Key Points
-
Central to the function of the MVB pathway for trafficking to lysosomes are the endosomal sorting complex required for transport-0 (ESCRT-0), -I, -II and –III complexes, which are represented in all eukaryotic taxa. Ubiquitylation is the best characterised signal for entry into this pathway, and all of the ESCRTs, except for ESCRT-III, recognize ubiquitin.
-
The ESCRT-0 complex recruits ubiquitylated cargo to flat clathrin-coated dynamic microdomains on endosomes and is essential to recruiting ESCRT-I. The endosomal recruitment of ESCRT-0 is mediated by binding to 3-phosphoinosides, which are enriched in early endosome membranes.
-
ESCRT-I is built around a heterotrimeric core to which flexibly connected modules that recognize ubiquitylated cargo and the downstream ESCRT-II complex are connected. This complex is essential for intralumenal vesicle formation and vacuolar stability.
-
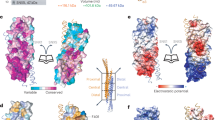
The four-subunit ESCRT-II core complex is built from winged-helix domains. The subunits link directly to the downstream VPS20 subunit of ESCRT-III, whereas the N-terminal GLUE domain of VPS36 recognizes ubiquitin, 3-phosphoinositides and, in yeast, the upstream ESCRT-I.
-
ESCRT-III subunits are represented in all eukaryotic taxa. These subunits heterodimerize via a long N-terminal helical hairpin to form lattices on endosomal membranes that require the AAA+ ATPase VPS4 for subsequent disassembly. The lattice has been proposed to facilitate membrane curvature and the formation of intralumenal vesicles.
-
De-ubiquitinases are recruited by ESCRT-III and can remove ubiquitin from cargo before and after it is committed to entry into intralumenal vesicles.
Abstract
The past two years have seen an explosion in the structural understanding of the endosomal sorting complex required for transport (ESCRT) machinery that facilitates the trafficking of ubiquitylated proteins from endosomes to lysosomes via multivesicular bodies (MVBs). A common organization of all ESCRTs is a rigid core attached to flexibly connected modules that recognize other components of the MVB pathway. Several previously unsuspected key links between multiple ESCRT subunits, phospholipids and ubiquitin have now been elucidated, which, together with the detailed morphological analyses of ESCRT-depletion phenotypes, provide new insights into the mechanism of MVB biogenesis.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$189.00 per year
only $15.75 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Luzio, J. P. et al. Lysosome-endosome fusion and lysosome biogenesis. J. Cell Sci. 113, 1515–1524 (2000).
Welsch, S. et al. Ultrastructural analysis of ESCRT proteins suggests a role for endosome-associated tubular–vesicular membranes in ESCRT function. Traffic 7, 1551–1566 (2006).
Morita, E. & Sundquist, W. I. Retrovirus budding. Annu. Rev. Cell Dev. Biol. 20, 395–425 (2004).
Odorizzi, G. The multiple personalities of Alix. J. Cell Sci. 119, 3025–3032 (2006).
Slagsvold, T., Pattni, K., Malerod, L. & Stenmark, H. Endosomal and non-endosomal functions of ESCRT proteins. Trends Cell Biol. 16, 317–326 (2006).
Penalva, M. A. & Arst, H. N. Jr. Recent advances in the characterization of ambient pH regulation of gene expression in filamentous fungi and yeasts. Annu Rev. Microbiol. 58, 425–451 (2004).
Clague, M. J. Membrane transport: a coat for ubiquitin. Curr. Biol. 12, R529–R531 (2002).
Clague, M. & Urbe, S. Hrs function: viruses provide the clue. Trends Cell Biol. 13, 603–606 (2003).
Bilodeau, P. S., Urbanowski, J. L., Winistorfer, S. C. & Piper, R. C. The Vps27p Hse1p complex binds ubiquitin and mediates endosomal protein sorting. Nature Cell Biol. 4, 534–539 (2002).
Raymond, C. K., Howald-Stevenson, I., Vater, C. A. & Stevens, T. H. Morphological classification of the yeast vacuolar protein sorting mutants: evidence for a prevacuolar compartment in class E vps mutants. Mol. Biol. Cell 3, 1389–1402 (1992).
Rieder, S. E., Banta, L. M., Kohrer, K., McCaffery, J. M. & Emr, S. D. Multilamellar endosome-like compartment accumulates in the yeast vps28 vacuolar protein sorting mutant. Mol. Biol. Cell 7, 985–999 (1996).
Lloyd, T. E. et al. Hrs regulates endosome membrane invagination and tyrosine kinase receptor signaling in Drosophila. Cell 108, 261–269 (2002).
