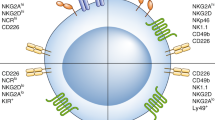
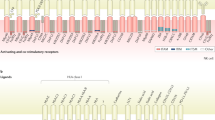
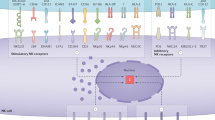
Abstract
Natural killer (NK) cells are innate lymphoid cells endowed with cytolytic activity and a capacity to secrete cytokines and chemokines. Several lines of evidence suggest that NK cells play an important role in anti-tumor immunity. Some therapies against hematological malignacies make use of the immune properties of NK cells, such as their ability to kill residual leukemic blasts efficiently after conditioning during haploidentical hematopoietic stem cell transplantation. However, knowledge on NK cell infiltration and the status of NK cell responsiveness in solid tumors is limited so far. The pro-angiogenic role of the recently described NK cell-like type 1 innate lymphoid cells (ILC1s) and their phenotypic resemblance to NK cells are confounding factors that add a level of complexity, at least in mice. Here, we review the current knowledge on the presence and function of NK cells in solid tumors as well as the immunotherapeutic approaches designed to harness NK cell functions in these conditions, including those that aim to reinforce conventional anti-tumor therapies to increase the chances of successful treatment.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
References
Vivier, E. et al. Innate lymphoid cells: 10 years on. Cell 174, 1054–1066 (2018).
Shi, F. D., Ljunggren, H. G., La Cava, A. & Van Kaer, L. Organ-specific features of natural killer cells. Nat. Rev. Immunol. 11, 658–671 (2011).
Walzer, T. et al. Identification, activation, and selective in vivo ablation of mouse NK cells via NKp46. Proc. Natl. Acad. Sci. USA 104, 3384–3389 (2007).
Biassoni, R., Bottino, C., Cantoni, C. & Moretta, A. Human natural killer receptors and their ligands. Curr. Protoc. Immunol. Chapter 14, Unit 14.10 (2002).
Moretta, L. et al. Surface NK receptors and their ligands on tumor cells. Semin. Immunol. 18, 151–158 (2006).
Guia, S., Fenis, A., Vivier, E. & Narni-Mancinelli, E. Activating and inhibitory receptors expressed on innate lymphoid cells. Semin. Immunopathol. 40, 331–341 (2018).
Moretta, A. et al. Receptors for HLA class-I molecules in human natural killer cells. Annu. Rev. Immunol. 14, 619–648 (1996).
Wu, N. & Veillette, A. SLAM family receptors in normal immunity and immune pathologies. Curr. Opin. Immunol. 38, 45–51 (2016).
Bryceson, Y. T., Ljunggren, H. G. & Long, E. O. Minimal requirement for induction of natural cytotoxicity and intersection of activation signals by inhibitory receptors. Blood 114, 2657–2666 (2009).
Bryceson, Y. T., March, M. E., Ljunggren, H. G. & Long, E. O. Activation, coactivation, and costimulation of resting human natural killer cells. Immunol. Rev. 214, 73–91 (2006).
Vivier, E., Tomasello, E., Baratin, M., Walzer, T. & Ugolini, S. Functions of natural killer cells. Nat. Immunol. 9, 503–510 (2008).
Smyth, M. J. et al. Perforin-mediated cytotoxicity is critical for surveillance of spontaneous lymphoma. J. Exp. Med. 192, 755–760 (2000).
Street, S. E. et al. Host perforin reduces tumor number but does not increase survival in oncogene-driven mammary adenocarcinoma. Cancer Res. 67, 5454–5460 (2007).
Vivier, E. et al. Innate or adaptive immunity? The example of natural killer cells. Science 331, 44–49 (2011).
Moretta, A. et al. Early liaisons between cells of the innate immune system in inflamed peripheral tissues. Trends Immunol. 26, 668–675 (2005).
Martin-Fontecha, A. et al. Induced recruitment of NK cells to lymph nodes provides IFN-gamma for T(H)1 priming. Nat. Immunol. 5, 1260–1265 (2004).
