrade, F. et al. Granzyme B directly and efficiently cleaves several downstream caspase substrates: implications for CTL-induced apoptosis. Immunity 8, 451–460 (1998).
Browne, K. A., Johnstone, R. W., Jans, D. A. & Trapani, J. A. Filamin (280-kDa actin-binding protein) is a caspase substrate and is also cleaved directly by the cytotoxic T lymphocyte protease granzyme B during apoptosis. J. Biol. Chem. 275, 39262–39266 (2000).
Casciola-Rosen, L., Andrade, F., Ulanet, D., Wong, W. B. & Rosen, A. Cleavage by granzyme B is strongly predictive of autoantigen status: implications for initiation of autoimmunity. J. Exp. Med. 190, 815–826 (1999).
Kroemer, G. & Reed, J. C. Mitochondrial control of cell death. Nature Med. 6, 513–519 (2000).
Heibein, J. A., Barry, M., Motyka, B. & Bleackley, R. C. Granzyme-B-induced loss of mitochondrial inner membrane potential (Delta Psi m) and cytochrome c release are caspase independent. J. Immunol. 163, 4683–4693 (1999).
MacDonald, G., Shi, L., Vande Velde, C., Lieberman, J. & Greenberg, A. H. Mitochondria-dependent and -independent regulation of granzyme-B-induced apoptosis. J. Exp. Med. 189, 131–144 (1999).
Alimonti, J. B., Shi, L., Baijal, P. K. & Greenberg, A. H. Granzyme B induces BID-mediated cytochrome c release and mitochondrial permeability transition. J. Biol. Chem. 276, 6974–6982 (2001).
Barry, M. et al. Granzyme B short-circuits the need for caspase 8 activity during granule-mediated cytotoxic T-lymphocyte killing by directly cleaving Bid. Mol. Cell. Biol. 20, 3781–3794 (2000).A demonstration that granzyme B can proteolytically cleave the pro-apoptotic protein BID in cells.
Heibein, J. A. et al. Granzyme-B-mediated cytochrome c release is regulated by the Bcl-2 family members Bid and Bax. J. Exp. Med. 192, 1391–1402 (2000).
Pinkoski, M. J. et al. Granzyme-B-mediated apoptosis proceeds predominantly through a Bcl-2-inhibitable mitochondrial pathway. J. Biol. Chem. 276, 12060–12067 (2001).
Sutton, V. R. et al. Initiation of apoptosis by granzyme B requires direct cleavage of bid, but not direct granzyme-B-mediated caspase activation. J. Exp. Med. 192, 1403–1414 (2000).This study shows that the cleavage of BID by granzyme B is required for initiation of apoptosis.
Li, H., Zhu, H., Xu, C. J. & Yuan, J. Cleavage of BID by caspase-8 mediates the mitochondrial damage in the Fas pathway of apoptosis. Cell 94, 491–501 (1998).
Wang, G. Q. et al. Resistance to granzyme-B-mediated cytochrome c release in Bak-deficient cells. J. Exp. Med. 194, 1325–1337 (2001).
Martinou, J. C. & Green, D. R. Breaking the mitochondrial barrier. Nature Rev. Mol. Cell Biol. 2, 63–67 (2001).A recent and articulate review that discusses current theories on the leakage of proteins from mitochondria.
Zamzami, N. & Kroemer, G. The mitochondrion in apoptosis: how Pandora's box opens. Nature Rev. Mol. Cell Biol. 2, 67–71 (2001).
Marzo, I. et al. Bax and adenine nucleotide translocator cooperate in the mitochondrial control of apoptosis. Science 281, 2027–2031 (1998).
Narita, M. et al. Bax interacts with the permeability transition pore to induce permeability transition and cytochrome c release in isolated mitochondria. Proc. Natl Acad. Sci. USA 95, 14681–14686 (1998).
Shimizu, S., Narita, M. & Tsujimoto, Y. Bcl-2 family proteins regulate the release of apoptogenic cytochrome c by the mitochondrial channel VDAC. Nature 399, 483–487 (1999).
Thomas, D. A., Scorrano, L., Putcha, G. V., Korsmeyer, S. J. & Ley, T. J. Granzyme B can cause mitochondrial depolarization and cell death in the absence of BID, BAX and BAK. Proc. Natl Acad. Sci. USA 98, 14985–14990 (2001).
