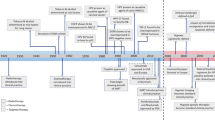
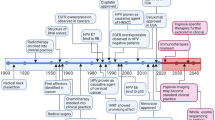
Abstract
Head and neck cancers are a heterogeneous collection of malignancies of the upper aerodigestive tract, salivary glands and thyroid. In this Review, we primarily focus on the changing therapeutic landscape of head and neck squamous cell carcinomas (HNSCCs) that can arise in the oral cavity, oropharynx, hypopharynx and larynx. We highlight developments in surgical and non-surgical therapies (mainly involving the combination of radiotherapy and chemotherapy), outlining how these treatments are being used in the current era of widespread testing for the presence of human papillomavirus infection in patients with HNSCC. Finally, we describe the clinical trials that led to the approval of the first immunotherapeutic agents for HNSCC, and discuss the development of strategies to decrease the toxicity of different treatment modalities.
Key points
-
Current treatments for human papillomavirus-driven (HPV+) oropharyngeal squamous cell carcinoma (OPSCC), primarily derived from those for the more aggressive HPV− head and neck squamous cell carcinoma (HSNCC), might be more intensive than necessary for patients with favourable risk features and an excellent prognosis.
-
Multiple prospective studies have investigated various treatment de-intensification strategies with promising early results; however, pending definitive conclusions, HPV+ OPSCC should continue to be treated with established standard-of-care therapies, known to yield very high survival.
-
Minimally invasive transoral surgical techniques provide an alternative treatment option for OPSCC; retrospective evidence is promising, but results from prospective randomized trials are required to fully integrate these approaches into the treatment armamentarium.
-
More-precisely targeted radiotherapy (with incorporation of intensity-modulated radiation therapy, molecular imaging-guided therapy, adaptive therapy and proton beam therapy) has the potential to decrease the long-term toxicity of radiotherapy.
-
Anti-EGFR therapy with cetuximab improves survival in the curative and recurrent and/or metastatic settings; however, no other molecularly targeted approach has prolonged survival in HNSCC.
-
Anti-programmed cell death 1 (PD-1) therapies, currently approved for platinum-refractory recurrent and/or metastatic HNSCC, offer the prospect of long-term remissions with fewer toxicities than traditional cytotoxic chemotherapy for a minority of patients, and are expected to advance to earlier lines of therapy.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout



Similar content being viewed by others
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424 (2018).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2017. CA Cancer J. Clin. 67, 7–30 (2017).
Wyss, A. et al. Cigarette, cigar, and pipe smoking and the risk of head and neck cancers: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Am. J. Epidemiol. 178, 679–690 (2013).
Maier, H. et al. Tobacco and alcohol and the risk of head and neck cancer. Clin. Investig. 70, 320–327 (1992).
Blot, W. J. et al. Smoking and drinking in relation to oral and pharyngeal cancer. Cancer Res. 48, 3282–3287 (1988).
Chaturvedi, A. K. et al. Human papillomavirus and rising oropharyngeal cancer incidence in the United States. J. Clin. Oncol. 29, 4294–4301 (2011).
World Health Organization. IARC Monographs on the Evaluation of Carcinogenic Risks to Humans, in Human Papillomaviruses (WHO, 2007).
Sturgis, E. M. & Cinciripini, P. M. Trends in head and neck cancer incidence in relation to smoking prevalence: an emerging epidemic of human papillomavirus-associated cancers? Cancer 110, 1429–1435 (2007).
Gillison, M. L. et al. Evidence for a causal association between human papillomavirus and a subset of head and neck cancers. J. Natl Cancer Inst. 92, 709–720 (2000).
Ang, K. K. et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N. Engl. J. Med. 363, 24–35 (2010).
D’Souza, G. et al. Case-control study of human papillomavirus and oropharyngeal cancer. N. Engl. J. Med. 356, 1944–1956 (2007).
Gillison, M. L. et al. Epidemiology of human papillomavirus-positive head and neck squamous cell carcinoma. J. Clin. Oncol. 33, 3235–3242 (2015).
Mehanna, H. et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer — systematic review and meta-analysis of trends by time and region. Head Neck 35, 747–755 (2013).
Carlander, A. F. et al. Continuing rise in oropharyngeal cancer in a high HPV prevalence area: a Danish population-based study from 2011 to 2014. Eur. J. Cancer 70, 75–82 (2017).
Ribeiro, K. B. et al. Low human papillomavirus prevalence in head and neck cancer: results from two large case-control studies in high-incidence regions. Int. J. Epidemiol. 40, 489–502 (2011).
Chung, C. H. et al. p16 protein expression and human papillomavirus status as prognostic biomarkers of nonoropharyngeal head and neck squamous cell carcinoma. J. Clin. Oncol. 32, 3930–3938 (2014).