Hayakawa, Y. et al. IFN-gamma-mediated inhibition of tumor angiogenesis by natural killer T-cell ligand, alpha-galactosylceramide. Blood 100, 1728–1733 (2002).
Krebs, P. et al. NK-cell-mediated killing of target cells triggers robust antigen-specific T-cell-mediated and humoral responses. Blood 113, 6593–6602 (2009).
Roder, J. C. et al. A new immunodeficiency disorder in humans involving NK cells. Nature 284, 553–555 (1980).
Sullivan, J. L., Byron, K. S., Brewster, F. E. & Purtilo, D. T. Deficient natural killer cell activity in x-linked lymphoproliferative syndrome. Science 210, 543–545 (1980).
Gorelik, E., Wiltrout, R. H., Okumura, K., Habu, S. & Herberman, R. B. Role of NK cells in the control of metastatic spread and growth of tumor cells in mice. Int J. Cancer 30, 107–112 (1982).
Talmadge, J. E., Meyers, K. M., Prieur, D. J. & Starkey, J. R. Role of natural killer cells in tumor growth and metastasis: C57BL/6 normal and beige mice. J. Natl. Cancer Inst. 65, 929–935 (1980).
Nakajima, T., Mizushima, N., Nakamura, J. & Kanai, K. Surface markers of NK cells in peripheral blood of patients with cirrhosis and hepatocellular carcinoma. Immunol. Lett. 13, 7–10 (1986).
Pross, H. F. & Lotzova, E. Role of natural killer cells in cancer. Nat. Immun. 12, 279–292 (1993).
Schantz, S. P., Shillitoe, E. J., Brown, B. & Campbell, B. Natural killer cell activity and head and neck cancer: a clinical assessment. J. Natl. Cancer Inst. 77, 869–875 (1986).
Orange, J. S. Natural killer cell deficiency. J. Allergy Clin. Immunol. 132, 515–525 (2013).
Spinner, M. A. et al. GATA2 deficiency: a protean disorder of hematopoiesis, lymphatics, and immunity. Blood 123, 809–821 (2014).
Gineau, L. et al. Partial MCM4 deficiency in patients with growth retardation, adrenal insufficiency, and natural killer cell deficiency. J. Clin. Invest. 122, 821–832 (2012).
Ebbo, M. et al. Low circulating natural killer cell counts are associated with severe disease in patients with common variable immunodeficiency. EBioMedicine 6, 222–230 (2016).
Vely, F. et al. Evidence of innate lymphoid cell redundancy in humans. Nat. Immunol. 17, 1291–1299 (2016).
Smyth, M. J., Hayakawa, Y., Takeda, K. & Yagita, H. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2, 850–861 (2002).
Lee, U., Santa, K., Habu, S. & Nishimura, T. Murine asialo GM1+CD8+T cells as novel interleukin-12-responsive killer T cell precursors. Jpn J. Cancer Res. 87, 429–432 (1996).
Trambley, J. et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J. Clin. Invest. 104, 1715–1722 (1999).
Kataoka, S., Konishi, Y., Nishio, Y., Fujikawa-Adachi, K. & Tominaga, A. Antitumor activity of eosinophils activated by IL-5 and eotaxin against hepatocellular carcinoma. DNA Cell Biol. 23, 549–560 (2004).
Nishikado, H., Mukai, K., Kawano, Y., Minegishi, Y. & Karasuyama, H. NK cell-depleting anti-asialo GM1 antibody exhibits a lethal off-target effect on basophils in vivo. J. Immunol. 186, 5766–5771 (2011).
Slifka, M. K., Pagarigan, R. R. & Whitton, J. L. NK markers are expressed on a high percentage of virus-specific CD8+and CD4+T cells. J. Immunol. 164, 2009–2015 (2000).
Wiltrout, R. H. et al. Reactivity of anti-asialo GM1 serum with tumoricidal and non-tumoricidal mouse macrophages. J. Leukoc. Biol. 37, 597–614 (1985).
Cooley, S. et al. Donor selection for natural killer cell receptor genes leads to superior survival after unrelated transplantation for acute myelogenous leukemia. Blood 116, 2411–2419 (2010).
