Abstract
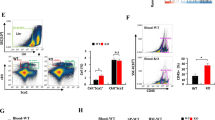
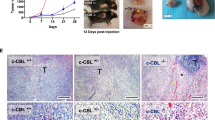
Inflammation critically contributes to cancer metastasis, in which myeloid-derived suppressor cells (MDSCs) are an important participant. Although MDSCs are known to suppress immune surveillance, their roles in directly stimulating cancer cell proliferation and metastasis currently remain unclear. Lysosomal acid lipase (LAL) deficiency causes systemic expansion and infiltration of MDSCs in multiple organs and subsequent inflammation. In the LAL-deficient (lal−/−) mouse model, melanoma metastasized massively in allogeneic lal−/− mice, which was suppressed in allogeneic lal+/+ mice owing to immune rejection. Here we report for the first time that MDSCs from lal−/− mice directly stimulated B16 melanoma cell in vitro proliferation and in vivo growth and metastasis. Cytokines, that is, interleukin-1β and tumor necrosis factor-α from MDSCs are required for B16 melanoma cell proliferation in vitro. Myeloid-specific expression of human LAL (hLAL) in lal−/− mice rescues these malignant phenotypes in vitro and in vivo. The tumor-promoting function of lal−/− MDSCs is mediated, at least in part, through overactivation of the mammalian target of rapamycin (mTOR) pathway. Knockdown of mTOR, Raptor or Rictor in lal−/− MDSCs suppressed their stimulation on proliferation of cancer cells, including B16 melanoma, Lewis lung carcinoma and transgenic mouse prostate cancer-C2 cancer cells. Our results indicate that LAL has a critical role in regulating MDSCs’ ability to directly stimulate cancer cell proliferation and overcome immune rejection of cancer metastasis in allogeneic mice through modulation of the mTOR pathway, which provides a mechanistic basis for targeting MDSCs to reduce the risk of cancer metastasis. Therefore MDSCs possess dual functions to facilitate cancer metastasis: suppress immune surveillance and stimulate cancer cell proliferation and growth.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Grivennikov SI, Greten FR, Karin M . Immunity, inflammation, and cancer. Cell 2010; 140: 883–899.
Qu P, Shelley WC, Yoder MC, Wu L, Du H, Yan C . Critical roles of lysosomal acid lipase in myelopoiesis. Am J Pathol 2010; 176: 2394–2404.
Qu P, Yan C, Blum JS, Kapur R, Du H . Myeloid-specific expression of human lysosomal acid lipase corrects malformation and malfunction of myeloid-derived suppressor cells in lal−/− mice. J Immunol 2011; 187: 3854–3866.
Qu P, Yan C, Du H . Matrix metalloproteinase 12 overexpression in myeloid lineage cells plays a key role in modulating myelopoiesis, immune suppression, and lung tumorigenesis. Blood 2011; 117: 4476–4489.
Qu P, Du H, Li Y, Yan C . Myeloid-specific expression of Api6/AIM/Sp alpha induces systemic inflammation and adenocarcinoma in the lung. J Immunol 2009; 182: 1648–1659.
Wu L, Yan C, Czader M, Foreman O, Blum JS, Kapur R et al. Inhibition of PPARgamma in myeloid-lineage cells induces systemic inflammation, immunosuppression, and tumorigenesis. Blood 2012; 119: 115–126.
Yan C, Lian X, Li Y, Dai Y, White A, Qin Y et al. Macrophage-specific expression of human lysosomal acid lipase corrects inflammation and pathogenic phenotypes in lal−/− mice. Am J Pathol 2006; 169: 916–926.
Gabrilovich DI, Nagaraj S . Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol 2009; 9: 162–174.
Mandruzzato S, Solito S, Falisi E, Francescato S, Chiarion-Sileni V, Mocellin S et al. IL4Rα+ myeloid-derived suppressor cell expansion in cancer patients. J Immunol 2009; 182: 6562–6568.
Diaz-Montero CM, Salem M, Nishimura M, Garrett-Mayer E, Cole D, Montero A . Increased circulating myeloid-derived suppressor cells correlate with clinical cancer stage, metastatic tumor burden, and doxorubicin–cyclophosphamide chemotherapy. Cancer Immunol Immunother 2009; 58: 49–59.
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P et al. Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 2009; 15: 2148–2157.
Hoechst B, Voigtlaender T, Ormandy L, Gamrekelashvili J, Zhao F, Wedemeyer H et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 2009; 50: 799–807.
Wang L, Chang EWY, Wong SC, Ong S-M, Chong DQY, Ling KL . Increased myeloid-derived suppressor cells in gastric cancer correlate with cancer stage and plasma S100A8/A9 proinflammatory proteins. J Immunol 2013; 190: 794–804.
Ostrand-Rosenberg S, Sinha P . Myeloid-derived suppressor cells: linking inflammation and cancer. J Immunol 2009; 182: 4499–4506.