Li, H. et al. Association of human papillomavirus status at head and neck carcinoma subsites with overall survival. JAMA Otolaryngol. Head Neck Surg. 144, 519–525 (2018).
Fakhry, C. et al. Associations between oral HPV16 infection and cytopathology: evaluation of an oropharyngeal “pap-test equivalent” in high-risk populations. Cancer Prev. Res. (Phila) 4, 1378–1384 (2011).
Harper, D. M. et al. Sustained efficacy up to 4.5 years of a bivalent L1 virus-like particle vaccine against human papillomavirus types 16 and 18: follow-up from a randomised control trial. Lancet 367, 1247–1255 (2006).
Food and Drug Administration. FDA approves expanded use of Gardasil 9 to include individuals 27 through 45 years old. FDA https://www.fda.gov/news-events/press-announcements/fda-approves-expanded-use-gardasil-9-include-individuals-27-through-45-years-old (2018).
Chaturvedi, A. K. et al. Effect of prophylactic human papillomavirus (HPV) vaccination on oral HPV infections among young adults in the United States. J. Clin. Oncol. 36, 262–267 (2018).
Herrero, R. et al. Reduced prevalence of oral human papillomavirus (HPV) 4 years after bivalent HPV vaccination in a randomized clinical trial in Costa Rica. PLOS ONE 8, e68329 (2013).
Huang, S. H. et al. Refining American Joint Committee on Cancer/Union for International Cancer Control TNM stage and prognostic groups for human papillomavirus-related oropharyngeal carcinomas. J. Clin. Oncol. 33, 836–845 (2015).
O’Sullivan, B. et al. Development and validation of a staging system for HPV-related oropharyngeal cancer by the International Collaboration on Oropharyngeal cancer Network for Staging (ICON-S): a multicentre cohort study. Lancet Oncol. 17, 440–451 (2016).
American Joint Committee on Cancer. AJCC Cancer Staging Manual 7th edn (eds Edge, S. et al.) (Springer, 2010).
American Joint Committee on Cancer. AJCC Cancer Staging Manual 8th edn (eds Amin, M. B. et al.) (Springer, 2017).
Cramer, J. D. et al. Validation of the eighth edition American Joint Committee on Cancer staging system for human papillomavirus-associated oropharyngeal cancer. Head Neck 40, 457–466 (2017).
Fakhry, C. et al. Development and validation of nomograms predictive of overall and progression-free survival in patients with oropharyngeal cancer. J. Clin. Oncol. 35, 4057–4065 (2017).
Gleber-Netto, F. O. et al. Variations in HPV function are associated with survival in squamous cell carcinoma. JCI Insight 4, 124762 (2019).
Tamura, Y. et al. Therapeutic outcomes of laryngeal cancer at Kyoto University Hospital for 10 years. Acta Otolaryngol. Suppl. 127, 62–65 (2007).
Cognetti, D. M., Weber, R. S. & Lai, S. Y. Head and neck cancer: an evolving treatment paradigm. Cancer 113, 1911–1932 (2008).
Gatta, G. et al. Prognoses and improvement for head and neck cancers diagnosed in Europe in early 2000s: the EUROCARE-5 population-based study. Eur. J. Cancer 51, 2130–2143 (2015).
Blanchard, P. et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): a comprehensive analysis by tumour site. Radiother. Oncol. 100, 33–40 (2011).
Machtay, M. et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J. Clin. Oncol. 26, 3582–3589 (2008).
Chinn, S. B. & Myers, J. N. Oral cavity carcinoma: current management, controversies, and future directions. J. Clin. Oncol. 33, 3269–3276 (2015).
National Comprehensive Cancer Network. NCCN clinical practice guidelines in oncology: head and neck cancers. NCCN https://oncolife.com.ua/doc/nccn/Head_and_Neck_Cancers.pdf (2018).
Hanasono, M. M. et al. Impact of reconstructive microsurgery in patients with advanced oral cavity cancers. Head Neck 31, 1289–1296 (2009).
Calais, G. et al. Randomized trial of radiation therapy versus concomitant chemotherapy and radiation therapy for advanced-stage oropharynx carcinoma. J. Natl Cancer Inst. 91, 2081–2086 (1999).
Haigentz, M. et al. Current trends in initial management of oropharyngeal cancer: the declining use of open surgery. Eur. Arch. Otorhinolaryngol. 266, 1845–1855 (2009).
White, H. et al. Salvage surgery for recurrent cancers of the oropharynx: comparing TORS with standard open surgical approaches. JAMA Otolaryngol. Head Neck Surg. 139, 773–778 (2013).
O’Malley, B. W. et al. Transoral robotic surgery (TORS) for base of tongue neoplasms. Laryngoscope 116, 1465–1472 (2006).
de Almeida, J. R. et al. Oncologic outcomes after transoral robotic surgery: a multi-institutional study. JAMA Otolaryngol. Head Neck Surg. 141, 1043–1051 (2015).
