Key Points
-
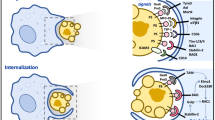
Apoptosis, a programmed and physiological form of cell death, is known to shape the immune system by regulating populations of effector lymphocytes. However, the binding and ingestion of dying cells by monocytes/macrophages and dendritic cells can also influence immune responses markedly by inducing or suppressing inflammation. Therefore, dead cells, which are a reflection of an organism's immediate past, can control its immunological future.
-
Dying cells are recognized by phagocytes as being non-self, altered-self or non-motile self, using innate-immune recognition, scavenger receptors or immunoglobulin-superfamily molecules, respectively.
-
Cell clearance by apoptosis has anti-inflammatory properties, by suppressing the release of pro-inflammatory cytokines by monocytes/macrophages and by the direct release of immunosuppressive cytokines, such as interleukin-10 and transforming growth factor-β1, by apoptotic cells.
-
Dendritic-cell maturation and presentation of antigen are suppressed by the uptake of apoptotic cells, which leads to the promotion of tolerance.
-
Defects in the clearance of apoptotic cells are associated with spontaneous/persistent tissue inflammation and autoimmunity to cell contents.
-
Strategies to promote the safe, anti-inflammatory and immunosuppressive clearance of dying cells are discussed.
-
There is a need to understand the mechanisms that, under certain circumstances, paradoxically allow apoptotic cells to stimulate the release of pro-inflammatory cytokines, such as tumour-necrosis factor, by macrophages and that allow dendritic cells to present antigen derived from apoptotic cells.
Abstract
Apoptosis, which is a programmed and physiological form of cell death, is known to shape the immune system by regulating populations of effector lymphocytes. However, the binding and ingestion of dying cells by monocytes/macrophages and dendritic cells can also influence immune responses markedly by enhancing or suppressing inflammation. Therefore, dead cells, which are a reflection of an organism's immediate past, can control its immunological future.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Kerr, J. F. R., Wyllie, A. H. & Currie, A. R. Apoptosis: a basic biological phenomenon with widespread implications in tissue kinetics. Br. J. Cancer 26, 239–257 (1972). The seminal description of apoptosis.
Krammer, P. H. CD95's deadly mission in the immune system. Nature 407, 789–795 (2000).
Savill, J. & Fadok, V. Corpse clearance defines the meaning of cell death. Nature 407, 784–788 (2000).
Taylor, P. R. et al. A hierarchical role for classical pathway complement proteins in the clearance of apoptotic cells. J. Exp. Med. 192, 359–366 (2000). A crucial demonstration of the links between defective clearance of apoptotic cells and autoimmunity (see also reference 103).
Albert, M. L., Sauter, B. & Bhardwaj, N. Dendritic cells acquire antigen from apoptotic cells and induce class-I-restricted CTLs. Nature 392, 86–89 (1998). The first report of mechanisms by which antigens that are expressed by apoptotic cells can be presented to T cells, thereby accounting for cross-presentation.
Savill, J. S., Dransfield, I., Hogg, N. & Haslett, C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature 343, 170–173 (1990). A description of the first phagocyte receptor for apoptotic cells to be identified, as shown by antibody- and peptide-mediated blockade and the selection of vitronectin-receptor-bearing macrophages.
Fadok, V. A., Bratton, D. L., Henson, P. M. Phagocyte receptors for apoptotic cells: recognition, uptake and consequences. J. Clin. Invest. 108, 957–962 (2001).
Hu, B., Sonstein, J., Christensen, P. J., Punturieri, A. & Curtis, J. L. Deficient in vitro and in vivo phagocytosis of apoptotic T cells by resident murine alveolar macrophages. J. Immunol. 165, 2124–2133 (2000).
Schagat, T. L., Wofford, J. A. & Wright, J. R. Surfactant protein A enhances alveolar macrophage phagocytosis of apoptotic neutrophils. J. Immunol. 166, 2727–2733 (2001).
