Abstract
Cancer stem cells (CSCs) have important roles in tumour development, relapse and metastasis; the intrinsic self-renewal characteristics and tumorigenic properties of these cells provide them with unique capabilities to resist diverse forms of anticancer therapy, seed recurrent tumours, and disseminate to and colonize distant tissues. The findings of several studies indicate that CSCs originate from non-malignant stem or progenitor cells. Accordingly, inhibition of developmental signalling pathways that are crucial for stem and progenitor cell homeostasis and function, such as the Notch, WNT, Hedgehog and Hippo signalling cascades, continues to be pursued across multiple cancer types as a strategy for targeting the CSCs hypothesized to drive cancer progression — with some success in certain malignancies. In addition, with the renaissance of anticancer immunotherapy, a better understanding of the interplay between CSCs and the tumour immune microenvironment might be the key to unlocking a new era of oncological treatments associated with a reduced propensity for the development of resistance and with enhanced antimetastatic activity, thus ultimately resulting in improved patient outcomes. Herein, we provide an update on the progress to date in the clinical development of therapeutics targeting the Notch, WNT, Hedgehog and Hippo pathways. We also discuss the interactions between CSCs and the immune system, including the potential immunological effects of agents targeting CSC-associated developmental signalling pathways, and provide an overview of the emerging approaches to CSC-targeted immunotherapy.
Key points
The results of laboratory-based research have demonstrated the fundamental roles of cancer stem cells (CSCs) in mediating the resistance of cancers to therapy, tumour recurrence and metastasis.
CSCs harbour important alterations affecting developmental signalling pathways that control cell renewal and differentiation, including the Notch, WNT and Hedgehog pathways.
Crosstalk with the WNT pathways has led to the molecular characterization of abnormalities in the core components of the Hippo pathway in multiple malignancies; transcriptional co-activation or co-repression by the Hippo pathway effectors YAP1 and TAZ serves as an important regulatory hub for multiple pathways and has potential context-dependent oncogene or tumour suppressor activity.
Multiple clinical trials using single agents and combination therapies have been performed to study the safety and efficacy of targeting CSCs via the inhibition of these development pathways.
Various interactions of CSCs with the tumour immune microenvironment result in evasion of CSC detection by the immune system and are therefore key candidates for novel approaches to immunotherapy.
Further improvements in targeting CSCs as a specific population are fundamental to improving the efficacy of new anticancer therapies.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Lapidot, T. et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature 367, 645–648 (1994).
Vermeulen, L., Sprick, M. R., Kemper, K., Stassi, G. & Medema, J. P. Cancer stem cells — old concepts, new insights. Cell Death Differ. 15, 947–958 (2008).
Espinoza, I. & Miele, L. Deadly crosstalk: notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 341, 41–45 (2013).
Pattabiraman, D. R. & Weinberg, R. A. Tackling the cancer stem cells — what challenges do they pose? Nat. Rev. Drug Discov. 13, 497–512 (2014).
Ge, Y. et al. Stem cell lineage infidelity drives wound repair and cancer. Cell 169, 636–650.e14 (2017).
Visvader, J. E. & Lindeman, G. J. Cancer stem cells: current status and evolving complexities. Cell Stem Cell 10, 717–728 (2012).
Ishizawa, K. et al. Tumor-initiating cells are rare in many human tumors. Cell Stem Cell 7, 279–282 (2010).
Sachs, N. & Clevers, H. Organoid cultures for the analysis of cancer phenotypes. Curr. Opin. Genet. Dev. 24, 68–73 (2014).
Schulenburg, A. et al. Cancer stem cells in basic science and in translational oncology: can we translate into clinical application? J. Hematol. Oncol. 8, 16 (2015).
Zhou, B. B. et al. Tumour-initiating cells: challenges and opportunities for anticancer drug discovery. Nat. Rev. Drug Discov. 8, 806–823 (2009).
Bao, S. et al. Glioma stem cells promote radioresistance by preferential activation of the DNA damage response. Nature 444, 756–760 (2006).
Li, X. et al. Intrinsic resistance of tumorigenic breast cancer cells to chemotherapy. J. Natl Cancer Inst. 100, 672–679 (2008).
Plaks, V., Kong, N. & Werb, Z. The cancer stem cell niche: how essential is the niche in regulating stemness of tumor cells? Cell Stem Cell 16, 225–238 (2015).
Wiechert, A. et al. Cisplatin induces stemness in ovarian cancer. Oncotarget 7, 30511–30522 (2016).
Saygin, C., Matei, D., Majeti, R., Reizes, O. & Lathia, J. D. Targeting cancer stemness in the clinic: from hype to hope. Cell Stem Cell 24, 25–40 (2019).
Nishio, M., Otsubo, K., Maehama, T., Mimori, K. & Suzuki, A. Capturing the mammalian Hippo: elucidating its role in cancer. Cancer Sci. 104, 1271–1277 (2013).
Pelullo, M. et al. Wnt, Notch, and TGF-β pathways impinge on Hedgehog signaling complexity: an open window on cancer. Front. Genet. 10, 711 (2019).
Korkaya, H., Paulson, A., Iovino, F. & Wicha, M. S. HER2 regulates the mammary stem/progenitor cell population driving tumorigenesis and invasion. Oncogene 27, 6120–6130 (2008).