Haughey, B. H. et al. Transoral laser microsurgery as primary treatment for advanced-stage oropharyngeal cancer: a United States multicenter study. Head Neck 33, 1683–1694 (2011).
US Food and Drug Administration. 501(k) summary, section III: indications for use for intuitive surgical endoscopic instrument control system for transoral otolaryngology procedures. FDA https://www.accessdata.fda.gov/cdrh_docs/pdf9/K090993.pdf (2009).
Liederbach, E. et al. A contemporary analysis of surgical trends in the treatment of squamous cell carcinoma of the oropharynx from 1998 to 2012: a report from the National Cancer Database. Ann. Surg. Oncol. 22, 4422–4431 (2015).
US Food and Drug Administration. 510k summary medrobotics flex system (K150776). FDA https://www.accessdata.fda.gov/cdrh_docs/pdf15/K150776.pdf (2015).
Weinstein, G. S. et al. Transoral robotic surgery: a multicenter study to assess feasibility, safety, and surgical margins. Laryngoscope 122, 1701–1707 (2012).
Weinstein, G. S. et al. Selective neck dissection and deintensified postoperative radiation and chemotherapy for oropharyngeal cancer: a subset analysis of the University of Pennsylvania transoral robotic surgery trial. Laryngoscope 120, 1749–1755 (2010).
Holsinger, F. C. & Ferris, R. L. Transoral endoscopic head and neck surgery and its role within the multidisciplinary treatment paradigm of oropharynx cancer: robotics, lasers, and clinical trials. J. Clin. Oncol. 33, 3285–3292 (2015).
Albergotti, W. G. et al. A prospective evaluation of short-term dysphagia after transoral robotic surgery for squamous cell carcinoma of the oropharynx. Cancer 123, 3132–3140 (2017).
Sharma, A. et al. Survival and gastrostomy prevalence in patients with oropharyngeal cancer treated with transoral robotic surgery versus chemoradiotherapy. JAMA Otolaryngol. Head Neck Surg. 142, 691–697 (2016).
Kelly, J. R. et al. Upfront surgery versus definitive chemoradiotherapy in patients with human papillomavirus-associated oropharyngeal squamous cell cancer. Oral Oncol. 79, 64–70 (2018).
Grau, C. et al. Cervical lymph node metastases from unknown primary tumours. Results from a national survey by the Danish Society for Head and Neck Oncology. Radiother. Oncol. 55, 121–129 (2000).
Galloway, T. J. & Ridge, J. A. Management of squamous cancer metastatic to cervical nodes with an unknown primary site. J. Clin. Oncol. 33, 3328–3337 (2015).
Kim, S. H. et al. HPV integration begins in the tonsillar crypt and leads to the alteration of p16, EGFR and c-myc during tumor formation. Int. J. Cancer 120, 1418–1425 (2007).
Fakhry, C. et al. Human papillomavirus testing in head and neck carcinomas: ASCO Clinical Practice Guideline Endorsement of the College of American Pathologists Guideline. J. Clin. Oncol. 36, 3152–3161 (2018).
Graboyes, E. M. et al. Management of human papillomavirus-related unknown primaries of the head and neck with a transoral surgical approach. Head Neck 37, 1603–1611 (2015).
Durmus, K. et al. Transoral robotic approach to carcinoma of unknown primary. Head Neck 36, 848–852 (2014).
Geltzeiler, M. et al. Transoral robotic surgery for management of cervical unknown primary squamous cell carcinoma: updates on efficacy, surgical technique and margin status. Oral Oncol. 66, 9–13 (2017).
Hatten, K. M. et al. Transoral robotic surgery-assisted endoscopy with primary site detection and treatment in occult mucosal primaries. JAMA Otolaryngol. Head Neck Surg. 143, 267–273 (2017).
Patel, S. A. et al. Robotic surgery for primary head and neck squamous cell carcinoma of unknown site. JAMA Otolaryngol. Head Neck Surg. 139, 1203–1211 (2013).
Weiss, M. H., Harrison, L. B. & Isaacs, R. S. Use of decision analysis in planning a management strategy for the stage N0 neck. Arch. Otolaryngol. Head Neck Surg. 120, 699–702 (1994).
D’Cruz, A. K. et al. Elective versus therapeutic neck dissection in node-negative oral cancer. N. Engl. J. Med. 373, 521–529 (2015).
Brennan, S. et al. Prospective trial to evaluate staged neck dissection or elective neck radiotherapy in patients with CT-staged T1-2 N0 squamous cell carcinoma of the oral tongue. Head Neck 32, 191–198 (2010).
Schiefke, F. et al. Function, postoperative morbidity, and quality of life after cervical sentinel node biopsy and after selective neck dissection. Head Neck 31, 503–512 (2009).
Murer, K. et al. Comparison of morbidity between sentinel node biopsy and elective neck dissection for treatment of the N0 neck in patients with oral squamous cell carcinoma. Head Neck 33, 1260–1264 (2011).