Mevorach, D., Mascarenhas, J. O., Gershov, D. & Elkon, K. B. Complement-dependent clearance of apoptotic cells by human macrophages. J. Exp. Med. 188, 2313–2320 (1998).
Gaipl, U. S. et al. Complement binding is an early feature of necrotic and a rather late event during apoptotic cell death. Cell Death Differ. 8, 327–334 (2001).
Savill, J. S., Henson, P. M. & Haslett, C. Phagocytosis of aged human neutrophils by macrophages is mediated by a novel 'charge-sensitive' recognition mechanism. J. Clin. Invest. 84, 1518–1527 (1989).
Ren, Y. et al. Non-phlogistic clearance of late apoptotic neutrophils by macrophages: efficient phagocytosis independent of β2-integrins. J. Immunol. 166, 4743–4750 (2001).
Scott, R. S. et al. Phagocytosis and clearance of apoptotic cells is mediated by MER. Nature 411, 207–211 (2001). The first data to indicate a role in the phagocytosis of apoptotic cells for receptor tyrosine kinases that normally keep immune responses in check (see also reference 104).
Hamon, Y. et al. ABC1 promotes engulfment of apoptotic cells and transbilayer redistribution of phosphatidylserine. Nature Cell Biol. 2, 399–406 (2000). An important demonstration of a role for the CED7 homologue ABC1 in altering plasma-membrane lipid distribution in dying cells and phagocytes to promote the clearance of apoptotic cells in vitro and in vivo (see also reference 37).
Pickering, M. C. et al. Ultraviolet radiation-induced keratinocyte apoptosis in C1q-deficient mice. J. Invest. Dermatol. 117, 52–58 (2001).
Ogden, C. A. et al. C1q and mannose-binding lectin engagement of cell-surface calreticulin and CD91 initiates macropinocytosis and uptake of apoptotic cells. J. Exp. Med. 194, 781–795 (2001).
Devitt, A. et al. Human CD14 mediates recognition and phagocytosis of apoptotic cells. Nature 392, 505–509 (1998). A pioneering report implicating recognition mechanisms of innate immunity in the clearance of dying cells.
Savill, J. Apoptosis: phagocytic docking without shocking. Nature 392, 442–443 (1998).
Gershov, D., Kim, S., Brot, N. & Elkon, K. B. C-reactive protein binds to apoptotic cells, protects the cells from assembly of the terminal complement components and sustains an anti-inflammatory innate immune response: implications for systemic autoimmunity. J. Exp. Med. 192, 1353–1364 (2001).
Savill, J. S., Hogg, N., Ren, Y. & Haslett, C. Thrombospondin co-operates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J. Clin. Invest. 90, 1513–1522 (1992).
Ren, Y., Silverstein, R. L., Allen, J. & Savill, J. CD36 gene transfer confers capacity for phagocytosis of cells undergoing apoptosis. J. Exp. Med. 181, 1857–1862 (1995). This study was the first to show the principle of 'gain of phagocytic function'.
Sambrano, G. R. & Steinberg, D. Recognition of oxidatively damaged and apoptotic cells by an oxidized low-density lipoprotein receptor on mouse peritoneal macrophages: role of membrane phosphatidylserine. Proc. Natl Acad. Sci. USA 92, 1396–1400 (1995).
Chang, M. -K. et al. Monoclonal antibodies against oxidized low-density lipoprotein bind to apoptotic cells and inhibit their phagocytosis by elicited macrophages: evidence that oxidation-specific epitopes mediate macrophage recognition. Proc. Natl Acad. Sci. USA 96, 6353–6358 (1999).
Kagan, V. E. et al. A role for oxidative stress in apoptosis: oxidation and externalisation of phosphatidylserine is required for macrophage clearance of cells undergoing Fas-mediated apoptosis. J. Immunol. 169, 487–499 (2002).