Davis, J. E., Sutton, V. R., Smyth, M. J. & Trapani, J. A. Dependence of granzyme-B-mediated cell death on a pathway regulated by Bcl-2 or its viral homolog, BHRF1. Cell. Death Differ. 7, 973–983 (2000).
Sutton, V. R., Vaux, D. L. & Trapani, J. A. Bcl-2 prevents apoptosis induced by perforin and granzyme B, but not that mediated by whole cytotoxic lymphocytes. J. Immunol. 158, 5783–5790 (1997).
Vaux, D. L., Aguila, H. L. & Weissman, I. L. Bcl-2 prevents death of factor-deprived cells but fails to prevent apoptosis in targets of cell-mediated killing. Int. Immunol. 4, 821–824 (1992).
Scaffidi, C. et al. Two CD95 (APO-1/Fas) signaling pathways. EMBO J. 17, 1675–1687 (1998).
Heusel, J. W., Wesselschmidt, R. L., Shresta, S., Russell, J. H. & Ley, T. J. Cytotoxic lymphocytes require granzyme B for the rapid induction of DNA fragmentation and apoptosis in allogeneic target cells. Cell 76, 977–987 (1994).Granzyme-B-deficient cells are defective in rapid DNA fragmentation.
Shresta, S., MacIvor, D. M., Heusel, J. W., Russell, J. H. & Ley, T. J. Natural killer and lymphokine-activated killer cells require granzyme B for the rapid induction of apoptosis in susceptible target cells. Proc. Natl Acad. Sci. USA 92, 5679–5683 (1995).
Enari, M. et al. A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391, 43–50 (1998).
Li, L. Y., Luo, X. & Wang, X. Endonuclease G is an apoptotic DNase when released from mitochondria. Nature 412, 95–99 (2001).
Liu, X., Zou, H., Slaughter, C. & Wang, X. DFF, a heterodimeric protein that functions downstream of caspase-3 to trigger DNA fragmentation during apoptosis. Cell 89, 175–184 (1997).
Sharif-Askari, E. et al. Direct cleavage of the human DNA fragmentation factor-45 by granzyme B induces caspase-activated DNase release and DNA fragmentation. EMBO J. 20, 3101–3113 (2001).
Thomas, D. A., Du, C., Xu, M., Wang, X. & Ley, T. J. DFF45/ICAD can be directly processed by granzyme B during the induction of apoptosis. Immunity 12, 621–632 (2000).
Wolf, B. B., Schuler, M., Echeverri, F. & Green, D. R. Caspase-3 is the primary activator of apoptotic DNA fragmentation via DNA fragmentation factor-45/inhibitor of caspase-activated DNase inactivation. J. Biol. Chem. 274, 30651–30656 (1999).
Bird, C. H. et al. Selective regulation of apoptosis: the cytotoxic lymphocyte serpin proteinase inhibitor 9 protects against granzyme-B-mediated apoptosis without perturbing the Fas cell-death pathway. Mol. Cell. Biol. 18, 6387–6398 (1998).
Medema, J. P. et al. Blockade of the granzyme-B/perforin pathway through overexpression of the serine protease inhibitor PI-9/SPI-6 constitutes a mechanism for immune escape by tumors. Proc. Natl Acad. Sci. USA 98, 11515–11520 (2001).
Medema, J. P. et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: differential modulation by T helper type 1 and type 2 cells. J. Exp. Med. 194, 657–667 (2001).
Ebnet, K. et al. Granzyme-A-deficient mice retain potent cell-mediated cytotoxicity. EMBO J. 14, 4230–4239 (1995).
Shresta, S., Goda, P., Wesselschmidt, R. & Ley, T. J. Residual cytotoxicity and granzyme-K expression in granzyme-A-deficient cytotoxic lymphocytes. J. Biol. Chem. 272, 20236–20244 (1997).
Mullbacher, A. et al. Granzyme A is critical for recovery of mice from infection with the natural cytopathic viral pathogen, ectromelia. Proc. Natl Acad. Sci. USA 93, 5783–5787 (1996).The first report to indicate that granzyme A is necessary for recovery from virus infection by the poxvirus ectromelia.