Alkureishi, L. W. et al. Sentinel node biopsy in head and neck squamous cell cancer: 5-year follow-up of a European multicenter trial. Ann. Surg. Oncol. 17, 2459–2464 (2010).
Civantos, F. J. et al. Sentinel lymph node biopsy accurately stages the regional lymph nodes for T1-T2 oral squamous cell carcinomas: results of a prospective multi-institutional trial. J. Clin. Oncol. 28, 1395–1400 (2010).
Schilling, C. et al. Sentinel European Node Trial (SENT): 3-year results of sentinel node biopsy in oral cancer. Eur. J. Cancer 51, 2777–2784 (2015).
Thompson, C. F. et al. Diagnostic value of sentinel lymph node biopsy in head and neck cancer: a meta-analysis. Eur. Arch. Otorhinolaryngol. 270, 2115–2122 (2013).
Mehanna, H. et al. PET-CT surveillance versus neck dissection in advanced head and neck cancer. N. Engl. J. Med. 374, 1444–1454 (2016).
Stack, B. C. et al. The negative predictive value (NPV) of FDG-PET/CT in the head and neck squamous cell carcinoma (HNSCC) N0 patient, the first report of the ACRIN 6685 trial. J. Clin. Oncol. 35, 6041–6041 (2017).
Lowe, V. J. et al. Multicenter trial of [18F]fluorodeoxyglucose positron emission tomography/computed tomography staging of head and neck cancer and negative predictive value and surgical impact in the N0 neck: results from ACRIN 6685. J. Clin. Oncol. https://doi.org/10.1200/JCO.18.01182 (2019).
Lacas, B. et al. Role of radiotherapy fractionation in head and neck cancers (MARCH): an updated meta-analysis. Lancet Oncol. 18, 1221–1237 (2017).
Nutting, C. M. et al. Parotid-sparing intensity modulated versus conventional radiotherapy in head and neck cancer (PARSPORT): a phase 3 multicentre randomised controlled trial. Lancet Oncol. 12, 127–136 (2011).
Kam, M. K. et al. Prospective randomized study of intensity-modulated radiotherapy on salivary gland function in early-stage nasopharyngeal carcinoma patients. J. Clin. Oncol. 25, 4873–4879 (2007).
Pow, E. H. et al. Xerostomia and quality of life after intensity-modulated radiotherapy versus conventional radiotherapy for early-stage nasopharyngeal carcinoma: initial report on a randomized controlled clinical trial. Int. J. Radiat. Oncol. Biol. Phys. 66, 981–991 (2006).
Peng, G. et al. A prospective, randomized study comparing outcomes and toxicities of intensity-modulated radiotherapy versus conventional two-dimensional radiotherapy for the treatment of nasopharyngeal carcinoma. Radiother. Oncol. 104, 286–293 (2012).
Gregoire, V., Langendijk, J. A. & Nuyts, S. Advances in radiotherapy for head and neck cancer. J. Clin. Oncol. 33, 3277–3284 (2015).
Castadot, P. et al. Assessment by a deformable registration method of the volumetric and positional changes of target volumes and organs at risk in pharyngo-laryngeal tumors treated with concomitant chemo-radiation. Radiother. Oncol. 95, 209–217 (2010).
Schwartz, D. L. et al. Adaptive radiotherapy for head-and-neck cancer: initial clinical outcomes from a prospective trial. Int. J. Radiat. Oncol. Biol. Phys. 83, 986–993 (2012).
Kim, J. K. et al. Proton therapy for head and neck cancer. Curr. Treat. Options Oncol. 19, 28 (2018).
Setton, J. et al. A multi-institution pooled analysis of gastrostomy tube dependence in patients with oropharyngeal cancer treated with definitive intensity-modulated radiotherapy. Cancer 121, 294–301 (2015).
Blanchard, P. et al. Intensity-modulated proton beam therapy (IMPT) versus intensity-modulated photon therapy (IMRT) for patients with oropharynx cancer — a case matched analysis. Radiother. Oncol. 120, 48–55 (2016).
Vlacich, G. et al. Dose to the inferior pharyngeal constrictor predicts prolonged gastrostomy tube dependence with concurrent intensity-modulated radiation therapy and chemotherapy for locally-advanced head and neck cancer. Radiother. Oncol. 110, 435–440 (2014).
Feng, F. Y. et al. Intensity-modulated chemoradiotherapy aiming to reduce dysphagia in patients with oropharyngeal cancer: clinical and functional results. J. Clin. Oncol. 28, 2732–2738 (2010).
Pignon, J. P. et al. Meta-analysis of chemotherapy in head and neck cancer (MACH-NC): an update on 93 randomised trials and 17,346 patients. Radiother. Oncol. 92, 4–14 (2009).