Shaw, P. X. et al. Natural antibodies with the T15 idiotype may act in atherosclerosis, apoptotic clearance and protective immunity. J. Clin. Invest. 105, 1731–1740 (2000).
Oka, K. et al. Lectin-like oxidized low-density lipoprotein receptor 1 mediates phagocytosis of aged/apoptotic cells in endothelial cells. Proc. Natl Acad. Sci. USA 95, 9535–9540 (1998).
Platt, N., Suzuki, H., Kurihara, Y., Kodama, T. & Gordon, S. Role for the class A macrophage scavenger receptor in the phagocytosis of apoptotic thymocytes in vitro. Proc. Natl Acad. Sci. USA 93, 12456–12460 (1996).
Platt, N., Suzuki, H., Kodama, T. & Gordon, S. Apoptotic thymocyte clearance in scavenger receptor class A-deficient mice is apparently normal. J. Immunol. 164, 4861–4867 (2000).
Dini, L., Autori, F., Lentini, A., Olivierio, S. & Piacentini, M. The clearance of apoptotic cells in the liver is mediated by the asialoglycoprotein receptor. FEBS Lett. 296, 174–178 (1992).
Duvall, E., Wyllie, A. H. & Morris, R. G. Macrophage recognition of cells undergoing programmed cell death. Immunology 56, 351–358 (1985).
Fadok, V. A., Bratton, D. L., Frasch, S. C., Warner, M. L. & Henson, P. M. The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 5, 557–563 (1998).
Fadok, V. A. et al. Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal of macrophages. J. Immunol. 148, 2207–2216 (1992).
Fadok, V. A. et al. A receptor for phosphatidylserine-specific clearance of apoptotic cells. Nature 405, 85–90 (2000). References 33 and 34 describe the discovery of the first 'eat-me' flag on dying cells.
Verhoven, B., Schlegel, R. A. & Williamson, P. Mechanisms of phosphatidylserine exposure, a phagocyte recognition signal, on apoptotic T lymphocytes. J. Exp. Med. 182, 1597–1601 (1995).
Frasch, S. C. et al. Regulation of phospholipid scramblase activity during apoptosis and cell activation by protein kinase Cδ. J. Biol. Chem. 275, 23065–23073 (2000).
Marguet, D., Luciani, M. F., Moynault, A., Williamson, P. & Chimini, G. Engulfment of apoptotic cells involves the redistribution of membrane phosphatidlyserine on phagocyte and prey. Nature Cell Biol. 1, 454–456 (1999).
Balasubramanian, K., Chandra, J. & Schroit, A. J. Immune clearance of phosphatidylserine-expressing cells by phagocytes. The role of β2-glycoprotein I in macrophage recognition. J. Biol. Chem. 272, 31113–31117 (1997).
Cocca, B. A. et al. Structural basis for autoantibody recognition of phosphatidylserine-β2 glycoprotein 1 and apoptotic cells. Proc. Natl Acad. Sci. 98, 13826–13831 (2001).
Moffatt, O. D., Devitt, A., Bell, E. D., Simmons, D. L. & Gregory, C. D. Macrophage recognition of ICAM-3 on apoptotic leukocytes. J. Immunol. 162, 6800–6810 (1999).
Hughes, J., Liu, Y., Ren, Y. & Savill, J. Human glomerular mesangial cell phagocytosis of apoptotic cells is mediated by a CD36-independent vitronectin receptor/thrombospondin recognition mechanism. J. Immunol. 158, 4389–4397 (1997).
Parnaik, R., Raff, M. C. & Scholes, J. Differences between the clearance of apoptotic cells by professional and non-professional phagocytes. Curr. Biol. 10, 857–860 (2000).
Brown, S. et al. Apoptosis disables CD31-mediated cell detachment from phagocytes promoting binding and engulfment. Nature (in the press). A new insight into discrimination between living and dying cells — apoptosis switches the function of an immunoglobulin-superfamily molecule so that detachment is disabled, which converts a repulsive interaction to an adhesive one.