Komada, M. & Soriano, P. Hrs, a FYVE finger protein localized to early endosomes, is implicated in vesicular traffic and required for ventral folding morphogenesis. Genes Dev. 13, 1475–1485 (1999).
Kanazawa, C. et al. Effects of deficiencies of STAMs and Hrs, mammalian class E Vps proteins, on receptor downregulation. Biochem. Biophys. Res. Commun. 309, 848–856 (2003).
Razi, M. & Futter, C. E. Distinct roles for Tsg101 and Hrs in multivesicular body formation and inward vesiculation. Mol. Biol. Cell 17, 3469–3483 (2006).
Hammond, D. E. et al. Endosomal dynamics of Met determine signaling output. Mol. Biol. Cell 14, 1346–1354 (2003).
Bache, K., Raiborg, C., Mehlum, A. & Stenmark, H. STAM and Hrs are subunits of a multivalent ubiquitin-binding complex on early endosomes. J. Biol. Chem. 278, 12513–12521 (2003).
Gillooly, D. J. et al. Localization of phosphatidylinositol 3-phosphate in yeast and mammalian cells. EMBO J. 19, 4577–4588 (2000).
Raiborg, C. et al. FYVE and coiled-coil domains determine the specific localisation of Hrs to early endosomes. J. Cell Sci. 114, 2255–2263 (2001).
Urbe, S., Mills, I. G., Stenmark, H., Kitamura, N. & Clague, M. J. Endosomal localization and receptor dynamics determine tyrosine phosphorylation of hepatocyte growth factor-regulated tyrosine kinase substrate. Mol. Cell. Biol. 20, 7685–7692 (2000).
Raiborg, C. et al. Hrs sorts ubiquitinated proteins into clathrin-coated microdomains of early endosomes. Nature Cell Biol. 4, 394–398 (2002).
Urbe, S. et al. The UIM domain of Hrs couples receptor sorting to vesicle formation. J. Cell Sci. 116, 4169–4179 (2003).
Murk, J. L. et al. Endosomal compartmentalization in three dimensions: implications for membrane fusion. Proc. Natl Acad. Sci. USA 100, 13332–13337 (2003).
Sachse, M., Urbe, S., Oorschot, V., Strous, G. J. & Klumperman, J. Bilayered clathrin coats on endosomal vacuoles are involved in protein sorting toward lysosomes. Mol. Biol. Cell 13, 1313–1328 (2002). References 21 and 24 provide evidence that ubiquitylated receptors are sorted at endosomes via HRS and clathrin, and clathrin in turn restricts the distribution of HRS (reference 26).
McCullough, J. et al. Activation of the endosome-associated ubiquitin isopeptidase AMSH by STAM, a component of the multivesicular body-sorting machinery. Curr. Biol. 16, 160–165 (2006).
Raiborg, C., Wesche, J., Malerod, L. & Stenmark, H. Flat clathrin coats on endosomes mediate degradative protein sorting by scaffolding Hrs in dynamic microdomains. J. Cell Sci. 119, 2414–2424 (2006).
Asao, H. et al. Hrs is associated with STAM, a signal-transducing adaptor molecule. Its suppressive effect on cytokine-induced cell growth. J. Biol. Chem. 272, 32785–32791 (1997).
Takata, H., Kato, M., Denda, K. & Kitamura, N. A Hrs binding protein having a Src homology 3 domain is involved in intracellular degradation of growth factors and their receptors. Genes Cells 5, 57–69 (2000).
Kobayashi, H. et al. Hrs, a mammalian master molecule in vesicular transport and protein sorting, suppresses the degradation of ESCRT proteins signal transducing adaptor molecule 1 and 2. J. Biol. Chem. 280, 10468–10477 (2005).
Mizuno, E., Kawahata, K., Okamoto, A., Kitamura, N. & Komada, M. Association with Hrs is required for the early endosomal localization, stability, and function of STAM. J. Biochem. 135, 385–396 (2004).
Mao, Y. et al. Crystal structure of the VHS and FYVE tandem domains of Hrs, a protein involved in membrane trafficking and signal transduction. Cell 100, 447–456 (2000).
Hanyaloglu, A., McCullagh, E. & von Zastrow, M. Essential role of Hrs in a recycling mechanism mediating functional resensitization of cell signaling. EMBO J. 24, 2265–2283 (2005).
Mizuno, E., Kawahata, K., Kato, M., Kitamura, N. & Komada, M. STAM proteins bind ubiquitinated proteins on the early endosome via the VHS domain and ubiquitin-interacting motif. Mol. Biol. Cell 14, 3675–3689 (2003).
Misra, S. & Hurley, J. H. Crystal structure of a phosphatidylinositol 3-phosphate-specific membrane-targeting motif, the FYVE domain of Vps27p. Cell 97, 657–666 (1999).