Hsu, K. C. et al. Improved outcome in HLA-identical sibling hematopoietic stem-cell transplantation for acute myelogenous leukemia predicted by KIR and HLA genotypes. Blood 105, 4878–4884 (2005).
Ruggeri, L. et al. Role of natural killer cell alloreactivity in HLA-mismatched hematopoietic stem cell transplantation. Blood 94, 333–339 (1999).
Ruggeri, L. et al. Effectiveness of donor natural killer cell alloreactivity in mismatched hematopoietic transplants. Science 295, 2097–2100 (2002).
Chiossone, L., Dumas, P. Y., Vienne, M. & Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 18, 671–688 (2018).
Brittenden, J., Heys, S. D., Ross, J. & Eremin, O. Natural killer cells and cancer. Cancer 77, 1226–1243 (1996).
Garcia-Iglesias, T. et al. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer 9, 186 (2009).
Tartter, P. I., Steinberg, B., Barron, D. M. & Martinelli, G. The prognostic significance of natural killer cytotoxicity in patients with colorectal cancer. Arch. Surg. 122, 1264–1268 (1987).
Halama, N. et al. Natural killer cells are scarce in colorectal carcinoma tissue despite high levels of chemokines and cytokines. Clin. Cancer Res. 17, 678–689 (2011).
Erdag, G. et al. Immunotype and immunohistologic characteristics of tumor-infiltrating immune cells are associated with clinical outcome in metastatic melanoma. Cancer Res. 72, 1070–1080 (2012).
Ali, T. H. et al. Enrichment of CD56(dim)KIR+CD57+highly cytotoxic NK cells in tumour-infiltrated lymph nodes of melanoma patients. Nat. Commun. 5, 5639 (2014).
Messaoudene, M. et al. Mature cytotoxic CD56(bright)/CD16(+) natural killer cells can infiltrate lymph nodes adjacent to metastatic melanoma. Cancer Res. 74, 81–92 (2014).
Denkert, C. et al. Tumour-infiltrating lymphocytes and prognosis in different subtypes of breast cancer: a pooled analysis of 3771 patients treated with neoadjuvant therapy. Lancet Oncol. 19, 40–50 (2018).
Salgado, R. et al. Tumor-infiltrating lymphocytes and associations with pathological complete response and event-free survival in HER2-positive early-stage breast cancer treated with lapatinib and trastuzumab: a secondary analysis of the NeoALTTO Trial. JAMA Oncol. 1, 448–454 (2015).
Arnould, L. et al. Trastuzumab-based treatment of HER2-positive breast cancer: an antibody-dependent cellular cytotoxicity mechanism? Br. J. Cancer 94, 259–267 (2006).
Gennari, R. et al. Pilot study of the mechanism of action of preoperative trastuzumab in patients with primary operable breast tumors overexpressing HER2. Clin. Cancer Res. 10, 5650–5655 (2004).
Muller, P. et al. Trastuzumab emtansine (T-DM1) renders HER2+breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci. Transl. Med. 7, 315ra188 (2015).
Muntasell, A. et al. NK cell infiltrates and HLA class I expression in primary HER2+breast cancer predict and uncouple pathological response and disease-free survival. Clin. Cancer Res. 25, 1535–1545 (2019).
Eckl, J. et al. Transcript signature predicts tissue NK cell content and defines renal cell carcinoma subgroups independent of TNM staging. J. Mol. Med. 90, 55–66 (2012).
Schleypen, J. S. et al. Cytotoxic markers and frequency predict functional capacity of natural killer cells infiltrating renal cell carcinoma. Clin. Cancer Res. 12, 718–725 (2006).
Schleypen, J. S. et al. Renal cell carcinoma-infiltrating natural killer cells express differential repertoires of activating and inhibitory receptors and are inhibited by specific HLA class I allotypes. Int. J. Cancer 106, 905–912 (2003).
Schantz, S. P. & Ordonez, N. G. Quantitation of natural killer cell function and risk of metastatic poorly differentiated head and neck cancer. Nat. Immun. Cell Growth Regul. 10, 278–288 (1991).