Dai, M. et al. CDK4 regulates cancer stemness and is a novel therapeutic target for triple-negative breast cancer. Sci. Rep. 6, 35383 (2016).
Venkatesh, V. et al. Targeting Notch signalling pathway of cancer stem cells. Stem Cell Investig. 5, 5 (2018).
Gomez-del Arco, P. et al. Alternative promoter usage at the Notch1 locus supports ligand-independent signaling in T cell development and leukemogenesis. Immunity 33, 685–698 (2010).
Andersson, E. R., Sandberg, R. & Lendahl, U. Notch signaling: simplicity in design, versatility in function. Development 138, 3593–3612 (2011).
Thompson, B. J. et al. The SCFFBW7 ubiquitin ligase complex as a tumor suppressor in T cell leukemia. J. Exp. Med. 204, 1825–1835 (2007).
Weng, A. P. et al. Activating mutations of NOTCH1 in human T cell acute lymphoblastic leukemia. Science 306, 269–271 (2004).
Qiao, L. & Wong, B. C. Role of Notch signaling in colorectal cancer. Carcinogenesis 30, 1979–1986 (2009).
Kannan, S. et al. Notch activation inhibits AML growth and survival: a potential therapeutic approach. J. Exp. Med. 210, 321–337 (2013).
Pandya, K. et al. Targeting both Notch and ErbB-2 signalling pathways is required for prevention of ErbB-2-positive breast tumour recurrence. Br. J. Cancer 105, 796–806 (2011).
Samon, J. B. et al. Preclinical analysis of the γ-secretase inhibitor PF-03084014 in combination with glucocorticoids in T-cell acute lymphoblastic leukemia. Mol. Cancer Ther. 11, 1565–1575 (2012).
Wei, P. et al. Evaluation of selective γ-secretase inhibitor PF-03084014 for its antitumor efficacy and gastrointestinal safety to guide optimal clinical trial design. Mol. Cancer Ther. 9, 1618–1628 (2010).
Sosa Iglesias, V. et al. Synergistic effects of NOTCH/γ-secretase inhibition and standard of care treatment modalities in non-small cell lung cancer cells. Front. Oncol. 8, 460 (2018).
Morgan, K. M. et al. Gamma secretase inhibition by BMS-906024 enhances efficacy of paclitaxel in lung adenocarcinoma. Mol. Cancer Ther. 16, 2759–2769 (2017).
Messersmith, W. A. et al. A phase I, dose-finding study in patients with advanced solid malignancies of the oral γ-secretase inhibitor PF-03084014. Clin. Cancer Res. 21, 60–67 (2015).
Kummar, S. et al. Clinical activity of the γ-secretase inhibitor PF-03084014 in adults with desmoid tumors (aggressive fibromatosis). J. Clin. Oncol. 35, 1561–1569 (2017).
Papayannidis, C. et al. A phase 1 study of the novel γ-secretase inhibitor PF-03084014 in patients with T-cell acute lymphoblastic leukemia and T-cell lymphoblastic lymphoma. Blood Cancer J. 5, e350 (2015).
Zweidler-McKay, P. A. et al. The safety and activity of BMS-906024, a gamma secretase inhibitor (GSI) with anti-notch activity, in patients with relapsed T-cell acute lymphoblastic leukemia (T-ALL): initial results of a phase 1 trial. Blood 124, 968 (2014).
Cook, N. et al. A phase I trial of the γ-secretase inhibitor MK-0752 in combination with gemcitabine in patients with pancreatic ductal adenocarcinoma. Br. J. Cancer 118, 793–801 (2018).
Kappes, D. J., He, X. & He, X. CD4-CD8 lineage commitment: an inside view. Nat. Immunol. 6, 761–766 (2005).
Yun, J. et al. Crosstalk between PKCα and Notch-4 in endocrine-resistant breast cancer cells. Oncogenesis 2, e60 (2013).
Kuhnert, F., Kirshner, J. R. & Thurston, G. Dll4-Notch signaling as a therapeutic target in tumor angiogenesis. Vasc. Cell 3, 20 (2011).
Gu, J. W. et al. Notch signals in the endothelium and cancer "stem-like" cells: opportunities for cancer therapy. Vasc. Cell 4, 7 (2012).
Huang, J. et al. Dll4 inhibition plus aflibercept markedly reduces ovarian tumor growth. Mol. Cancer Ther. 15, 1344–1352 (2016).
Miles, K. M. et al. Dll4 blockade potentiates the anti-tumor effects of VEGF inhibition in renal cell carcinoma patient-derived xenografts. PLOS ONE 9, e112371 (2014).
Chiorean, E. G. et al. A phase I first-in-human study of enoticumab (REGN421), a fully human delta-like ligand 4 (Dll4) monoclonal antibody in patients with advanced solid tumors. Clin. Cancer Res. 21, 2695–2703 (2015).
McKeage, M. J. et al. Phase Ib trial of the anti-cancer stem cell DLL4-binding agent demcizumab with pemetrexed and carboplatin as first-line treatment of metastatic non-squamous NSCLC. Target Oncol. 13, 89–98 (2018).
Hughes, B. et al. Abstract A084: DENALI: a 3-arm double-blind randomized phase 2 study of carboplatin, pemetrexed, and placebo (CPP) versus carboplatin, pemetrexed, and either 1 or 2 truncated courses of demcizumab (CPD) in patients with non-squamous non-small cell lung cancer (NSCLC). Mol. Cancer Ther. 17, A084 (2018).