Dumas, J. J. et al. Multivalent endosome targeting by homodimeric EEA1. Mol. Cell 8, 947–958 (2001).
Stahelin, R. V. et al. Phosphatidylinositol-3-phosphate induces the membrane penetration of the FYVE domains of Vps27p and Hrs. J. Biol. Chem. 277, 26379–26388 (2002).
Pullan, L. et al. The endosome-associated protein Hrs is hexameric and controls cargo sorting as a “master molecule”. Structure 14, 661–671 (2006).
Lu, Q., Hope, L. W., Brasch, M., Reinhard, C. & Cohen, S. N. TSG101 interaction with HRS mediates endosomal trafficking and receptor down-regulation. Proc. Natl Acad. Sci. USA 100, 7626–7631 (2003).
Bache, K. G., Brech, A., Mehlum, A. & Stenmark, H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 162, 435–442 (2003).
Pornillos, O., Alam, S., Davis, D. & Sundquist, W. Structure of the Tsg101 UEV domain in complex with the PTAP motif of the HIV-1 p6 protein. Nature Struct. Biol. 9, 812–817 (2002).
Pornillos, O. et al. Structure and functional interactions of the Tsg101 UEV domain. EMBO J. 21, 2397–2406 (2002).
Pornillos, O. et al. HIV Gag mimics the Tsg101-recruiting activity of the human Hrs protein. J. Cell Biol. 162, 425–434 (2003).
Katzmann, D., Stefan, C., Babst, M. & Emr, S. Vps27 recruits ESCRT machinery to endosomes during MVB sorting. J. Cell Biol. 162, 413–423 (2003).
Bilodeau, P., Winistorfer, S., Kearney, W., Robertson, A. & Piper, R. Vps27–Hse1 and ESCRT-I complexes cooperate to increase efficiency of sorting ubiquitinated proteins at the endosome. J. Cell Biol. 163, 237–243 (2003).
Katzmann, D., Babst, M. & Emr, S. Ubiquitin-dependent sorting into the multivesicular body pathway requires the function of a conserved endosomal protein sorting complex, ESCRT-I. Cell 106, 145–155 (2001).
Oestreich, A. J., Davies, B. A., Payne, J. A. & Katzmann, D. J. Mvb12 is a novel member of ESCRT-I involved in cargo selection by the MVB pathway. Mol. Biol. Cell 18, 646–657 (2006).
Chu, T., Sun, J., Saksena, S. & Emr, S. D. New component of ESCRT-I regulates endosomal sorting complex assembly. J. Cell Biol. 175, 815–823 (2006).
Curtiss, M., Jones, C. & Babst, M. Efficient cargo sorting by ESCRT-I and the subsequent release of ESCRT-I from MVBs requires the subunit Mvb12. Mol. Biol. Cell 18, 636–645 (2007).
Gill, D. J. et al. Structural insight into the ESCRT-I/-II link and its role in MVB trafficking. EMBO J. 26, 600–612 (2007).
Bache, K. G. et al. The growth-regulatory protein HCRP1/hVps37A is a subunit of mammalian ESCRT-I and mediates receptor downregulation. Mol. Biol. Cell 15, 4337–4346 (2004).
Bishop, N. & Woodman, P. TSG101/mammalian VPS23 and mammalian VPS28 interact directly and are recruited to VPS4-induced endosomes. J. Biol. Chem. 276, 11735–11742 (2001).
Eastman, S. W., Martin-Serrano, J., Chung, W., Zang, T. & Bieniasz, P. D. Identification of human VPS37C, a component of endosomal sorting complex required for transport-I important for viral budding. J. Biol. Chem. 280, 628–636 (2005).
Stuchell, M. D. et al. The human endosomal sorting complex required for transport (ESCRT-I) and its role in HIV-1 budding. J. Biol. Chem. 279, 36059–36071 (2004).
Doyotte, A., Russell, M. R., Hopkins, C. R. & Woodman, P. G. Depletion of TSG101 forms a mammalian “Class E” compartment: a multicisternal early endosome with multiple sorting defects. J. Cell Sci. 118, 3003–3017 (2005). References 15 and 54 show that in mammalian cells ESCRT-I depletion results in extensive tubulation and prevents ILVs formation, whereas HRS depletion produces enlarged, spherical MVBs with few ILVs.
Kostelansky, M. S. et al. Structural and functional organization of the ESCRT-I trafficking complex. Cell 125, 113–126 (2006).
Teo, H. et al. ESCRT-I Core and ESCRT-II GLUE domain structures reveal role for GLUE in linking to ESCRT-I and membranes. Cell 125, 99–111 (2006).