Knepper-Nicolai, B., Brown, S. B. & Savill, J. Constitutive apoptosis in human neutrophils requires synergy between calpains and the proteasome downstream of caspases. J. Biol. Chem. 273, 30530–30536 (1998).
Chimini, G. Apoptosis: repulsive encounters. Nature 418, 139–142 (2002).
Reddien, P. W., Cameron, S. & Horvitz, H. R. Phagocytosis promotes programmed cell death in C. elegans. Nature 412, 198–202 (2001).
Hoeppner, D. J., Hentgartner, M. O. & Schnabel, R. Engulfment genes cooperate with ced-3 to promote cell death in Caenorhabditis elegans. Nature 412, 202–206 (2001).
Brown, S. B. & Savill, J. Phagocytosis triggers macrophage release of Fas-ligand and induces apoptosis of bystander leucocytes. J. Immunol. 162, 480–485 (1999).
Hanayama, R. et al. Identification of a factor that links apoptotic cells to phagocytes. Nature 417, 182–187 (2002).
Hoffmann, P. R. et al. Phosphatidylserine (PS) induces PS receptor-mediated macropinocytosis and promotes clearance of apoptotic cells. J. Cell Biol. 155, 649–660 (2001). A key study indicating that the phosphatidylserine receptor promotes ingestion of tethered apoptotic cells and fluid through macropinocytosis.
Meagher, L. C., Savill, J. S., Baker, A. & Haslett, C. Phagocytosis of apoptotic neutrophils does not induce macrophage release of thromboxane B2 . J. Leukocyte Biol. 52, 269–273 (1992). The original demonstration of neutral clearance of apoptotic cells without activating macrophages.
Stern, M., Savill, J. & Haslett, C. Human monocyte-derived macrophage phagocytosis of senescent eosinophils undergoing apoptosis: mediation by αvβ3/CD36/thrombospondin recognition mechanism and lack of phlogistic response. Am. J. Pathol. 149, 911–921 (1996).
Wright, S. D. & Silverstein, S. C. Receptors for C3b and C3bi promote phagocytosis but not the release of toxic oxygen from human phagocytes. J. Exp. Med. 158, 2016–2023 (1983).
Marth, T. & Kelsall, B. L. Regulation of interleukin-12 by complement receptor 3 signalling. J. Exp. Med. 185, 1987–1995 (1997).
Voll, R. E., Herrmann, M., Roth, E. A., Stach, C. & Kalden, J. R. Immunosuppressive effects of apoptotic cells. Nature 390, 350–351 (1997). The title of this paper highlights a key discovery in the field of apoptosis.
Newman, S. L., Henson, J. E. & Henson, P. M. Phagocytosis of senescent neutrophils by human monocyte-derived macrophages and rabbit inflammatory macrophages. J. Exp. Med. 156, 430–442 (1982).
Byrne, A. & Reen, D. J. Lipopolysaccharide induces rapid production of IL-10 by monocytes in the presence of apoptotic neutrophils. J. Immunol. 168, 1968–1997 (2002).
Fadok, V. A. et al. Macrophages that have ingested apoptotic cells in vitro inhibit proinflammatory cytokine production through autocrine/paracrine mechanisms involving TGF-β, PGE2 and PAF. J. Clin. Invest. 101, 890–898 (1998). A classical paper defining a role for TGF-β1 in the anti-inflammatory clearance of dying cells.
McDonald, P. P., Fadok, V. A., Bratton, D. & Henson, P. M. Transcriptional and translational regulation of inflammatory mediator production by endogenous TGF-β in macrophages that have ingested apoptotic cells. J. Immunol. 163, 6164–6172 (1999).
Cocco, R. E. & Ucker, D. S. Distinct modes of macrophage recognition for apoptotic and necrotic cells are not specified exclusively by phosphatidylserine exposure. Mol. Biol. Cell 12, 919–930 (2001).