Hidalgo, M. et al. A phase 1b study of the anti-cancer stem cell agent demcizumab (DEM) and gemcitabine (GEM) +/- nab-paclitaxel in patients with pancreatic cancer. J. Clin. Oncol. 34, 341 (2016).
Cubillo Gracian, A. et al. 620PDYOSEMITE: a 3 arm double-blind randomized phase 2 study of gemcitabine, paclitaxel protein-bound particles for injectable suspension, and placebo (GAP) versus gemcitabine, paclitaxel protein-bound particles for injectable suspension and either 1 or 2 truncated courses of demcizumab (GAD). Ann. Oncol. 28 (Suppl. 5), mdx369.004 (2017).
Rudin, C. M. et al. Rovalpituzumab tesirine, a DLL3-targeted antibody–drug conjugate, in recurrent small-cell lung cancer: a first-in-human, first-in-class, open-label, phase 1 study. Lancet Oncol. 18, 42–51 (2017).
Carbone, D. P. et al. Efficacy and safety of rovalpituzumab tesirine in patients with DLL3-expressing, ≥ 3rd line small cell lung cancer: results from the phase 2 TRINITY study. J. Clin. Oncol. 36, 8507 (2018).
Casulo, C. et al. Safety and preliminary efficacy results of a phase I first-in-human study of the novel Notch-1 targeting antibody brontictuzumab (OMP-52M51) administered intravenously to patients with hematologic malignancies. Blood 128, 5108 (2016).
Ferrarotto, R. et al. A phase I dose-escalation and dose-expansion study of brontictuzumab in subjects with selected solid tumors. Ann. Oncol. 29, 1561–1568 (2018).
Pietanza, M. C. et al. Final results of phase Ib of tarextumab (TRXT, OMP-59R5, anti-Notch2/3) in combination with etoposide and platinum (EP) in patients (pts) with untreated extensive-stage small-cell lung cancer (ED-SCLC). J. Clin. Oncol. 33, 7508 (2015).
Hu, Z. I. et al. A randomized phase II trial of nab-paclitaxel and gemcitabine with tarextumab or placebo in patients with untreated metastatic pancreatic cancer. Cancer Med. 8, 5148–5157 (2019).
Weber, D. et al. 66P Pharmacological activity of CB-103: an oral pan-NOTCH inhibitor targeting the NOTCH transcription complex. Ann. Oncol. 29, mdy047.015 (2018).
Astudillo, L. et al. The small molecule IMR-1 inhibits the notch transcriptional activation complex to suppress tumorigenesis. Cancer Res. 76, 3593–3603 (2016).
Westhoff, B. et al. Alterations of the Notch pathway in lung cancer. Proc. Natl Acad. Sci. USA 106, 22293–22298 (2009).
Pine, S. R. Rethinking γ-secretase inhibitors for treatment of non-small cell lung cancer: is Notch the target? Clin. Cancer Res. 24, 6136–6141 (2018).
Katoh, M. & Katoh, M. Molecular genetics and targeted therapy of WNT-related human diseases (review). Int. J. Mol. Med. 40, 587–606 (2017).
Zhan, T., Rindtorff, N. & Boutros, M. Wnt signaling in cancer. Oncogene 36, 1461–1473 (2017).
Vermeulen, L. et al. Wnt activity defines colon cancer stem cells and is regulated by the microenvironment. Nat. Cell Biol. 12, 468–476 (2010).
Giannakis, M. et al. RNF43 is frequently mutated in colorectal and endometrial cancers. Nat. Genet. 46, 1264–1266 (2014).
Zhang, Y. et al. Canonical Wnt signaling is required for pancreatic carcinogenesis. Cancer Res. 73, 4909–4922 (2013).
Morris, J. P. 4th, Cano, D. A., Sekine, S., Wang, S. C. & Hebrok, M. β-catenin blocks Kras-dependent reprogramming of acini into pancreatic cancer precursor lesions in mice. J. Clin. Invest. 120, 508–520 (2010).
Jiang, X. et al. Inactivating mutations of RNF43 confer Wnt dependency in pancreatic ductal adenocarcinoma. Proc. Natl Acad. Sci. USA 110, 12649–12654 (2013).
Seshagiri, S. et al. Recurrent R-spondin fusions in colon cancer. Nature 488, 660–664 (2012).
Llovet, J. M., Montal, R., Sia, D. & Finn, R. S. Molecular therapies and precision medicine for hepatocellular carcinoma. Nat. Rev. Clin. Oncol. 15, 599–616 (2018).
Palacios, J. & Gamallo, C. Mutations in the β-catenin gene (CTNNB1) in endometrioid ovarian carcinomas. Cancer Res. 58, 1344–1347 (1998).
Morin, P. J. et al. Activation of β-catenin-Tcf signaling in colon cancer by mutations in β-catenin or APC. Science 275, 1787–1790 (1997).
Rubinfeld, B. et al. Stabilization of β-catenin by genetic defects in melanoma cell lines. Science 275, 1790–1792 (1997).
Delmas, V. et al. β-catenin induces immortalization of melanocytes by suppressing p16 INK4a expression and cooperates with N-Ras in melanoma development. Genes Dev. 21, 2923–2935 (2007).
Chien, A. J. et al. Targeted BRAF inhibition impacts survival in melanoma patients with high levels of Wnt/β-catenin signaling. PLOS ONE 9, e94748 (2014).