Tsan, D. L. et al. The comparison between weekly and three-weekly cisplatin delivered concurrently with radiotherapy for patients with postoperative high-risk squamous cell carcinoma of the oral cavity. Radiat. Oncol. 7, 215 (2012).
Nguyen-Tan, P. F. et al. Randomized phase III trial to test accelerated versus standard fractionation in combination with concurrent cisplatin for head and neck carcinomas in the Radiation Therapy Oncology Group 0129 trial: long-term report of efficacy and toxicity. J. Clin. Oncol. 32, 3858–3866 (2014).
Szturz, P. et al. Weekly low-dose versus three-weekly high-dose cisplatin for concurrent chemoradiation in locoregionally advanced non-nasopharyngeal head and neck cancer: a systematic review and meta-analysis of aggregate data. Oncologist 22, 1056–1066 (2017).
Uygun, K. et al. The comparison of weekly and three-weekly cisplatin chemotherapy concurrent with radiotherapy in patients with previously untreated inoperable non-metastatic squamous cell carcinoma of the head and neck. Cancer Chemother. Pharmacol. 64, 601–605 (2009).
Quon, H. et al. Phase III study of radiation therapy with or without cis-platinum in patients with unresectable squamous or undifferentiated carcinoma of the head and neck: an intergroup trial of the Eastern Cooperative Oncology Group (E2382). Int. J. Radiat. Oncol. Biol. Phys. 81, 719–725 (2011).
Noronha, V. et al. Once-a-week versus once-every-3-weeks cisplatin chemoradiation for locally advanced head and neck cancer: a phase III randomized noninferiority trial. J. Clin. Oncol. 36, 1064–1072 (2018).
Bauml, J. M. et al. Cisplatin every 3 weeks versus weekly with definitive concurrent radiotherapy for squamous cell carcinoma of the head and neck. J. Natl Cancer Inst. 111, 490–497 (2019).
Kunieda, F. et al. Randomized phase II/III trial of post-operative chemoradiotherapy comparing 3-weekly cisplatin with weekly cisplatin in high-risk patients with squamous cell carcinoma of head and neck: Japan Clinical Oncology Group Study (JCOG1008). Jpn J. Clin. Oncol. 44, 770–774 (2014).
Quon, H. et al. Radiation therapy for oropharyngeal squamous cell carcinoma: American Society of Clinical Oncology Endorsement of the American Society for Radiation Oncology evidence-based clinical practice guideline. J. Clin. Oncol. 35, 4078–4090 (2017).
Blanchard, P. et al. Taxane-cisplatin-fluorouracil as induction chemotherapy in locally advanced head and neck cancers: an individual patient data meta-analysis of the Meta-Analysis of Chemotherapy in Head and Neck Cancer Group. J. Clin. Oncol. 31, 2854–2860 (2013).
Posner, M. R. et al. Cisplatin and fluorouracil alone or with docetaxel in head and neck cancer. N. Engl. J. Med. 357, 1705–1715 (2007).
Vermorken, J. B. et al. Cisplatin, fluorouracil, and docetaxel in unresectable head and neck cancer. N. Engl. J. Med. 357, 1695–1704 (2007).
Haddad, R. et al. Induction chemotherapy followed by concurrent chemoradiotherapy (sequential chemoradiotherapy) versus concurrent chemoradiotherapy alone in locally advanced head and neck cancer (PARADIGM): a randomised phase 3 trial. Lancet Oncol. 14, 257–264 (2013).
Cohen, E. E. et al. Phase III randomized trial of induction chemotherapy in patients with N2 or N3 locally advanced head and neck cancer. J. Clin. Oncol. 32, 2735–2743 (2014).
Geoffrois, L. et al. Induction chemotherapy followed by cetuximab radiotherapy is not superior to concurrent chemoradiotherapy for head and neck carcinomas: results of the GORTEC 2007-02 phase III randomized trial. J. Clin. Oncol. 36, 3077–3083 (2018).
Kalyankrishna, S. & Grandis, J. R. Epidermal growth factor receptor biology in head and neck cancer. J. Clin. Oncol. 24, 2666–2672 (2006).
Bossi, P. et al. Prognostic and predictive value of EGFR in head and neck squamous cell carcinoma. Oncotarget 7, 74362–74379 (2016).
Ferris, R. L., Jaffee, E. M. & Ferrone, S. Tumor antigen-targeted, monoclonal antibody-based immunotherapy: clinical response, cellular immunity, and immunoescape. J. Clin. Oncol. 28, 4390–4399 (2010).
Vermorken, J. B. et al. Open-label, uncontrolled, multicenter phase II study to evaluate the efficacy and toxicity of cetuximab as a single agent in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck who failed to respond to platinum-based therapy. J. Clin. Oncol. 25, 2171–2177 (2007).
Burtness, B. et al. Phase III randomized trial of cisplatin plus placebo compared with cisplatin plus cetuximab in metastatic/recurrent head and neck cancer: an Eastern Cooperative Oncology Group study. J. Clin. Oncol. 23, 8646–8654 (2005).