Huynh, M. -L. N., Fadok, V. A. & Henson, P. M. Phosphatidylserine-dependent ingestion of apoptotic cells promoted TGF-β1 secretion and the resolution of inflammation. J. Clin. Invest. 109, 41–50 (2002). The first in vivo demonstration that the clearance of apoptotic cells can suppress inflammatory responses.
Duffield, J. A. et al. Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J. Immunol. 164, 2110–2119 (2000).
Reiter, I., Krammer, B. & Schwamberger, G. Differential effect of apoptotic versus necrotic tumor cells on macrophage antitumor activities. J. Immunol. 163, 1730–1732 (1999).
Duffield, J. S., Ware, C. F., Ryffel, B. & Savill, J. Suppression by apoptotic cells defines tumour necrosis factor-mediated induction of glomerular mesangial cell apoptosis by activated macrophages. Am. J. Pathol. 159, 1397–1404 (2001).
Freire-de-Lima, C. G. et al. Uptake of apoptotic cells drives the growth of a pathogenic trypanosome in macrophages. Nature 403, 199–203 (2000).
Gao, Y., Herndon, J. M., Zhang, H., Griffith, T. S. & Ferguson, T. A. Anti-inflammatory effects of CD95 ligand (FasL)-induced apoptosis. J. Exp. Med. 188, 887–896 (1998).
Chen, W. -J., Frank, M. E., Jin, W. & Wahl, S. M. TGF-β released by apoptotic T cells contributes to an immunosuppressive milieu. Immunity 14, 715–725 (2001).
Lorimore, S. A., Coates, P. J., Scobie, G. E., Milne, G. & Wright, E. G. Inflammatory-type responses after exposure to ionizing radiation in vivo: a mechanism for radiation-induced bystander effects? Oncogene 20, 7085–7095 (2001).
Kurosaka, K., Watanabe, N. & Kobayashi, Y. Production of proinflammatory cytokines by phorbol myristate acetate-treated THP-1 cells and monocyte-derived macrophages after phagocytosis of apoptotic CTLL-2 cells. J. Immunol. 161, 6245–6249 (1998).
Basu, S., Binder, R. J., Ramalingam, T. & Srivastava, P. K. CD91 is a common receptor for heat-shock proteins pg96, hsp70 and calreticulin. Immunity 14, 303–313 (2001).
Miwa, K. et al. Caspase-1-independent IL-1β release and inflammation induced by the apoptosis inducer Fas ligand. Nature Med. 4, 1287–1292 (1998).
Sansonetti, P. J. et al. Caspase-1 activation of IL-1β and IL-8 are essential for Shigella flexneri-induced inflammation. Immunity 12, 581–590 (2000).
Restifo, N. P. Building better vaccines: how apoptotic cell death can induce inflammation and activate innate and adaptive immunity. Curr. Opin. Immunol. 12, 597–603 (2000).
Horino, K. et al. A monocyte chemotactic factor S19 ribosomal protein dimer in phagocytic clearance of apoptotic cells. Lab. Invest. 78, 603–617 (1998).
Rubartelli, A., Foggi, A. & Zocchi, M. K. The selective engulfment of apoptotic bodies by dendritic cells is mediated by the αvβ3 integrin and requires intracellular and extracellular calcium. Eur. J. Immunol. 27, 1893–1900 (1997). The first report of phagocytosis of apoptotic cells by dendritic cells.
Albert, M. L. et al. Immature dendritic cells phagocytose apoptotic cells via αvβ5 and CD36, and cross-present antigens to cytotoxic T lymphocytes. J. Exp. Med. 188, 1359–1368 (1998).
Urban, B. C., Willcox, N. & Roberts, D. J. A role for CD36 in the regulation of dendritic-cell function. Proc. Natl Acad. Sci. USA 98, 8750–8755 (2001).