Khramtsov, A. I. et al. Wnt/β-catenin pathway activation is enriched in basal-like breast cancers and predicts poor outcome. Am. J. Pathol. 176, 2911–2920 (2010).
Miranda Kuzan-Fischer, C., Juraschka, K. & Taylor, M. D. Medulloblastoma in the molecular era. J. Korean Neurosurg. Soc. 61, 292–301 (2018).
Yeung, J. et al. β-Catenin mediates the establishment and drug resistance of MLL leukemic stem cells. Cancer Cell 18, 606–618 (2010).
Lane, S. W. et al. Differential niche and Wnt requirements during acute myeloid leukemia progression. Blood 118, 2849–2856 (2011).
Muller-Tidow, C. et al. Translocation products in acute myeloid leukemia activate the Wnt signaling pathway in hematopoietic cells. Mol. Cell Biol. 24, 2890–2904 (2004).
Griffiths, E. A. et al. Acute myeloid leukemia is characterized by Wnt pathway inhibitor promoter hypermethylation. Leuk. Lymphoma 51, 1711–1719 (2010).
Valencia, A. et al. Wnt signaling pathway is epigenetically regulated by methylation of Wnt antagonists in acute myeloid leukemia. Leukemia 23, 1658–1666 (2009).
van Andel, H., Kocemba, K. A., Spaargaren, M. & Pals, S. T. Aberrant Wnt signaling in multiple myeloma: molecular mechanisms and targeting options. Leukemia 33, 1063–1075 (2019).
Chim, C. S., Pang, R., Fung, T. K., Choi, C. L. & Liang, R. Epigenetic dysregulation of Wnt signaling pathway in multiple myeloma. Leukemia 21, 2527–2536 (2007).
Kocemba, K. A. et al. Transcriptional silencing of the Wnt-antagonist DKK1 by promoter methylation is associated with enhanced Wnt signaling in advanced multiple myeloma. PLOS ONE 7, e30359 (2012).
Zhao, C. et al. Loss of β-catenin impairs the renewal of normal and CML stem cells in vivo. Cancer Cell 12, 528–541 (2007).
Gregory, M. A. et al. Wnt/Ca2+/NFAT signaling maintains survival of Ph+ leukemia cells upon inhibition of Bcr-Abl. Cancer Cell 18, 74–87 (2010).
Heidel, F. H. et al. Genetic and pharmacologic inhibition of β-catenin targets imatinib-resistant leukemia stem cells in CML. Cell Stem Cell 10, 412–424 (2012).
Guo, W. et al. Multi-genetic events collaboratively contribute to Pten-null leukaemia stem-cell formation. Nature 453, 529–533 (2008).
Kaveri, D. et al. β-Catenin activation synergizes with Pten loss and Myc overexpression in Notch-independent T-ALL. Blood 122, 694–704 (2013).
Moskalev, E. A. et al. Concurrent epigenetic silencing of Wnt/β-catenin pathway inhibitor genes in B cell chronic lymphocytic leukaemia. BMC Cancer 12, 213 (2012).
Wang, L. et al. Somatic mutation as a mechanism of Wnt/β-catenin pathway activation in CLL. Blood 124, 1089–1098 (2014).
Kagey, M. H. & He, X. Rationale for targeting the Wnt signalling modulator Dickkopf-1 for oncology. Br. J. Pharmacol. 174, 4637–4650 (2017).
Yamabuki, T. et al. Dikkopf-1 as a novel serologic and prognostic biomarker for lung and esophageal carcinomas. Cancer Res. 67, 2517–2525 (2007).
Ryan, D. P. et al. Current results of a phase I study of DKN-01, an anti-DKK1 antibody, in combination with paclitaxel (P) in patients (pts) with advanced DKK1+ esophageal cancer (EC) or gastro-esophageal junction tumors (GEJ). J. Clin. Oncol. 34, e15525 (2016).
Jimeno, A. et al. A first-in-human phase I study of the anticancer stem cell agent ipafricept (OMP-54F28), a decoy receptor for Wnt ligands, in patients with advanced solid tumors. Clin. Cancer Res. 23, 7490–7497 (2017).
Gurney, A. et al. Wnt pathway inhibition via the targeting of Frizzled receptors results in decreased growth and tumorigenicity of human tumors. Proc. Natl Acad. Sci. USA 109, 11717–11722 (2012).
Smith, D. C. et al. First-in-human evaluation of the human monoclonal antibody vantictumab (OMP-18R5; anti-Frizzled) targeting the WNT pathway in a phase I study for patients with advanced solid tumors. J. Clin. Oncol. 31, 2540 (2013).
Messersmith, W. et al. Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with nab-paclitaxel (Nab-P) and gemcitabine (G) in patients (pts) with previously untreated stage IV pancreatic cancer (PC). Ann. Oncol. 27, 677P (2016).
Mita, M. M. et al. Phase 1b study of WNT inhibitor vantictumab (VAN, human monoclonal antibody) with paclitaxel (P) in patients (pts) with 1st- to 3rd-line metastatic HER2-negative breast cancer (BC). J. Clin. Oncol. 34, 2516 (2016).
Karvonen, H. et al. Wnto5a and ROR1 activate non-canonical Wnt signaling via RhoA in TCF3-PBX1 acute lymphoblastic leukemia and highlight new treatment strategies via Bcl-2 co-targeting. Oncogene 38, 3288–3300 (2019).