Pereira, R. A., Simon, M. M. & Simmons, A. Granzyme A, a noncytolytic component of CD8+ cell granules, restricts the spread of herpes simplex virus in the peripheral nervous systems of experimentally infected mice. J. Virol. 74, 1029–1032 (2000).
Simon, M. M. et al. In vitro- and ex vivo-derived cytolytic leukocytes from granzyme A × B double-knockout mice are defective in granule-mediated apoptosis but not lysis of target cells. J. Exp. Med. 186, 1781–1786 (1997).
Beresford, P. J., Xia, Z., Greenberg, A. H. & Lieberman, J. Granzyme A loading induces rapid cytolysis and a novel form of DNA damage independently of caspase activation. Immunity 10, 585–594 (1999).Granzyme A mediates a new form of DNA damage.
Nakajima, H., Park, H. L. & Henkart, P. A. Synergistic roles of granzymes A and B in mediating target-cell death by rat basophilic leukemia mast-cell tumors also expressing cytolysin/perforin. J. Exp. Med. 181, 1037–1046 (1995).
Zhang, D. et al. Induction of rapid histone degradation by the cytotoxic T lymphocyte protease granzyme A. J. Biol. Chem. 276, 3683–3690 (2001).
Zhang, D., Beresford, P. J., Greenberg, A. H. & Lieberman, J. Granzymes A and B directly cleave lamins and disrupt the nuclear lamina during granule-mediated cytolysis. Proc. Natl Acad. Sci. USA 98, 5746–5751 (2001).
Tortorella, D., Gewurz, B. E., Furman, M. H., Schust, D. J. & Ploegh, H. L. Viral subversion of the immune system. Annu. Rev. Immunol. 18, 861–926 (2000).A comprehensive review of viral strategies of immune evasion.
Loenen, W. A., Bruggeman, C. A. & Wiertz, E. J. Immune evasion by human cytomegalovirus: lessons in immunology and cell biology. Semin. Immunol. 13, 41–49 (2001).
Roulston, A., Marcellus, R. C. & Branton, P. E. Viruses and apoptosis. Annu. Rev. Microbiol. 53, 577–628 (1999).
Bump, N. J. et al. Inhibition of ICE family proteases by baculovirus antiapoptotic protein p35. Science 269, 1885–1888 (1995).
Tewari, M. & Dixit, V. M. Fas- and tumor-necrosis-factor-induced apoptosis is inhibited by the poxvirus crmA gene product. J. Biol. Chem. 270, 3255–3260 (1995).
Everett, H. et al. M11L: a novel mitochondria-localized protein of myxoma virus that blocks apoptosis of infected leukocytes. J. Exp. Med. 191, 1487–1498 (2000).
Goldmacher, V. S. et al. A cytomegalovirus-encoded mitochondria-localized inhibitor of apoptosis structurally unrelated to Bcl-2. Proc. Natl Acad. Sci. USA 96, 12536–12541 (1999).The first demonstration of a new mitochondria-localized anti-apoptotic protein encoded by human cytomegalovirus.
Wasilenko, S. T., Meyers, A. F., Vander Helm, K. & Barry, M. Vaccinia-virus infection disarms the mitochondrion-mediated pathway of the apoptotic cascade by modulating the permeability transition pore. J. Virol. 75, 11437–11448 (2001).
Andrade, F. et al. Adenovirus L4-100K assembly protein is a granzyme-B substrate that potently inhibits granzyme-B-mediated cell death. Immunity 14, 751–761 (2001).
Krensky, A. M. Granulysin: a novel antimicrobial peptide of cytolytic T lymphocytes and natural killer cells. Biochem. Pharmacol. 59, 317–320 (2000).
Stenger, S., Rosat, J. P., Bloom, B. R., Krensky, A. M. & Modlin, R. L. Granulysin: a lethal weapon of cytolytic T cells. Immunol. Today 20, 390–394 (1999).This article reviews the role of granulysin in CTL-mediated killing.
Stenger, S. et al. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science 282, 121–125 (1998).
Thoma-Uszynski, S., Stenger, S. & Modlin, R. L. CTL-mediated killing of intracellular Mycobacterium tuberculosis is independent of target-cell nuclear apoptosis. J. Immunol. 165, 5773–5779 (2000).