Stuart, L. M. et al. Inhibitory effects of apoptotic-cell ingestion upon endotoxin-driven myeloid dendritic-cell maturation. J. Immunol. 168, 1627–1635 (2002). References 77 and 78 show the suppressive effects of apoptotic-cell ingestion on immature dendritic cells, which might contribute to cross-tolerization (see also reference 94).
Urban, B. C. et al. Plasmodium falciparum-infected erythrocytes modulate the maturation of dendritic cells. Nature 400, 73–77 (1999).
Bellone, M. et al. Processing of engulfed apoptotic bodies yields T-cell epitopes. J. Immunol. 159, 5391–5399 (1997). The first demonstration that the phagocytosis of apoptotic cells might promote the presentation of antigen to primed T cells.
Rodriguez, A., Regnault, A., Kleijmeer, M., Ricciardi-Castagnoli, P. & Amigorena, S. Selective transport of internalized antigens to the cytosol for MHC class I presentation in dendritic cells. Nature Cell Biol. 1, 362–368 (1999).
Schulz, O., Pennington, D. J., Hodivala-Dilke, K., Febbraio, M. & Reis e Sousa, C. CD36 or αvβ3 and αvβ5 integrins are not essential for MHC class I cross-presentation of cell-associated antigen by CD8α+ murine dendritic cells. J. Immunol. 168, 6057–6065 (2002).
Belz, G. T. et al. CD36 is differentially expressed by CD8+ splenic dendritic cells but is not required for cross-presentation in vivo. J. Immunol. 168, 6066–6070 (2002).
Inaba, K. et al. Efficient presentation of phagocytosed cellular fragments on the major histocompatibility complex class II products of dendritic cells. J. Exp. Med. 188, 2163–2169 (1998).
Casciola-Rosen, L. A., Annhalt, G. J. & Rosen, A. DNA-dependent protein kinase is one of a subset of autoantigens specifically cleaved early during apoptosis. J. Exp. Med. 182, 1625–1634 (1995).
Gallucci, S., Lolkema, M. & Matzinger, P. Natural adjuvants: endogenous activators of dendritic cells. Nature Med. 5, 1249–1255 (1999).
Sauter, B. B. et al. Consequences of cell death: exposure to necrotic tumor cells, but not primary tissue cells or apoptotic cells, induces the maturation of immunostimulatory dendritic cells. J. Exp. Med. 191, 423–433 (2000).
Basu, S., Binder, R. J., Suto, R., Anderson, K. M. & Srivastava, P. K. Necrotic but not apoptotic cell death releases heat-shock proteins which deliver a partial maturation signal to dendritic cells and activate the NF-κB pathway. Int. Immunol. 12, 1539–1546 (2000).
Fadok, V. A., Bratton, D. L., Guthrie, L. & Henson, P. M. Differential effects of apoptotic versus lysed cells on macrophage production of cytokines: role of proteases. J. Immunol. 166, 6847–6854 (2001).
Rovere, P. et al. Bystander apoptosis triggers dendritic-cell maturation and antigen-presenting function. J. Immunol. 161, 4467–4471 (1998).
Steinman, R. M., Turley, S., Mellman, I. & Inaba, K. The induction of tolerance by dendritic cells that have captured apoptotic cells. J. Exp. Med. 191, 411–416 (2000). A fundamental position statement in the field.
Huang, F. -P. et al. A discrete subpopulation of dendritic cells transports apoptotic intestinal epithelial cells to T-cell areas of mesenteric lymph nodes. J. Exp. Med. 191, 435–443 (2000).
Nakamura, K. et al. Unresponsiveness of peripheral T cells induced by apoptotic bodies derived from autologous T cells. Cell. Immunol. 193, 147–154 (1999).
Albert, M. L., Jegathesan, M. & Darnell, R. B. Dendritic cells acquire antigen from apoptotic cells and cross-tolerize antigen-specific CD8+ T cells. Nature Immunol. 2, 1010–1017 (2001). A crucial piece in the puzzle of how dendritic-cell handling of apoptotic cells might regulate immune responses.