Choi, M. Y. et al. Phase I trial: cirmtuzumab inhibits ROR1 signaling and stemness signatures in patients with chronic lymphocytic leukemia. Cell Stem Cell 22, 951–959.e3 (2018).
Yu, J. et al. Cirmtuzumab inhibits Wnt5a-induced Rac1 activation in chronic lymphocytic leukemia treated with ibrutinib. Leukemia 31, 1333–1339 (2017).
Soerensen, P. G. et al. Phase I dose-escalating study to evaluate the safety, tolerability, and pharmacokinetic and pharmacodynamic profiles of Foxy-5 in patients with metastatic breast, colorectal, or prostate cancer. J. Clin. Oncol. 32, TPS1140 (2014).
Pai, S. G. et al. Wnt/β-catenin pathway: modulating anticancer immune response. J. Hematol. Oncol. 10, 101 (2017).
DeBruine, Z. J. et al. Wnt5a promotes Frizzled-4 signalosome assembly by stabilizing cysteine-rich domain dimerization. Genes Dev. 31, 916–926 (2017).
Agarwal, P. et al. Enhanced targeting of CML stem and progenitor cells by inhibition of porcupine acyltransferase in combination with TKI. Blood 129, 1008–1020 (2017).
Janku, F. et al. Abstract C45: Phase I study of WNT974, a first-in-class Porcupine inhibitor, in advanced solid tumors. Mol. Cancer Ther. 14, C45 (2015).
Yoon, S.-S. et al. Novel phase 1a/1b dose-finding study design of CWP232291 (CWP291) in relapsed or refractory myeloma (MM). J. Clin. Oncol. 35, TPS8058 (2017).
Manasanch, E. E. et al. Interim results from the phase 1a/1b dose-finding study of CWP232291 (CWP291) in relapsed or refractory myeloma (RRMM) alone or in combination with lenalidomide and dexamethasone. Blood 130, 3091 (2017).
Cortes, J. E. et al. Phase 1 study of CWP232291 in relapsed/refractory acute myeloid leukemia (AML) and myelodysplastic syndrome (MDS). J. Clin. Oncol. 33, 7044 (2015).
Menon, M. et al. A novel tankyrase inhibitor, MSC2504877, enhances the effects of clinical CDK4/6 inhibitors. Sci. Rep. 9, 201 (2019).
Wang, W. et al. Tankyrase inhibitors target YAP by stabilizing angiomotin family proteins. Cell Rep. 13, 524–532 (2015).
Schoumacher, M. et al. Inhibiting tankyrases sensitizes KRAS-mutant cancer cells to MEK inhibitors via FGFR2 feedback signaling. Cancer Res. 74, 3294–3305 (2014).
Plummer, E. R. et al. First-in-human phase 1 study of the PARP/tankyrase inhibitor 2X-121 (E7449) as monotherapy in patients with advanced solid tumors and validation of a novel drug response predictor (DRP) mRNA biomarker. J. Clin. Oncol. 36, 2505 (2018).
Ko, A. H. et al. Final results of a phase Ib dose-escalation study of PRI-724, a CBP/beta-catenin modulator, plus gemcitabine (GEM) in patients with advanced pancreatic adenocarcinoma (APC) as second-line therapy after FOLFIRINOX or FOLFOX. J. Clin. Oncol. 34, e15721 (2016).
Yamada, K. et al. Abstract 2927: antitumor and antiangiogenesis activities of E7386, an orally active CBP/β-catenin modulator, as a single agent and in combination with lenvatinib in human HCC xenograft models. Cancer Res. 78, 2927 (2018).
Steg, A. D. et al. Smoothened antagonists reverse taxane resistance in ovarian cancer. Mol. Cancer Ther. 11, 1587–1597 (2012).
Lim, Y. & Matsui, W. Hedgehog signaling in hematopoiesis. Crit. Rev. Eukaryot. Gene Expr. 20, 129–139 (2010).
Theunissen, J. W. & de Sauvage, F. J. Paracrine Hedgehog signaling in cancer. Cancer Res. 69, 6007–6010 (2009).
Murone, M., Rosenthal, A. & de Sauvage, F. J. Hedgehog signal transduction: from flies to vertebrates. Exp. Cell Res. 253, 25–33 (1999).
Pasca di Magliano, M. & Hebrok, M. Hedgehog signalling in cancer formation and maintenance. Nat. Rev. Cancer 3, 903–911 (2003).
Ng, J. M. & Curran, T. The Hedgehog's tale: developing strategies for targeting cancer. Nat. Rev. Cancer 11, 493–501 (2011).
Amakye, D., Jagani, Z. & Dorsch, M. Unraveling the therapeutic potential of the Hedgehog pathway in cancer. Nat. Med. 19, 1410–1422 (2013).
Rudin, C. M. et al. Treatment of medulloblastoma with Hedgehog pathway inhibitor GDC-0449. N. Engl. J. Med. 361, 1173–1178 (2009).
Aberger, F., Hutterer, E., Sternberg, C., Del Burgo, P. J. & Hartmann, T. N. Acute myeloid leukemia — strategies and challenges for targeting oncogenic Hedgehog/GLI signaling. Cell Commun. Signal. 15, 8 (2017).
Queiroz, K. C. et al. Hedgehog signaling maintains chemoresistance in myeloid leukemic cells. Oncogene 29, 6314–6322 (2010).