Feng, G. H., Lih, C. J. & Cohen, S. N. TSG101 protein steady-state level is regulated posttranslationally by an evolutionarily conserved COOH-terminal sequence. Cancer Res. 60, 1736–1741 (2000).
Teo, H., Veprintsev, D. B. & Williams, R. L. Structural insights into endosomal sorting complex required for transport (ESCRT-I) recognition of ubiquitinated proteins. J. Biol. Chem. 279, 28689–28696 (2004).
Sundquist, W. I. et al. Ubiquitin recognition by the human TSG101 protein. Mol. Cell 13, 783–789 (2004).
Pineda-Molina, E. et al. The crystal structure of the C-terminal domain of Vps28 reveals a conserved surface required for Vps20 recruitment. Traffic 7, 1007–1016 (2006).
Langelier, C. et al. Human ESCRT-II complex and its role in human immunodeficiency virus type 1 release. J. Virol. 80, 9465–9680 (2006).
Babst, M., Katzmann, D. J., Snyder, W. B., Wendland, B. & Emr, S. D. Endosome-associated complex, ESCRT-II, recruits transport machinery for protein sorting at the multivesicular body. Dev. Cell 3, 283–289 (2002).
Teo, H., Perisic, O., Gonzalez, B. & Williams, R. L. ESCRT-II, an endosome-associated complex required for protein sorting: crystal structure and interactions with ESCRT-III and membranes. Dev. Cell 7, 559–569 (2004).
Hierro, A. et al. Structure of the ESCRT-II endosomal trafficking complex. Nature 431, 221–225 (2004). References 55, 56, 63 and 64 show the structural organization of multisubunit ESCRT-I and -II cores, as well as the structure of the ESCRT-II Ptdins3P-binding GLUE domain, and demonstrate a direct link between yeast ESCRT-I and ESCRT-II.
Wernimont, A. K. & Weissenhorn, W. Crystal structure of subunit VPS25 of the endosomal trafficking complex ESCRT-II. BMC Struct. Biol. 4, 10 (2004).
Yorikawa, C. et al. Human CHMP6, a myristoylated ESCRT-III protein, interacts directly with an ESCRT-II component EAP20 and regulates endosomal cargo sorting. Biochem. J. 387, 17–26 (2005).
Slagsvold, T. et al. Eap45 in mammalian ESCRT-II binds ubiquitin via a phosphoinositide-interacting GLUE domain. J. Biol. Chem. 280, 19600–19606 (2005).
Hirano, S. et al. Structural basis of ubiquitin recognition by mammalian Eap45 GLUE domain. Nature Struct. Mol. Biol. 13, 1031–1032 (2006).
Alam, S. L. et al. Structural basis for ubiquitin recognition by the human ESCRT-II EAP45 GLUE domain. Nature Struct. Mol. Biol. 13, 1029–1030 (2006).
Alam, S. L. et al. Ubiquitin interactions of NZF zinc fingers. EMBO J. 23, 1411–1421 (2004).
Thompson, B. J. et al. Tumor suppressor properties of the ESCRT-II complex component Vps25 in Drosophila. Dev. Cell 9, 711–720 (2005).
Herz, H. M. et al. vps25 mosaics display non-autonomous cell survival and overgrowth, and autonomous apoptosis. Development 133, 1871–1880 (2006).
Vaccari, T. & Bilder, D. The Drosophila tumor suppressor Vps25 prevents nonautonomous overproliferation by regulating notch trafficking. Dev. Cell 9, 687–698 (2005). References 71, 72 and 73 demonstrate an important role for ESCRT-II in metazoan cells.
Moberg, K. H., Schelble, S., Burdick, S. K. & Hariharan, I. K. Mutations in erupted, the Drosophila ortholog of mammalian tumor susceptibility gene 101, elicit non-cell-autonomous overgrowth. Dev. Cell 9, 699–710 (2005).
Bowers, K. et al. Degradation of endocytosed epidermal growth factor and virally ubiquitinated major histocompatibility complex class I is independent of mammalian ESCRTII. J. Biol. Chem. 281, 5094–5105 (2006).
Babst, M., Katzmann, D. J., Estepa-Sabal, E. J., Meerloo, T. & Emr, S. D. ESCRT-III: an endosome-associated heterooligomeric protein complex required for MVB sorting. Dev. Cell 3, 271–282 (2002). References 45, 62 and 76 first defined the functions and compositions of ESCRT-I, -II and -III, respectively.
Amerik, A., Nowak, J., Swaminathan, S. & Hochstrasser, M. The Doa4 deubiquitinating enzyme is functionally linked to the vacuolar protein-sorting and endocytic pathways. Mol. Biol. Cell 11, 3365–3380 (2000). References 77, 111 and 113 demonstrate a requirement for a DUB in the endocytic pathway in yeast.