Ernst, W. A. et al. Granulysin, a T-cell product, kills bacteria by altering membrane permeability. J. Immunol. 165, 7102–7108 (2000).
Gamen, S. et al. Granulysin-induced apoptosis. I. Involvement of at least two distinct pathways. J. Immunol. 161, 1758–1764 (1998).
Kaspar, A. A. et al. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J. Immunol. 167, 350–356 (2001).
Pardo, J. et al. A role of the mitochondrial apoptosis-inducing factor in granulysin-induced apoptosis. J. Immunol. 167, 1222–1229 (2001).
Pena, S. V., Hanson, D. A., Carr, B. A., Goralski, T. J. & Krensky, A. M. Processing, subcellular localization and function of 519 (granulysin), a human late T-cell activation molecule with homology to small, lytic, granule proteins. J. Immunol. 158, 2680–2688 (1997).
Lowin, B., Hahne, M., Mattmann, C. & Tschopp, J. Cytolytic T-cell cytotoxicity is mediated through perforin and Fas lytic pathways. Nature 370, 650–652 (1994).
Pham, C. T. & Ley, T. J. Dipeptidyl peptidase I is required for the processing and activation of granzymes A and B in vivo. Proc. Natl Acad. Sci. USA 96, 8627–8632 (1999).
Metkar, S. S. et al. Cytotoxic cell granule-mediated apoptosis: perforin delivers granzyme-B–serglycin complexes into target cells without plasma membrane pore formation. Immunity 16, 417–428 (2002).
Russell, J. H. & Ley, T. J. Lymphocyte-mediated cytotoxicity. Annu. Rev. Immunol. 20, 323–370 (2002).
Acknowledgements
We dedicate this review to the memory of A. Greenberg: sometimes a collaborator, often a competitor, always a friend. R.C.B. is an Alberta Heritage Foundation for Medical Research Scientist, Canadian Institute Health Research Distinguished Scientist and Howard Hughes International Research Scholar. M.B. is an Alberta Heritage Foundation for Medical Research Scholar.
Author information
Authors and Affiliations
Related links
Glossary
- MICROTUBULE-ORGANIZING CENTRE
-
A region of the cell from which microtubules grow. Motor proteins that are associated with the microtubles are responsible for the directed movement of organelles in the cytoplasm.
- LPR MICE
-
These are naturally occurring mutants that bear a deletion of the Fas gene.
- GLD MICE
-
These mice have a naturally occurring mutation of Fas ligand that causes a generalized lymphoproliferative disease.
- CASPASES
-
A family of cysteine proteinases that are involved in the initiation and effector stages of apoptosis.
- TYPE I/II SYSTEM
-
A classification of cells on the basis of their sensitivity to FAS-mediated killing. Type I cells recruit caspase-8, which results in the subsequent cleavage of caspase-3. Type II cells activate caspase-3 through a mitochondria-dependent step.
- GRAFT-VERSUS-HOST DISEASE
-
The immune reaction against a graft recipient that is mounted by immune-competent cells of a graft.
- IMMUNOLOGICAL SYNAPSE
-
A distinct region formed at the contact zone between the cytotoxic T lymphocyte and target cell due to the specific reorganization of cell-surface membrane proteins.
Rights and permissions
About this article
Cite this article
Barry, M., Bleackley, R. Cytotoxic T lymphocytes: all roads lead to death. Nat Rev Immunol 2, 401–409 (2002). https://doi.org/10.1038/nri819
Issue Date:
DOI: https://doi.org/10.1038/nri819
This article is cited by
-
Immune modulation in malignant pleural effusion: from microenvironment to therapeutic implications
Cancer Cell International (2024)
-
In silico formulation of a next-generation multiepitope vaccine for use as a prophylactic candidate against Crimean-Congo hemorrhagic fever
BMC Medicine (2023)
-
A mitochondrial function-related LncRNA signature predicts prognosis and immune microenvironment for breast cancer
Scientific Reports (2023)
-
A spike-targeting bispecific T cell engager strategy provides dual layer protection against SARS-CoV-2 infection in vivo
Communications Biology (2023)
-
Targeted Therapy of Spinal Cord Injury: Inhibition of Apoptosis Is a Promising Therapeutic Strategy
Molecular Neurobiology (2023)