Vermorken, J. B. et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N. Engl. J. Med. 359, 1116–1127 (2008).
Bonner, J. A. et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 354, 567–578 (2006).
Vermorken, J. B. et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. 14, 697–710 (2013).
Machiels, J. P. et al. Afatinib versus methotrexate as second-line treatment in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck progressing on or after platinum-based therapy (LUX-Head & Neck 1): an open-label, randomised phase 3 trial. Lancet Oncol. 16, 583–594 (2015).
Machiels, J. P. et al. Zalutumumab plus best supportive care versus best supportive care alone in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck after failure of platinum-based chemotherapy: an open-label, randomised phase 3 trial. Lancet Oncol. 12, 333–343 (2011).
Patil, V. M. et al. Results of a randomized phase III study of nimotuzumab in combination with concurrent radiotherapy and cisplatin versus radiotherapy and cisplatin alone, in locally advanced squamous cell carcinoma of the head and neck [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 6000 (2018).
Harari, P. M. et al. Postoperative chemoradiotherapy and cetuximab for high-risk squamous cell carcinoma of the head and neck: Radiation Therapy Oncology Group RTOG-0234. J. Clin. Oncol. 32, 2486–2495 (2014).
Chung, C. H. et al. Molecular classification of head and neck squamous cell carcinomas using patterns of gene expression. Cancer Cell 5, 489–500 (2004).
Cancer Genome Atlas Network. Comprehensive genomic characterization of head and neck squamous cell carcinomas. Nature 517, 576–582 (2015).
Agrawal, N. et al. Exome sequencing of head and neck squamous cell carcinoma reveals inactivating mutations in NOTCH1. Science 333, 1154–1157 (2011).
Keck, M. K. et al. Integrative analysis of head and neck cancer identifies two biologically distinct HPV and three non-HPV subtypes. Clin. Cancer Res. 21, 870–881 (2015).
Stransky, N. et al. The mutational landscape of head and neck squamous cell carcinoma. Science 333, 1157–1160 (2011).
Soulieres, D. et al. Buparlisib and paclitaxel in patients with platinum-pretreated recurrent or metastatic squamous cell carcinoma of the head and neck (BERIL-1): a randomised, double-blind, placebo-controlled phase 2 trial. Lancet Oncol. 18, 323–335 (2017).
Beck, T. N. et al. Phospho-T356RB1 predicts survival in HPV-negative squamous cell carcinoma of the head and neck. Oncotarget 6, 18863–18874 (2015).
Michel, L. et al. Phase I trial of palbociclib, a selective cyclin dependent kinase 4/6 inhibitor, in combination with cetuximab in patients with recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 58, 41–48 (2016).
Maxwell, J. H. et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastases and tumor recurrence. Clin. Cancer Res. 16, 1226–1235 (2010).
Gillison, M. L. et al. Tobacco smoking and increased risk of death and progression for patients with p16-positive and p16-negative oropharyngeal cancer. J. Clin. Oncol. 30, 2102–2111 (2012).
O’Sullivan, B. et al. Deintensification candidate subgroups in human papillomavirus-related oropharyngeal cancer according to minimal risk of distant metastasis. J. Clin. Oncol. 31, 543–550 (2013).
Marur, S. et al. E1308: phase II trial of induction chemotherapy followed by reduced-dose radiation and weekly cetuximab in patients with HPV-associated resectable squamous cell carcinoma of the oropharynx — ECOG-ACRIN Cancer Research Group. J. Clin. Oncol. 35, 490–497 (2017).
Gillison, M. L. et al. Radiotherapy plus cetuximab Zor cisplatin in human papillomavirus-positive oropharyngeal cancer (NRG Oncology RTOG 1016): a randomised, multicentre, non-inferiority trial. Lancet 393, P40–P50 (2018).
Hajek, M. et al. TRAF3/CYLD mutations identify a distinct subset of human papillomavirus-associated head and neck squamous cell carcinoma. Cancer 123, 1778–1790 (2017).
Chen, A. M. et al. Reduced-dose radiotherapy for human papillomavirus-associated squamous-cell carcinoma of the oropharynx: a single-arm, phase 2 study. Lancet Oncol. 18, 803–811 (2017).
Seiwert, T. Y. et al. OPTIMA: a phase II dose and volume de-escalation trial for human papillomavirus-positive oropharyngeal cancer. Ann. Oncol. 30, 297–302 (2019).
Hegde, J. V. et al. Patient-reported quality-of-life outcomes after de-escalated chemoradiation for human papillomavirus-positive oropharyngeal carcinoma: findings from a phase 2 trial. Cancer 124, 521–529 (2018).
Mehanna, H. et al. Radiotherapy plus cisplatin or cetuximab in low-risk human papillomavirus-positive oropharyngeal cancer (De-ESCALaTE HPV): an open-label randomised controlled phase 3 trial. Lancet 393, P51–P60 (2018).