Lim, Y. et al. Integration of Hedgehog and mutant FLT3 signaling in myeloid leukemia. Sci. Transl Med. 7, 291ra296 (2015).
Dierks, C. et al. Expansion of Bcr-Abl-positive leukemic stem cells is dependent on Hedgehog pathway activation. Cancer Cell 14, 238–249 (2008).
Jorgensen, H. G., Allan, E. K., Jordanides, N. E., Mountford, J. C. & Holyoake, T. L. Nilotinib exerts equipotent antiproliferative effects to imatinib and does not induce apoptosis in CD34+ CML cells. Blood 109, 4016–4019 (2007).
Peacock, C. D. et al. Hedgehog signaling maintains a tumor stem cell compartment in multiple myeloma. Proc. Natl Acad. Sci. USA 104, 4048–4053 (2007).
Hegde, G. V. et al. Hedgehog-induced survival of B-cell chronic lymphocytic leukemia cells in a stromal cell microenvironment: a potential new therapeutic target. Mol. Cancer Res. 6, 1928–1936 (2008).
Desch, P. et al. Inhibition of GLI, but not Smoothened, induces apoptosis in chronic lymphocytic leukemia cells. Oncogene 29, 4885–4895 (2010).
Kim, J. E. et al. Sonic Hedgehog signaling proteins and ATP-binding cassette G2 are aberrantly expressed in diffuse large B-cell lymphoma. Mod. Pathol. 22, 1312–1320 (2009).
Singh, R. R. et al. ABCG2 is a direct transcriptional target of Hedgehog signaling and involved in stroma-induced drug tolerance in diffuse large B-cell lymphoma. Oncogene 30, 4874–4886 (2011).
Yang, L., Xie, G., Fan, Q. & Xie, J. Activation of the Hedgehog-signaling pathway in human cancer and the clinical implications. Oncogene 29, 469–481 (2010).
Sekulic, A. et al. Efficacy and safety of vismodegib in advanced basal-cell carcinoma. N. Engl. J. Med. 366, 2171–2179 (2012).
Basset-Seguin, N. et al. Vismodegib in patients with advanced basal cell carcinoma: primary analysis of STEVIE, an international, open-label trial. Eur. J. Cancer 86, 334–348 (2017).
Lear, J. T. et al. Long-term efficacy and safety of sonidegib in patients with locally advanced and metastatic basal cell carcinoma: 30-month analysis of the randomized phase 2 BOLT study. J. Eur. Acad. Dermatol. Venereol. 32, 372–381 (2018).
Xie, P. & Lefrancois, P. Efficacy, safety, and comparison of sonic Hedgehog inhibitors in basal cell carcinomas: a systematic review and meta-analysis. J. Am. Acad. Dermatol. 79, 1089–1100.e17 (2018).
Bendell, J. et al. Phase I study of LY2940680, a SMO antagonist, in patients with advanced cancer including treatment-naive and previously treated basal cell carcinoma. Clin. Cancer Res. 24, 2082–2091 (2018).
Berlin, J. et al. A randomized phase II trial of vismodegib versus placebo with FOLFOX or FOLFIRI and bevacizumab in patients with previously untreated metastatic colorectal cancer. Clin. Cancer Res. 19, 258–267 (2013).
Catenacci, D. V. T. et al. Final analysis of a phase IB/randomized phase II study of gemcitabine (G) plus placebo (P) or vismodegib (V), a Hedgehog (Hh) pathway inhibitor, in patients (pts) with metastatic pancreatic cancer (PC): A University of Chicago phase II consortium study. J. Clin. Oncol. 31, 4012 (2013).
Cohen, D. J. et al. Vismodegib (V), a Hedgehog (HH) pathway inhibitor, combined with FOLFOX for first-line therapy of patients (pts) with advanced gastric and gastroesophageal junction (GEJ) carcinoma: a New York cancer consortium led phase II randomized study. J. Clin. Oncol. 31, 4011 (2013).
Kaye, S. B. et al. A phase II, randomized, placebo-controlled study of vismodegib as maintenance therapy in patients with ovarian cancer in second or third complete remission. Clin. Cancer Res. 18, 6509–6518 (2012).
Robinson, G. W. et al. Vismodegib exerts targeted efficacy against recurrent sonic Hedgehog-subgroup medulloblastoma: results from phase ii pediatric brain tumor consortium studies PBTC-025B and PBTC-032. J. Clin. Oncol. 33, 2646–2654 (2015).
Gajjar, A. et al. Phase I study of vismodegib in children with recurrent or refractory medulloblastoma: a pediatric brain tumor consortium study. Clin. Cancer Res. 19, 6305–6312 (2013).
Petrirena, G. J. et al. Recurrent extraneural sonic Hedgehog medulloblastoma exhibiting sustained response to vismodegib and temozolomide monotherapies and inter-metastatic molecular heterogeneity at progression. Oncotarget 9, 10175–10183 (2018).
Bowles, D. W. et al. A pilot study of cetuximab and the Hedgehog inhibitor IPI-926 in recurrent/metastatic head and neck squamous cell carcinoma. Oral Oncol. 53, 74–79 (2016).
Rudin, C. M. et al. A phase I study of IPI-926, a novel Hedgehog pathway inhibitor, in patients (pts) with advanced or metastatic solid tumors. J. Clin. Oncol. 29, 3014 (2011).