Kranz, A., Kinner, A. & Kolling, R. A family of small coiled-coil-forming proteins functioning at the late endosome in yeast. Mol. Biol. Cell 12, 711–723 (2001).
Howard, T. L., Stauffer, D. R., Degnin, C. R. & Hollenberg, S. M. CHMP1 functions as a member of a newly defined family of vesicle trafficking proteins. J. Cell Sci. 114, 2395–2404 (2001).
Nickerson, D. P., West, M. & Odorizzi, G. Did2 coordinates Vps4-mediated dissociation of ESCRT-III from endosomes. J. Cell Biol. 175, 715–720 (2006). Reference 80 demonstrates that Did2 has an important role in Vps4-mediated disassembly of ESCRT-III but not of ESCRT-I and-II, that ILV formation can be uncoupled from ESCRT-III disassembly and that class E compartments can have either multilammelar structures without ILVs ( vps4Δ ) or tubular structures with ILVs ( did2Δ).
Horii, M. et al. CHMP7, a novel ESCRT-III-related protein, associates with CHMP4b and functions in the endosomal sorting pathway. Biochem. J. 400, 23–32 (2006).
Muziol, T. et al. Structural basis for budding by the ESCRT-III factor CHMP3. Dev. Cell 10, 821–830 (2006). The structure of an ESCRT-III subunit provides a model for how activated subunits might assemble into dimers and arrays.
Zamborlini, A. et al. Release of autoinhibition converts ESCRT-III components into potent inhibitors of HIV-1 budding. Proc. Natl Acad. Sci. USA 103, 19140–19145 (2006).
Agromayor, M. & Martin-Serrano, J. Interaction of AMSH with ESCRT-III and deubiquitination of endosomal cargo. J. Biol. Chem. 281, 23083–23091 (2006).
McCullough, J., Clague, M. J. & Urbe, S. AMSH is an endosome-associated ubiquitin isopeptidase. J. Cell Biol. 166, 487–492 (2004). Reference 85 shows that AMSH is a Lys63-ubiquitin-chain specific DUB that has a role in sorting a growth-factor receptor at endosomes, and is recruited to CHMPs (shown in references 25, 84 and 88).
Scott, A. et al. Structural and mechanistic studies of VPS4 proteins. EMBO J. 24, 3658–3669 (2005). Structure-based mutagenesis suggests that during their disassembly by VPS4, ESCRT-III subunits are pulled into a central pore of the VPS4 double ring after the initial capture of ESCRT-III subunits by a three-helix bundle of VPS4 MIT domain (shown in reference 95).
von Schwedler, U. K. et al. The protein network of HIV budding. Cell 114, 701–713 (2003). References 87 and 90 were the first to characterize the network of interactions among the human ESCRT components and to show their roles in HIV-1 budding.
Tsang, H. T. et al. A systematic analysis of human CHMP protein interactions: additional MIT domain-containing proteins bind to multiple components of the human ESCRT III complex. Genomics 88, 333–346 (2006).
Bowers, K. et al. Protein–protein interactions of ESCRT complexes in the yeast Saccharomyces cerevisiae. Traffic 5, 194–210 (2004).
Martin-Serrano, J., Yarovoy, A., Perez-Caballero, D. & Bieniasz, P. D. Divergent retroviral late-budding domains recruit vacuolar protein sorting factors by using alternative adaptor proteins. Proc. Natl Acad. Sci. USA 100, 12414–12419 (2003).
Lin, Y., Kimpler, L. A., Naismith, T. V., Lauer, J. M. & Hanson, P. I. Interaction of the mammalian endosomal sorting complex required for transport (ESCRT) III protein hSnf7–1 with itself, membranes, and the AAA+ ATPase SKD1. J. Biol. Chem. 280, 12799–12809 (2005).
Eugster, A. et al. Ent5p is required with Ent3p and Vps27p for ubiquitin-dependent protein sorting into the multivesicular body. Mol. Biol. Cell 15, 3031–3041 (2004).
Whitley, P. et al. Identification of mammalian Vps24p as an effector of phosphatidylinositol-3,5-bisphosphate-dependent endosome compartmentalization. J. Biol. Chem. 278, 38786–38795 (2003).
Kyuuma, M. et al. AMSH, an ESCRT-III associated enzyme, deubiquitinates cargo on MVB/late endosomes. Cell Struct. Funct. 31, 159–172 (2007).
Scott, A. et al. Structure and ESCRT-III protein interactions of the MIT domain of human VPS4A. Proc. Natl Acad. Sci. USA 102, 13813–13818 (2005).