Chera, B. S. et al. Mature results of a prospective study of deintensified chemoradiotherapy for low-risk human papillomavirus-associated oropharyngeal squamous cell carcinoma. Cancer 124, 2347–2354 (2018).
Evans, M. et al. PATHOS: a phase II/III trial of risk-stratified, reduced intensity adjuvant treatment in patients undergoing transoral surgery for human papillomavirus (HPV)-positive oropharyngeal cancer [abstract]. J. Clin. Oncol. 36 (Suppl. 15), TPS6097 (2018).
Nichols, A. C. et al. Early-stage squamous cell carcinoma of the oropharynx: radiotherapy versus trans-oral robotic surgery (ORATOR) — study protocol for a randomized phase II trial. BMC Cancer 13, 133 (2013).
Ferris, R. L. Immunology and immunotherapy of head and neck cancer. J. Clin. Oncol. 33, 3293–3304 (2015).
Ferris, R. L. et al. Nivolumab for recurrent squamous-cell carcinoma of the head and neck. N. Engl. J. Med. 375, 1856–1867 (2016).
Seiwert, T. Y. et al. Safety and clinical activity of pembrolizumab for treatment of recurrent or metastatic squamous cell carcinoma of the head and neck (KEYNOTE-012): an open-label, multicentre, phase 1b trial. Lancet Oncol. 17, 956–965 (2016).
Chow, L. Q. M. et al. Antitumor activity of pembrolizumab in biomarker-unselected patients with recurrent and/or metastatic head and neck squamous cell carcinoma: results from the phase Ib KEYNOTE-012 expansion cohort. J. Clin. Oncol. 34, 3838–3845 (2016).
Harrington, K. J. et al. Nivolumab versus standard, single-agent therapy of investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck (CheckMate 141): health-related quality-of-life results from a randomised, phase 3 trial. Lancet Oncol. 18, 1104–1115 (2017).
Bauml, J. et al. Pembrolizumab for platinum- and cetuximab-refractory head and neck cancer: results from a single-arm, phase II study. J. Clin. Oncol. 35, 1542–1549 (2017).
Cohen, E. E. W. et al. Pembrolizumab versus methotrexate, docetaxel, or cetuximab for recurrent or metastatic head-and-neck squamous cell carcinoma (KEYNOTE-040): a randomised, open-label, phase 3 study. Lancet 393, P156–P167 (2019).
Segal, N. S. et al. Safety and efficacy of MEDI4736, an anti-PD-L1 antibody, in patients from a squamous cell carcinoma of the head and neck (SCCHN) expansion cohort [abstract]. J. Clin. Oncol. 33 (Suppl. 15), 3011 (2015).
Wolchok, J. D. et al. Nivolumab plus ipilimumab in advanced melanoma. N. Engl. J. Med. 369, 122–133 (2013).
Siu, L. L. et al. Safety and efficacy of durvalumab with or without tremelimumab in patients with PD-L1-low/negative recurrent or metastatic HNSCC: the phase 2 CONDOR randomized clinical trial. JAMA Oncol. 5, 195–203 (2018).
Chen, D. S. & Mellman, I. Elements of cancer immunity and the cancer-immune set point. Nature 541, 321–330 (2017).
Concha-Benavente, F. et al. Identification of the cell-intrinsic and -extrinsic pathways downstream of EGFR and IFNγ that induce PD-L1 expression in head and neck cancer. Cancer Res. 76, 1031–1043 (2016).
Dong, H. et al. Tumor-associated B7-H1 promotes T cell apoptosis: a potential mechanism of immune evasion. Nat. Med. 8, 793–800 (2002).
Badoual, C. et al. PD-1-expressing tumor-infiltrating T cells are a favorable prognostic biomarker in HPV-associated head and neck cancer. Cancer Res. 73, 128–138 (2013).
Burtness, B. et al. KEYNOTE-048: phase III study of first-line pembrolizumab (P) for recurrent/metastatic head and neck squamous cell carcinoma (R/M HNSCC) [abstract LBA8_PR]. Ann. Oncol. 29 (Suppl. 8), mdy424.045 (2018).
Rizvi, N. A. et al. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015).
Alexandrov, L. B. et al. Signatures of mutational processes in human cancer. Nature 500, 415–421 (2013).
Antonia, S. J. et al. Overall survival with durvalumab after chemoradiotherapy in stage III NSCLC. N. Engl. J. Med. 379, 2342–2350 (2018).
McBride, S. M. et al. A phase II randomized trial of nivolumab with stereotactic body radiotherapy (SBRT) versus nivolumab alone in metastatic (M1) head and neck squamous cell carcinoma (HNSCC) [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 6009 (2018).
Schuler, P. J. et al. Phase I dendritic cell p53 peptide vaccine for head and neck cancer. Clin. Cancer Res. 20, 2433–2444 (2014).