Bruchard M, Mignot G, Derangere V, Chalmin F, Chevriaux A, Vegran F et al. Chemotherapy-triggered cathepsin B release in myeloid-derived suppressor cells activates the Nlrp3 inflammasome and promotes tumor growth. Nat Med 2013; 19: 57–64.
Rothe G, Stohr J, Fehringer P, Gasche C, Schmitz G . Altered mononuclear phagocyte differentiation associated with genetic defects of the lysosomal acid lipase. Atherosclerosis 1997; 130: 215–221.
Elleder M, Chlumska A, Hyanek J, Poupetova H, Ledvinova J, Maas S et al. Subclinical course of cholesteryl ester storage disease in an adult with hypercholesterolemia, accelerated atherosclerosis, and liver cancer. J Hepatol 2000; 32: 528–534.
Guertin DA, Sabatini DM . Defining the role of mTOR in cancer. Cancer Cell 2007; 12: 9–22.
Ding X, Du H, Yoder MC, Yan C . Critical role of the mTOR pathway in development and function of myeloid-derived suppressor cells in lal(−/−) mice. Am J Pathol 2014; 184: 397–408.
Laplante M, Sabatini David M . mTOR signaling in growth control and disease. Cell 2012; 149: 274–293.
Yan C, Ding X, Dasgupta N, Wu L, Du H . Gene profile of myeloid-derived suppressive cells from the bone marrow of lysosomal acid lipase knock-out mice. PLoS ONE 2012; 7: e30701.
Talmadge JE, Gabrilovich DI . History of myeloid-derived suppressor cells. Nat Rev Cancer 2013; 13: 739–752.
Lian X, Yan C, Yang L, Xu Y, Du H . Lysosomal acid lipase deficiency causes respiratory inflammation and destruction in the lung. Am J Physiol Lung Cell Mol Physiol 2004; 286: L801–L807.
Qu P, Roberts J, Li Y, Albrecht M, Cummings OW, Eble JN et al. Stat3 downstream genes serve as biomarkers in human lung carcinomas and chronic obstructive pulmonary disease. Lung Cancer 2009; 63: 341–347.
Chambers AF, Groom AC, MacDonald IC . Metastasis: Dissemination and growth of cancer cells in metastatic sites. Nat Rev Cancer 2002; 2: 563–572.
Lian X, Yan C, Qin Y, Knox L, Li T, Du H . Neutral lipids and peroxisome proliferator-activated receptor-{gamma} control pulmonary gene expression and inflammation-triggered pathogenesis in lysosomal acid lipase knockout mice. Am J Pathol 2005; 167: 813–821.
Du H, Heur M, Duanmu M, Grabowski GA, Hui DY, Witte DP et al. Lysosomal acid lipase-deficient mice: depletion of white and brown fat, severe hepatosplenomegaly, and shortened life span. J Lipid Res 2001; 42: 489–500.
Joyce JA, Pollard JW . Microenvironmental regulation of metastasis. Nat Rev Cancer 2009; 9: 239–252.
Meyer C, Sevko A, Ramacher M, Bazhin AV, Falk CS, Osen W et al. Chronic inflammation promotes myeloid-derived suppressor cell activation blocking antitumor immunity in transgenic mouse melanoma model. Proc Natl Acad Sci 2011; 108: 17111–17116.
Bonauer A, Carmona G, Iwasaki M, Mione M, Koyanagi M, Fischer A et al. MicroRNA-92a controls angiogenesis and functional recovery of ischemic tissues in mice. Science 2009; 324: 1710–1713.
Zhao T, Li J, Chen AF . MicroRNA-34a induces endothelial progenitor cell senescence and impedes its angiogenesis via suppressing silent information regulator 1. Am J Physiol Endocrinol Metab 2010; 299: E110–E116.
Acknowledgements
This work was supported by the National Institutes of Health Grants CA138759, CA152099 (to CY) and HL087001 (to HD).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Competing interests
The authors declare no conflict of interest.
Additional information
Supplementary Table accompanies this paper on the Oncogene website
Supplementary information
Rights and permissions
About this article
Cite this article
Zhao, T., Du, H., Ding, X. et al. Activation of mTOR pathway in myeloid-derived suppressor cells stimulates cancer cell proliferation and metastasis in lal−/− mice. Oncogene 34, 1938–1948 (2015). https://doi.org/10.1038/onc.2014.143
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2014.143
This article is cited by
-
Selective autophagy in cancer: mechanisms, therapeutic implications, and future perspectives
Molecular Cancer (2024)
-
Tumor metabolism rewiring in epithelial ovarian cancer
Journal of Ovarian Research (2023)
-
Emerging role of lipophagy in liver disorders
Molecular and Cellular Biochemistry (2023)
-
The regulation, function, and role of lipophagy, a form of selective autophagy, in metabolic disorders
Cell Death & Disease (2022)
-
Roles of Myeloid-Derived Suppressor Cells in Cancer Metastasis: Immunosuppression and Beyond
Archivum Immunologiae et Therapiae Experimentalis (2019)