Ward, D. M. et al. The role of LIP5 and CHMP5 in multivesicular body formation and HIV-1 budding in mammalian cells. J. Biol. Chem. 280, 10548–10555 (2005).
Skibinski, G. et al. Mutations in the endosomal ESCRTIII-complex subunit CHMP2B in frontotemporal dementia. Nature Genet. 37, 806–808 (2005).
Bache, K. G. et al. The ESCRT-III subunit hVps24 is required for degradation but not silencing of the epidermal growth factor receptor. Mol. Biol. Cell 17, 2513–2523 (2006).
Shim, J. H. et al. CHMP5 is essential for late endosome function and down-regulation of receptor signaling during mouse embryogenesis. J. Cell Biol. 172, 1045–1056 (2006).
Azmi, I. et al. Recycling of ESCRTs by the AAA-ATPase Vps4 is regulated by a conserved VSL region in Vta1. J. Cell Biol. 172, 705–717 (2006).
Sachse, M., Strous, G. J. & Klumperman, J. ATPase-deficient hVPS4 impairs formation of internal endosomal vesicles and stabilizes bilayered clathrin coats on endosomal vacuoles. J. Cell Sci. 117, 1699–1708 (2004).
Scheuring, S. et al. Mammalian cells express two VPS4 proteins both of which are involved in intracellular protein trafficking. J. Mol. Biol. 312, 469–480 (2001).
Babst, M., Wendland, B., Estepa, E. J. & Emr, S. D. The Vps4p AAA ATPase regulates membrane association of a Vps protein complex required for normal endosome function. EMBO J. 17, 2982–2993 (1998).
Fujita, H. et al. A dominant negative form of the AAA ATPase SKD1/VPS4 impairs membrane trafficking out of endosomal/lysosomal compartments: class E vps phenotype in mammalian cells. J. Cell Sci. 116, 401–414 (2003).
DeLaBarre, B., Christianson, J. C., Kopito, R. R. & Brunger, A. T. Central pore residues mediate the p97/VCP activity required for ERAD. Mol. Cell 22, 451–462 (2006).
Fujita, H. et al. Mammalian class E Vps proteins, SBP1 and mVps2/CHMP2A, interact with and regulate the function of an AAA-ATPase SKD1/Vps4B. J. Cell Sci. 117, 2997–3009 (2004).
Yeo, S. C. et al. Vps20p and Vta1p interact with Vps4p and function in multivesicular body sorting and endosomal transport in Saccharomyces cerevisiae. J. Cell Sci. 116, 3957–3970 (2003).
Shiflett, S. et al. Characterization of Vta1p, a class E Vps protein in Saccharomyces cerevisiae. J. Biol. Chem. 279, 10982–10990 (2004).
Luhtala, N. & Odorizzi, G. Bro1 coordinates deubiquitination in the multivesicular body pathway by recruiting Doa4 to endosomes. J. Cell Biol. 166, 717–729 (2004).
Amerik, A. Y. & Hochstrasser, M. Mechanism and function of deubiquitinating enzymes. Biochim. Biophys. Acta 1695, 189–207 (2004).
Losko, S., Kopp, F., Kranz, A. & Kolling, R. Uptake of the ATP-binding cassette (ABC) transporter Ste6 into the yeast vacuole is blocked in the doa4 mutant. Mol. Biol. Cell 12, 1047–1059 (2001).
Reggiori, F. & Pelham, H. R. B. Sorting of proteins into multivesicular bodies: ubiquitin-dependent and-independent targeting. EMBO J. 20, 5176–5186 (2001). Shows that although ubiquitin functions as a key signal for the sorting of membrane proteins into the MVBs, ubiquitin-independent sorting can also occur.
Dupre, S. & Haguenauer-Tsapis, R. Deubiquitination step in the endocytic pathway of yeast plasma membrane proteins: crucial role of Doa4p ubiquitin isopeptidase. Mol. Cell. Biol. 21, 4482–4494 (2001).
Kim, J. et al. Structural basis for endosomal targeting by the Bro1 domain. Dev. Cell 8, 937–947 (2005).
Avvakumov, G. V. et al. Amino-terminal dimerization, NRDP1-rhodanese interaction, and inhibited catalytic domain conformation of the ubiquitin-specific protease 8 (USP8). J. Biol. Chem. 281, 38061–38070 (2006).
Hu, M. et al. Crystal structure of a UBP-family deubiquitinating enzyme in isolation and in complex with ubiquitin aldehyde. Cell 111, 1041–1054 (2002).
Kato, M., Miyazawa, K. & Kitamura, N. A deubiquitinating enzyme UBPY interacts with the Src homology 3 domain of Hrs-binding protein via a novel binding motif PX(V/I)(D/N)RXXKP. J. Biol. Chem. 275, 37481–37487 (2000).