Cortes, J. E. et al. Randomized comparison of low dose cytarabine with or without glasdegib in patients with newly diagnosed acute myeloid leukemia or high-risk myelodysplastic syndrome. Leukemia 33, 379–389 (2019).
Cortes, J. E. et al. Glasdegib in combination with cytarabine and daunorubicin in patients with AML or high-risk MDS: phase 2 study results. Am. J. Hematol. 93, 1301–1310 (2018).
Kim, J. et al. Itraconazole, a commonly used antifungal that inhibits Hedgehog pathway activity and cancer growth. Cancer Cell 17, 388–399 (2010).
Antonarakis, E. S. et al. Repurposing itraconazole as a treatment for advanced prostate cancer: a noncomparative randomized phase II trial in men with metastatic castration-resistant prostate cancer. Oncologist 18, 163–173 (2013).
Tsubamoto, H. et al. Repurposing itraconazole as an anticancer agent. Oncol. Lett. 14, 1240–1246 (2017).
Inoue, K., Tsubamoto, H., Isono-Nakata, R., Sakata, K. & Nakagomi, N. Itraconazole treatment of primary malignant melanoma of the vagina evaluated using positron emission tomography and tissue cDNA microarray: a case report. BMC Cancer 18, 630 (2018).
Ueda, T. et al. Itraconazole modulates Hedgehog, WNT/β-catenin, as well as Akt signalling, and inhibits proliferation of cervical cancer cells. Anticancer Res. 37, 3521–3526 (2017).
Tsubamoto, H., Sonoda, T. & Inoue, K. Impact of itraconazole on the survival of heavily pre-treated patients with triple-negative breast cancer. Anticancer Res. 34, 3839–3844 (2014).
Tsubamoto, H. et al. Combination chemotherapy with itraconazole for treating metastatic pancreatic cancer in the second-line or additional setting. Anticancer Res. 35, 4191–4196 (2015).
Rudin, C. M. et al. Phase 2 study of pemetrexed and itraconazole as second-line therapy for metastatic nonsquamous non-small-cell lung cancer. J. Thorac. Oncol. 8, 619–623 (2013).
Atwood, S. X. et al. Smoothened variants explain the majority of drug resistance in basal cell carcinoma. Cancer Cell 27, 342–353 (2015).
Sharpe, H. J. et al. Genomic analysis of Smoothened inhibitor resistance in basal cell carcinoma. Cancer Cell 27, 327–341 (2015).
Huang, L., Walter, V., Hayes, D. N. & Onaitis, M. Hedgehog-GLI signaling inhibition suppresses tumor growth in squamous lung cancer. Clin. Cancer Res. 20, 1566–1575 (2014).
Didiasova, M. et al. Pirfenidone exerts antifibrotic effects through inhibition of GLI transcription factors. FASEB J. 31, 1916–1928 (2017).
Polydorou, C., Mpekris, F., Papageorgis, P., Voutouri, C. & Stylianopoulos, T. Pirfenidone normalizes the tumor microenvironment to improve chemotherapy. Oncotarget 8, 24506–24517 (2017).
Zou, W. J. et al. Pirfenidone inhibits proliferation and promotes apoptosis of hepatocellular carcinoma cells by inhibiting the Wnt/β-catenin signaling pathway. Med. Sci. Monit. 23, 6107–6113 (2017).
Chen, Q. et al. Down-regulation of Gli transcription factor leads to the inhibition of migration and invasion of ovarian cancer cells via integrin β4-mediated FAK signaling. PLOS ONE 9, e88386 (2014).
Srivastava, R. K. et al. GLI inhibitor GANT-61 diminishes embryonal and alveolar rhabdomyosarcoma growth by inhibiting Shh/AKT-mTOR axis. Oncotarget 5, 12151–12165 (2014).
Lauth, M., Bergstrom, A., Shimokawa, T. & Toftgard, R. Inhibition of GLI-mediated transcription and tumor cell growth by small-molecule antagonists. Proc. Natl Acad. Sci. USA 104, 8455–8460 (2007).
Hou, X. et al. Inhibition of Hedgehog signaling by GANT58 induces apoptosis and shows synergistic antitumor activity with AKT inhibitor in acute T cell leukemia cells. Biochimie 101, 50–59 (2014).
Beauchamp, E. M. et al. Arsenic trioxide inhibits human cancer cell growth and tumor development in mice by blocking Hedgehog/GLI pathway. J. Clin. Invest. 121, 148–160 (2011).
Ally, M. S. et al. Effects of combined treatment with arsenic trioxide and itraconazole in patients with refractory metastatic basal cell carcinoma. JAMA Dermatol. 152, 452–456 (2016).
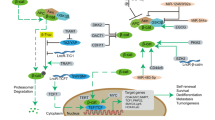
Huang, J., Wu, S., Barrera, J., Matthews, K. & Pan, D. The Hippo signaling pathway coordinately regulates cell proliferation and apoptosis by inactivating Yorkie, the Drosophila homolog of YAP. Cell 122, 421–434 (2005).
Yu, F. X., Zhao, B. & Guan, K. L. Hippo pathway in organ size control, tissue homeostasis, and cancer. Cell 163, 811–828 (2015).
Panciera, T. et al. Induction of expandable tissue-specific stem/progenitor cells through transient expression of YAP/TAZ. Cell Stem Cell 19, 725–737 (2016).