Harrington, K. J. et al. Phase I/II study of oncolytic HSV GM-CSF in combination with radiotherapy and cisplatin in untreated stage III/IV squamous cell cancer of the head and neck. Clin. Cancer Res. 16, 4005–4015 (2010).
Massarelli, E. et al. Combining immune checkpoint blockade and tumor-specific vaccine for patients with incurable human papillomavirus 16-related cancer: a phase 2 clinical trial. JAMA Oncol. 5, 67–73 (2019).
Davis, R. J., Ferris, R. L. & Schmitt, N. C. Costimulatory and coinhibitory immune checkpoint receptors in head and neck cancer: unleashing immune responses through therapeutic combinations. Cancers Head Neck 1, 12 (2016).
Ferris, R. L. et al. Effect of adding motolimod to standard combination chemotherapy and cetuximab treatment of patients with squamous cell carcinoma of the head and neck: the Active8 randomized clinical trial. JAMA Oncol. 4, 1583–1588 (2018).
Ma, D. J. et al. Two-year results for MC1273, a phase 2 evaluation of aggressive dose de-escalation for adjuvant chemoradiation in HPV+ oropharynx squamous cell carcinoma (OPSCC). Int. J. Radiat. Oncol. Biol. Phys. 99, 1320 (2017).
Siegel, R. et al. Phase II study: induction chemotherapy and transoral surgery as definitive treatment (Tx) for locally advanced oropharyngeal squamous cell carcinoma (OPSCC): a novel approach [abstract]. J. Clin. Oncol. 36 (Suppl. 15), 6004 (2018).
Ferris, R. L. et al. Nivolumab versus investigator’s choice in recurrent or metastatic squamous cell carcinoma of the head and neck: 2-year long-term survival update of CheckMate 141 with analyses by tumor PD-L1 expression. Oral Oncol. 81, 45–51 (2018).
Zandberg, D. et al. Durvalumab for recurrent/metastatic (R/M) head and neck squamous cell carcinoma (HNSCC): preliminary results from a single-arm, phase 2 study [abstract 10420]. Ann. Oncol. 28, (Suppl. 5), v372–v394 (2017).
Acknowledgements
The work of R.L.F. was supported by the US NIH grant P50 CA097190 and the University of Pittsburgh Cancer Center support grant P30CA047904.
Reviewer information
Nature Reviews Clinical Oncology thanks A. Eisbruch and the other anonymous reviewer(s), for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors made a substantial contribution to all aspects of the preparation of the manuscript.
Corresponding author
Ethics declarations
Competing interests
B.B. has received compensation for consulting from Aduro, Alligator Biosciences, AstraZeneca, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, DebioPharma, Genentech–Roche, Merck and TRM Oncology, has been a non-paid consultant of Adlai Norte and Celldex, has received compensation for serving on data safety monitoring committees for IDDI (AstraZeneca–Medimmune) and VentiRx, and for medical education activities from CCO, Chemo Advisor, PER, Peerview, Practice Update, Prime Oncology and OncLive, and has received travel support from Boehringer Ingelheim and Merck. Q.T.L. has received travel support for serving on the advisory board of Bristol-Myers Squibb, serves as a non-paid consultant of Merck, and serves on the data safety monitoring committee for a clinical trial sponsored by Pfizer. R.L.F. has received compensation for consulting and/or advisory board membership from AstraZeneca–Medimmune, Bristol-Myers Squibb, Merck, PER, Pfizer, Regeneron, Tesaro and TTMS Therapeutics. Through contracts with the UPMC Hillman Cancer Center, his laboratory has also been funded to perform sponsored research by AstraZeneca–Medimmune, Bristol-Myers Squibb and Tesaro. J.D.C. declares no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Related links
ClinicalTrials.gov: https://clinicaltrials.gov
Rights and permissions
About this article
Cite this article
Cramer, J.D., Burtness, B., Le, Q. et al. The changing therapeutic landscape of head and neck cancer. Nat Rev Clin Oncol 16, 669–683 (2019). https://doi.org/10.1038/s41571-019-0227-z
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-019-0227-z
This article is cited by
-
Resistance of HNSCC cell models to pan-FGFR inhibition depends on the EMT phenotype associating with clinical outcome
Molecular Cancer (2024)
-
Role of primary tumor volume and metastatic lymph node volume in response to curative effect of definitive radiotherapy for locally advanced head and neck cancer
European Journal of Medical Research (2024)
-
Molecular subtype identification and prognosis stratification based on golgi apparatus-related genes in head and neck squamous cell carcinoma
BMC Medical Genomics (2024)
-
Comparative response to PDT with methyl-aminolevulinate and temoporfin in cutaneous and oral squamous cell carcinoma cells
Scientific Reports (2024)
-
TRIB3 promotes malignancy of head and neck squamous cell carcinoma via inhibiting ferroptosis
Cell Death & Disease (2024)