Kaneko, T. et al. Structural insight into modest binding of a non-PXXP ligand to the signal transducing adaptor molecule-2 Src homology 3 domain. J. Biol. Chem. 278, 48162–48168 (2003).
Ren, J., Kee, Y., Huibregtse, J. M. & Piper, R. C. Hse1, a component of the yeast Hrs-STAM ubiquitin sorting complex, associates with ubiquitin peptidases and a ligase to control sorting efficiency into multivesicular bodies. Mol. Biol. Cell 18, 324–335 (2007).
Row, P. E., Prior, I. A., McCullough, J., Clague, M. J. & Urbe, S. The ubiquitin isopeptidase UBPY regulates endosomal ubiquitin dynamics and is essential for receptor down-regulation. J. Biol. Chem. 281, 12618–12624 (2006).
Mizuno, E., Kobayashi, K., Yamamoto, A., Kitamura, N. & Komada, M. A deubiquitinating enzyme UBPY regulates the level of protein ubiquitination on endosomes. Traffic 7, 1017–1031 (2006). References 120, 121 and 75 report on a key role for the DUB UBPY in receptor downregulation and endosomal ubiquitin dynamics.
Alwan, H. A. & van Leeuwen, J. E. UBPY-mediated epidermal growth factor receptor (EGFR) de-ubiquitination promotes EGFR degradation. J. Biol. Chem. 282, 1658–1669 (2007).
Kee, Y., Munoz, W., Lyon, N. & Huibregtse, J. M. The Ubp2 deubiquitinating enzyme modulates Rsp5-dependent K63-linked polyubiquitin conjugates in Saccharomyces cerevisiae. J. Biol. Chem. 281, 36724–36731 (2006).
Rink, J., Ghigo, E., Kalaidzidis, Y. & Zerial, M. Rab conversion as a mechanism of progression from early to late endosomes. Cell 122, 735–749 (2005).
Rusten, T. E. et al. Fab1 phosphatidylinositol 3-phosphate 5-kinase controls trafficking but not silencing of endocytosed receptors. Mol. Biol. Cell 17, 3989–4001 (2006).
White, I. J., Bailey, L. M., Aghakhani, M. R., Moss, S. E. & Futter, C. E. EGF stimulates annexin 1-dependent inward vesiculation in a multivesicular endosome subpopulation. EMBO J. 25, 1–12 (2006).
Field, M. C., Gabernet-Castello, C. & Dacks, J. B. in Origins and Evolution of Eukaryotic Endomembranes and Cytoskeleton (ed. Jékely, G.) (Landes Bioscience, Austin 2007).
Slater, R. & Bishop, N. E. Genetic structure and evolution of the Vps25 family, a yeast ESCRT-II component. BMC Evol. Biol. 6, 59 (2006).
Yang, M. et al. The Plasmodium falciparum Vps4 homolog mediates multivesicular body formation. J. Cell Sci. 117, 3831–3838 (2004).
Fisher, R. D. et al. Structure and ubiquitin binding of the ubiquitin-interacting motif. J. Biol. Chem. 278, 28976–28984 (2003).
Madshus, I. H. Ubiquitin binding in endocytosis — how tight should it be and where does it happen? Traffic 7, 258–261 (2006).
Hirano, S. et al. Double-sided ubiquitin binding of Hrs-UIM in endosomal protein sorting. Nature Struct. Mol. Biol. 13, 272–277 (2006).
Swanson, K. A., Kang, R. S., Stamenova, S. D., Hicke, L. & Radhakrishnan, I. Solution structure of Vps27 UIM–ubiquitin complex important for endosomal sorting and receptor downregulation. EMBO J. 22, 4597–4606 (2003).
Misra, S., Miller, G. J. & Hurley, J. H. Recognizing phosphatidylinositol 3-phosphate. Cell 107, 559–562 (2001).
Matsuo, H. et al. Role of LBPA and Alix in multivesicular liposome formation and endosome organization. Science 303, 531–534 (2004). Highlights that not only proteins, but also lipids (lysobisphosphatidic acid (LBPA) in this case) can have crucial roles in forming MVBs.
Johnson, E. E., Overmeyer, J. H., Gunning, W. T. & Maltese, W. A. Gene silencing reveals a specific function of hVps34 phosphatidylinositol 3-kinase in late versus early endosomes. J. Cell Sci. 119, 1219–1232 (2006).
Futter, C. E., Collinson, L. M., Backer, J. M. & Hopkins, C. R. Human VPS34 is required for internal vesicle formation within multivesicular endosomes. J. Cell Biol. 155, 1251–1264 (2001).