Schantz, S. P., Savage, H. E., Racz, T., Taylor, D. L. & Sacks, P. G. Natural killer cells and metastases from pharyngeal carcinoma. Am. J. Surg. 158, 361–366 (1989).
Andre, P. et al. Anti-NKG2A mAb is a checkpoint inhibitor that promotes anti-tumor immunity by unleashing both T and NK cells. Cell 175, 1731–1743.e13 (2018).
Baumeister, S. H., Freeman, G. J., Dranoff, G. & Sharpe, A. H. Coinhibitory pathways in immunotherapy for cancer. Annu Rev. Immunol. 34, 539–573 (2016).
Okazaki, T., Chikuma, S., Iwai, Y., Fagarasan, S. & Honjo, T. A rheostat for immune responses: the unique properties of PD-1 and their advantages for clinical application. Nat. Immunol. 14, 1212–1218 (2013).
Okazaki, T. & Honjo, T. PD-1 and PD-1 ligands: from discovery to clinical application. Int. Immunol. 19, 813–824 (2007).
Schumacher, T. N. & Schreiber, R. D. Neoantigens in cancer immunotherapy. Science 348, 69–74 (2015).
Sharma, P. & Allison, J. P. Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015).
Sharma, P. & Allison, J. P. The future of immune checkpoint therapy. Science 348, 56–61 (2015).
Barry, K. C. et al. A natural killer-dendritic cell axis defines checkpoint therapy-responsive tumor microenvironments. Nat. Med. 24, 1178–1191 (2018).
Hsu, J. et al. Contribution of NK cells to immunotherapy mediated by PD-1/PD-L1 blockade. J. Clin. Invest. 128, 4654–4668 (2018).
Dougall, W. C., Kurtulus, S., Smyth, M. J. & Anderson, A. C. TIGIT and CD96: new checkpoint receptor targets for cancer immunotherapy. Immunol. Rev. 276, 112–120 (2017).
Anderson, A. C., Joller, N. & Kuchroo, V. K. Lag-3, Tim-3, and TIGIT: co-inhibitory receptors with specialized functions in immune regulation. Immunity 44, 989–1004 (2016).
Lavin, Y. et al. Innate immune landscape in early lung adenocarcinoma by paired single-cell analyses. Cell 169, 750–765.e17 (2017).
Carrega, P. et al. Natural killer cells infiltrating human nonsmall-cell lung cancer are enriched in CD56 bright CD16(-) cells and display an impaired capability to kill tumor cells. Cancer 112, 863–875 (2008).
Platonova, S. et al. Profound coordinated alterations of intratumoral NK cell phenotype and function in lung carcinoma. Cancer Res 71, 5412–5422 (2011).
Castriconi, R. et al. Neuroblastoma-derived TGF-beta1 modulates the chemokine receptor repertoire of human resting NK cells. J. Immunol. 190, 5321–5328 (2013).
Gubbels, J. A. et al. MUC16 provides immune protection by inhibiting synapse formation between NK and ovarian tumor cells. Mol. Cancer 9, 11 (2010).
Balsamo, M. et al. Melanoma cells become resistant to NK-cell-mediated killing when exposed to NK-cell numbers compatible with NK-cell infiltration in the tumor. Eur. J. Immunol. 42, 1833–1842 (2012).
Pogge von Strandmann, E. et al. Human leukocyte antigen-B-associated transcript 3 is released from tumor cells and engages the NKp30 receptor on natural killer cells. Immunity 27, 965–974 (2007).
Reiners, K. S. et al. Soluble ligands for NK cell receptors promote evasion of chronic lymphocytic leukemia cells from NK cell anti-tumor activity. Blood 121, 3658–3665 (2013).
Horton, N. C., Mathew, S. O. & Mathew, P. A. Novel interaction between proliferating cell nuclear antigen and HLA I on the surface of tumor cells inhibits NK cell function through NKp44. PLoS ONE 8, e59552 (2013).
Rosental, B. et al. Proliferating cell nuclear antigen is a novel inhibitory ligand for the natural cytotoxicity receptor NKp44. J. Immunol. 187, 5693–5702 (2011).