Ronchetti, A. et al. Immunogenicity of apoptotic cells in vivo: role of antigen load, antigen-presenting cells and cytokines. J. Immunol. 163, 130–136 (1999).
Magnus, T., Chan, A., Grauer, O., Toyka, T. V. & Gold, R. Microglial phagocytosis of apoptotic inflammatory T cells leads to down-regulation of microglial immune activation. J. Immunol. 167, 5004–5010 (2001).
Mevorach, D., Zhou, J. L., Song, X. & Elkon, K. B. Systemic exposure to irradiated apoptotic cells induces autoantibody production. J. Exp. Med. 188, 387–392 (1998).
Licht, R., Jacobs, C. W. M., Tax, W. J. M. & Berden, J. H. M. No constitutive defect in phagocytosis of apoptotic cells by resident peritoneal macrophages from pre-morbid lupus mice. Lupus 10, 102–107 (2001).
Herrmann, M. et al. Impaired phagocytosis of apoptotic-cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 41, 1241–1250 (1998).
Napirei, M. et al. Features of systemic lupus erythematosus in DNase1-deficient mice. Nature Genet. 25, 177–181 (2000).
Bickerstaff, M. C. et al. Serum amyloid P component controls chromatin degradation and prevents antinuclear autoimmunity. Nature Med. 5, 694–697 (1999).
Familian, A. et al. Chromatin-independent binding of serum amyloid P component to apoptotic cells. J. Immunol. 167, 647–654 (2001).
Botto, M. et al. Homozygous C1q deficiency causes glomerulonephritis associated with multiple apoptotic bodies. Nature Genet. 19, 56–59 (1998).
Lu, Q. & Lemke, G. Homeostatic regulation of the immune system by receptor tyrosine kinases of the Tyro-3 family. Science 293, 306–311 (2001).
Wilkinson, R. et al. Platelet endothelial-cell adhesion molecule-1 (PECAM-1/CD31) acts as a regulator of B-cell development, B-cell antigen receptor (BCR)-mediated activation and autoimmune disease. Blood 100, 184–193 (2002).
Price, B. E. et al. Anti-phospholipid autoantibodies bind to apoptotic, but not viable, thymocytes in a β2-glycoprotein I-independent manner. J. Immunol. 157, 2201–2208 (1996).
Manfredi, A. A. et al. Apoptotic-cell clearance in systemic lupus erythematosus. I. Opsonization by antiphospholipid antibodies. Arthritis Rheum. 41, 205–214 ( 1998).
Miranda-Carus, M. -E. et al. Anti-SSA/Ro and anti-SSB/La autoantibodies bind the surface of apoptotic fetal cardiocytes and promote secretion of TNF-α by macrophages. J. Immunol. 165, 5345–5351 (2000).
Cocca, B. A., Cline, A. M. & Radic, M. Z. Blebs and apoptotic bodies are B-cell autoantigens. J. Immunol. 169, 159–166 (2002).
Rovere, P. et al. Dendritic-cell presentation of antigens from apoptotic cells in a proinflammatory context: role of opsonizing anti-β2-glycoprotein I antibodies. Arthritis Rheum. 42, 1412–1420 (1999).
Harper, L., Ren, Y., Savill, J., Adu, D. & Savage, C. Antineutrophil cytoplasmic antibodies induce reactive oxygen-dependent dysregulation of primed neutrophil apoptosis and clearance by macrophages. Am. J. Pathol. 157, 211–220 (2000).
Reddien, P. W. & Horvitz, H. R. CED-2/Crkll and CED-10/Rac control phagocytosis and cell migration in Caenorhabditis elegans. Nature Cell Biol. 2, 131–135 (2000).
Wu, Y. C. & Horvitz, H. R. C. elegans phagocytosis and cell-migration protein CED-5 is similar to human DOCK180. Nature 392, 501–504 (1998).