Bruckmann, A., Kunkel, W., Augsten, K., Wetzker, R. & Eck, R. The deletion of CaVPS34 in the human pathogenic yeast Candida albicans causes defects in vesicle-mediated protein sorting and nuclear segregation. Yeast 18, 343–353 (2001).
Acknowledgements
We would like to thank M. West, G. Odorizzi, C. Futter, J.-H. Shim, M. Russell, C. Hopkins, P. Woodman, S. Stuffers, A. Brech, H. Stenmark and L. Pullan, all for providing high-resolution EM images that, regrettably, could not be included in the final manuscript owing to space limitations.
Author information
Authors and Affiliations
Ethics declarations
Competing interests
The authors declare no competing financial interests.
Glossary
- Intralumenal vesicle
-
A small <50-nm vesicle that forms through inward budding of the endosomal membrane away from the cytosol.
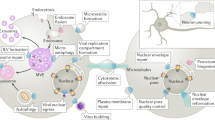
- Multivesicular body (MVB)
-
A morphologically defined endocytic organelle that is characterized by multiple internal vesicles in which internalized receptors are packaged before degradation.
- Lysosome
-
Membrane-bound organelle in higher eukaryotic cells that has an acidic interior and is the major storage site of the degradative enzymes (acidic hydrolases) that are responsible for the breakdown of internalized proteins and many membrane proteins. Functionally equivalent to the yeast vacuole.
- Ubiquitylation
-
Covalent attachment of ubiquitin via its C-terminal Gly to Lys side chains in target proteins. Ubiquitin can form seven distinct polyubiquitin chains through seven internal Lys residues.
- Endosomal sorting complex required for transport
-
(ESCRT). A multimeric protein complex that was first identified biochemically in yeast. ESCRTs control the sorting of endosomal cargo proteins into internal vesicles of multivesicular bodies.
- ESCRT-0
-
Nomenclature for the HRS–STAM complex, which was proposed as a recent extension of the originally defined ESCRT-I, -II and -III.
- De-ubiquitylating enzyme
-
Protease that can cleave the isopeptide bond between ubiquitin and Lys side chains of ubiquitylated substrates.
- Early endosome antigen-1
-
A protein that plays a part as a tether in the homotypic fusion of early endosomes.
- Phosphatidylinositol-3-phosphate
-
(PtdIns3P). A phospholipid that is highly enriched in endosomal membranes. Several ESCRT proteins encode PtdIns3P-binding domains, which allow these proteins to be recruited to endosomes.
- Clathrin
-
Coat protein that decorates clathrin-coated vesicles and is recruited by ESCRT-0 to sorting endosomes where it forms a flat double-layered coat, which is thought to sequester ubiquitylated cargo prior to incorporation into intralumenal vesicles.
- Vacuolar hydrolases
-
Enzymes that are responsible for protein degradation in late endosomes and lysosomes.
- Class E compartment
-
Aberrant multilamellar or multicisternal membrane-bound compartment in which both endocytic and biosynthetic proteins en route to the vacuole accumulate in Vps-mutant yeast of the class E subtype.
- CHMP
-
A family of structurally related proteins with a basic N terminus and an acidic C terminus that interact with each other to form ESCRT-III. Some CHMPs have also been associated with nuclear functions.
- MIT
-
A domain found in microtubule interacting and trafficking proteins that forms a three-helix bundle. Some MIT domains, including those of VPS4 and AMSH, bind to CHMPs.
- AAA+ ATPases
-
ATPases associated with various cellular activities: a superfamily of structurally related proteins that control diverse functions, including protein disassembly.
- Proteasome
-
Multi-enzyme complex responsible for the majority of protein turnover in eukaryotic cells that degrades proteins tagged with a particular type of ubiquitin chain.
Rights and permissions
About this article
Cite this article
Williams, R., Urbé, S. The emerging shape of the ESCRT machinery. Nat Rev Mol Cell Biol 8, 355–368 (2007). https://doi.org/10.1038/nrm2162
Issue Date:
DOI: https://doi.org/10.1038/nrm2162
This article is cited by
-
Versatile extracellular vesicle-mediated information transfer: intercellular synchronization of differentiation and of cellular phenotypes, and future perspectives
Inflammation and Regeneration (2024)
-
Embryonic signals mediate extracellular vesicle biogenesis and trafficking at the embryo–maternal interface
Cell Communication and Signaling (2023)
-
Extracellular vesicles derived from mesenchymal stem cells — a novel therapeutic tool in infectious diseases
Inflammation and Regeneration (2023)
-
Exosomal transmission of viruses, a two-edged biological sword
Cell Communication and Signaling (2023)
-
Shining the light on mesenchymal stem cell-derived exosomes in breast cancer
Stem Cell Research & Therapy (2023)