Hoechst, B. et al. Myeloid derived suppressor cells inhibit natural killer cells in patients with hepatocellular carcinoma via the NKp30 receptor. Hepatology 50, 799–807 (2009).
Li, T. et al. Hepatocellular carcinoma-associated fibroblasts trigger NK cell dysfunction via PGE2 and IDO. Cancer Lett. 318, 154–161 (2012).
Li, T. et al. Colorectal carcinoma-derived fibroblasts modulate natural killer cell phenotype and antitumor cytotoxicity. Med. Oncol. 30, 663 (2013).
Castriconi, R. et al. Transforming growth factor beta 1 inhibits expression of NKp30 and NKG2D receptors: consequences for the NK-mediated killing of dendritic cells. Proc. Natl. Acad. Sci. USA 100, 4120–4125 (2003).
Marcenaro, E. et al. IL-12 or IL-4 prime human NK cells to mediate functionally divergent interactions with dendritic cells or tumors. J. Immunol. 174, 3992–3998 (2005).
Pietra, G. et al. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 72, 1407–1415 (2012).
Faveeuw, C., Di Mauro, M. E., Price, A. A. & Ager, A. Roles of alpha(4) integrins/VCAM-1 and LFA-1/ICAM-1 in the binding and transendothelial migration of T lymphocytes and T lymphoblasts across high endothelial venules. Int. Immunol. 12, 241–251 (2000).
Lee, J. et al. An antibody designed to improve adoptive NK-cell therapy inhibits pancreatic cancer progression in a murine model. Cancer Immunol. Res. 7, 219–229 (2019).
Charych, D. H. et al. NKTR-214, an engineered cytokine with biased IL2 receptor binding, increased tumor exposure, and marked efficacy in mouse tumor models. Clin. Cancer Res. 22, 680–690 (2016).
Alvarez, I. B. et al. Role played by the programmed death-1-programmed death ligand pathway during innate immunity against Mycobacterium tuberculosis. J. Infect. Dis. 202, 524–532 (2010).
Norris, S. et al. PD-1 expression on natural killer cells and CD8(+) T cells during chronic HIV-1 infection. Viral Immunol. 25, 329–332 (2012).
Benson, D. M. et al. The PD-1/PD-L1 axis modulates the natural killer cell versus multiple myeloma effect: a therapeutic target for CT-011, a novel monoclonal anti-PD-1 antibody. Blood 116, 2286–2294 (2010).
Amaria, R. N. et al. Neoadjuvant immune checkpoint blockade in high-risk resectable melanoma. Nat. Med. 24, 1649–1654 (2018).
Liu, D. et al. Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV. Protein Cell 8, 861–877 (2017).
Chang, Y. H. et al. A chimeric receptor with NKG2D specificity enhances natural killer cell activation and killing of tumor cells. Cancer Res. 73, 1777–1786 (2013).
Lonez, C. et al. Study protocol for THINK: a multinational open-label phase I study to assess the safety and clinical activity of multiple administrations of NKR-2 in patients with different metastatic tumour types. BMJ Open 7, e017075 (2017).
Gasteiger, G., Fan, X., Dikiy, S., Lee, S. Y. & Rudensky, A. Y. Tissue residency of innate lymphoid cells in lymphoid and nonlymphoid organs. Science 350, 981–985 (2015).
Peng, H. et al. Liver-resident NK cells confer adaptive immunity in skin-contact inflammation. J. Clin. Invest. 123, 1444–1456 (2013).
Klose, C. S. N. et al. Differentiation of type 1 ILCs from a common progenitor to all helper-like innate lymphoid cell lineages. Cell 157, 340–356 (2014).
Abt, M. C. et al. Innate immune defenses mediated by two ILC subsets are critical for protection against acute Clostridium difficile infection. Cell Host Microbe 18, 27–37 (2015).
Weizman, O. E. et al. ILC1 confer early host protection at initial sites of viral infection. Cell 171, 795–808 e712 (2017).
Freud, A. G. et al. NKp80 defines a critical step during human natural killer cell development. Cell Rep. 16, 379–391 (2016).