Albert, M. L., Kim, J. -I. & Birge, R. B. The αvβ5 integrin recruits the CrkII/Dock180/Rac1 molecular complex for phagocytosis of apoptotic cells. Nature Cell Biol. 2, 899–905 (2000).
Leverrier, Y. & Ridley, A. J. Requirement for Rho GTPases and PI3-kinases during apoptotic-cell phagocytosis by macrophages. Curr. Biol. 11, 195–199 (2000).
Tosello-Trampont, A. -C., Brugnera, E. & Ravichandran, K. S. Evidence for a conserved role for CrkII and Rac in engulfment of apoptotic cells. J. Biol. Chem. 276, 13797–13802 (2000).
Leverrier, Y. et al. Cutting edge: the Wiskott-Aldrich syndrome protein is required for efficient phagocytosis of apoptotic cells. J. Immunol. 166, 4831–4834 (2001).
Caron, E. & Hall, A. Identification of two distinct mechanisms of phagocytosis controlled by different Rho GTPases. Science 282, 1717–1721 (1998).
Hart, S. P., Dougherty, G. J., Haslett, C. & Dransfield, I. CD44 regulates phagocytosis of apoptotic neutrophil granulocytes, but not apoptotic lymphocytes, by human macrophages. J. Immunol. 159, 919–925 (1997).
Meagher, L. C., Cousin, J. M., Seckl, J. R. & Haslett, C. Opposing effects of glucocorticoids on the rate of apoptosis in neutrophilic and eosinophilic granulocytes. J. Immunol. 156, 4422–4428 (1996).
Liu, Y. et al. Glucocorticoids promote non-phlogistic phagocytosis of apoptotic leukocytes. J. Immunol. 162, 3639–3646 (1999).
Giles, K. M. et al. Glucocorticoid augmentation of macrophage capacity for phagocytosis of apoptotic cells is associated with reduced p130Cas expression, loss of paxillin/pyk2 phosphorylation and high levels of active Rac. J. Immunol. 167, 976–986 (2001).
Godson, C. et al. Cutting edge: lipoxins rapidly stimulate nonphlogistic phagocytosis of apoptotic neutrophils by monocyte-derived macrophages. J. Immunol. 164, 1663–1667 (2000).
McMahon, B., Mitchell, S., Brady, H. R. & Godson, C. Lipoxins: revelations on resolution. Trends Pharmacol. Sci. 22, 391–395 (2001).
Mitchell, S. et al. Lipoxins stimulate macrophage phagocytosis of apoptotic neutrophils in acute inflammation in vivo. J. Am. Soc. Nephrol. (in the press).
Acknowledgements
We have received long-term support from the Wellcome Trust and the Medical Research Council. Many colleagues have been instrumental in developing the ideas that are presented here, not least those whose work could not be cited owing to space constraints. C. Gilchrist typed the manuscript.
Author information
Authors and Affiliations
Corresponding author
Related links
Related links
DATABASES
LocusLink
OMIM
Rights and permissions
About this article
Cite this article
Savill, J., Dransfield, I., Gregory, C. et al. A blast from the past: clearance of apoptotic cells regulates immune responses. Nat Rev Immunol 2, 965–975 (2002). https://doi.org/10.1038/nri957
Issue Date:
DOI: https://doi.org/10.1038/nri957
This article is cited by
-
Influence of CK2 protein kinase activity on the interaction between Trypanosoma cruzi and its vertebrate and invertebrate hosts
Parasitology Research (2024)
-
Mechanisms of ammonotelism, epithelium damage, cellular apoptosis, and proliferation in gill of Litopenaeus vannamei under NH4Cl exposure
Environmental Science and Pollution Research (2024)
-
Q fever immunology: the quest for a safe and effective vaccine
npj Vaccines (2023)
-
Mechanism of Cone Degeneration in Retinitis Pigmentosa
Cellular and Molecular Neurobiology (2023)
-
The role of extracellular vesicles in animal reproduction and diseases
Journal of Animal Science and Biotechnology (2022)