Gao, Y. et al. Tumor immunoevasion by the conversion of effector NK cells into type 1 innate lymphoid cells. Nat. Immunol. 18, 1004–1015 (2017).
Nussbaum, K. et al. Tissue microenvironment dictates the fate and tumor-suppressive function of type 3 ILCs. J. Exp. Med. 214, 2331–2347 (2017).
Vonarbourg, C. et al. Regulated expression of nuclear receptor RORgammat confers distinct functional fates to NK cell receptor-expressing RORgammat(+) innate lymphocytes. Immunity 33, 736–751 (2010).
Cortez, V. S. et al. SMAD4 impedes the conversion of NK cells into ILC1-like cells by curtailing non-canonical TGF-beta signaling. Nat. Immunol. 18, 995–1003 (2017).
Hazenberg, M. D. & Spits, H. Human innate lymphoid cells. Blood 124, 700–709 (2014).
Judge, C. J. et al. CD56(bright) NK IL-7Ralpha expression negatively associates with HCV level, and IL-7-induced NK function is impaired during HCV and HIV infections. J. Leukoc. Biol. 102, 171–184 (2017).
Fuchs, A. ILC1s in tissue inflammation and infection. Front. Immunol. 7, 104 (2016).
Michel, T. et al. Human CD56bright NK cells: an update. J. Immunol. 196, 2923–2931 (2016).
Poli, A. et al. CD56bright natural killer (NK) cells: an important NK cell subset. Immunology 126, 458–465 (2009).
Lima, M. et al. Chemokine receptor expression on normal blood CD56(+) NK-cells elucidates cell partners that comigrate during the innate and adaptive immune responses and identifies a transitional NK-cell population. J. Immunol. Res. 2015, 839684 (2015).
Mittal, D., Vijayan, D. & Smyth, M. J. Overcoming acquired PD-1/PD-L1 resistance with CD38 blockade. Cancer Discov. 8, 1066–1068 (2018).
Lopez-Verges, S. et al. CD57 defines a functionally distinct population of mature NK cells in the human CD56dimCD16+NK-cell subset. Blood 116, 3865–3874 (2010).
Kloverpris, H. N. et al. Innate lymphoid cells are depleted irreversibly during acute HIV-1 infection in the absence of viral suppression. Immunity 44, 391–405 (2016).
Cruz-Zarate, D. et al. Innate lymphoid cells have decreased HLA-DR expression but retain their responsiveness to TLR ligands during sepsis. J. Immunol. 201, 3401–3410 (2018).
Moretta, L. Dissecting CD56dim human NK cells. Blood 116, 3689–3691 (2010).
Acknowledgements
The laboratory of E.V. is supported by funding from the European Research Council (ERC) under the European Union Horizon 2020 research and innovation program (Targeting innate lymphoid cells (TILC), grant agreement number 694502), the Agence Nationale de la Recherche (PIONeeR Project (ANR-17-RHUS-0007)), Equipe Labellisée “La Ligue”, Ligue Nationale contre le Cancer, MSDAvenir, Innate Pharma and institutional grants to the CIML (Institut National Français de Recherche Médicale (INSERM), Centre National de la Recherche Scientifique (CNRS) and Aix-Marseille University) and to Marseille Immunopôle.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
G.H., P.A. and E.V. are employees of Innate Pharma. The other authors declare no competing interests.
Rights and permissions
About this article
Cite this article
Habif, G., Crinier, A., André, P. et al. Targeting natural killer cells in solid tumors. Cell Mol Immunol 16, 415–422 (2019). https://doi.org/10.1038/s41423-019-0224-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41423-019-0224-2
This article is cited by
-
New immune cell engagers for cancer immunotherapy
Nature Reviews Immunology (2024)
-
Neutrophils bearing adhesive polymer micropatches as a drug-free cancer immunotherapy
Nature Biomedical Engineering (2024)
-
Advances of medical nanorobots for future cancer treatments
Journal of Hematology & Oncology (2023)
-
The emerging role of glycolysis and immune evasion in gastric cancer
Cancer Cell International (2023)
-
Neoantigens: promising targets for cancer therapy
Signal Transduction and Targeted Therapy (2023)