Andl, T., Zhou, L., Yang, K., Kadekaro, A. L. & Zhang, Y. YAP and WWTR1: new targets for skin cancer treatment. Cancer Lett. 396, 30–41 (2017).
Zhang, Y. et al. VGLL4 selectively represses YAP-dependent gene induction and tumorigenic phenotypes in breast cancer. Sci. Rep. 7, 6190 (2017).
Cao, L., Sun, P. L., Yao, M., Jia, M. & Gao, H. Expression of YES-associated protein (YAP) and its clinical significance in breast cancer tissues. Hum. Pathol. 68, 166–174 (2017).
Allensworth, J. L., Sauer, S. J., Lyerly, H. K., Morse, M. A. & Devi, G. R. Smac mimetic Birinapant induces apoptosis and enhances TRAIL potency in inflammatory breast cancer cells in an IAP-dependent and TNF-α-independent mechanism. Breast Cancer Res. Treat. 137, 359–371 (2013).
Lamar, J. M., Motilal Nehru, V. & Weinberg, G. Epithelioid hemangioendothelioma as a model of YAP/TAZ-driven cancer: insights from a rare fusion sarcoma. Cancers 10, 229 (2018).
Tanas, M. R. et al. Identification of a disease-defining gene fusion in epithelioid hemangioendothelioma. Sci. Transl Med. 3, 98ra82 (2011).
Marsola, A. et al. Expression of Hippo signaling pathway and aurora kinase genes in chronic myeloid leukemia. Med. Oncol. 35, 26 (2018).
Cottini, F. et al. Rescue of Hippo coactivator YAP1 triggers DNA damage-induced apoptosis in hematological cancers. Nat. Med. 20, 599–606 (2014).
Bellam, N. & Pasche, B. TGF-β signaling alterations and colon cancer. Cancer Treat. Res. 155, 85–103 (2010).
Lobry, C., Oh, P. & Aifantis, I. Oncogenic and tumor suppressor functions of Notch in cancer: it's NOTCH what you think. J. Exp. Med. 208, 1931–1935 (2011).
Sordella, R., Bell, D. W., Haber, D. A. & Settleman, J. Gefitinib-sensitizing EGFR mutations in lung cancer activate anti-apoptotic pathways. Science 305, 1163–1167 (2004).
Van Raamsdonk, C. D. et al. Frequent somatic mutations of GNAQ in uveal melanoma and blue naevi. Nature 457, 599–602 (2009).
Wang, Y. et al. Comprehensive molecular characterization of the Hippo signaling pathway in cancer. Cell Rep. 25, 1304–1317.e5 (2018).
Kim, W. et al. Hippo signaling interactions with Wnt/β-catenin and Notch signaling repress liver tumorigenesis. J. Clin. Invest. 127, 137–152 (2017).
Hansen, C. G., Moroishi, T. & Guan, K. L. YAP and TAZ: a nexus for Hippo signaling and beyond. Trends Cell Biol. 25, 499–513 (2015).
Blume-Jensen, P. & Hunter, T. Oncogenic kinase signalling. Nature 411, 355–365 (2001).
Morales, F. & Giordano, A. Overview of CDK9 as a target in cancer research. Cell Cycle 15, 519–527 (2016).
Al-Moujahed, A. et al. Verteporfin inhibits growth of human glioma in vitro without light activation. Sci. Rep. 7, 7602 (2017).
Song, S. et al. A novel YAP1 inhibitor targets CSC-enriched radiation-resistant cells and exerts strong antitumor activity in esophageal adenocarcinoma. Mol. Cancer Ther. 17, 443–454 (2018).
Jiao, S. et al. A peptide mimicking VGLL4 function acts as a YAP antagonist therapy against gastric cancer. Cancer Cell 25, 166–180 (2014).
Jiao, S. et al. VGLL4 targets a TCF4-TEAD4 complex to coregulate Wnt and Hippo signalling in colorectal cancer. Nat. Commun. 8, 14058 (2017).
Sekido, Y. Targeting the Hippo pathway is a new potential therapeutic modality for malignant mesothelioma. Cancers 10, E90 (2018).
Swords, R. T. et al. Pevonedistat, a first-in-class NEDD8-activating enzyme inhibitor, combined with azacitidine in patients with AML. Blood 131, 1415–1424 (2018).
Swords, R. T. et al. Pevonedistat (MLN4924), a first-in-class NEDD8-activating enzyme inhibitor, in patients with acute myeloid leukaemia and myelodysplastic syndromes: a phase 1 study. Br. J. Haematol. 169, 534–543 (2015).
Lockhart, A. C. et al. Phase Ib study of pevonedistat, a NEDD8-activating enzyme inhibitor, in combination with docetaxel, carboplatin and paclitaxel, or gemcitabine, in patients with advanced solid tumors. Invest. New Drugs 37, 87–97 (2019).
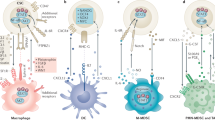
Wan, S. et al. Tumor-associated macrophages produce interleukin 6 and signal via STAT3 to promote expansion of human hepatocellular carcinoma stem cells. Gastroenterology 147, 1393–1404 (2014).
Lu, H. et al. A breast cancer stem cell niche supported by juxtacrine signalling from monocytes and macrophages. Nat. Cell Biol. 16, 1105–1117 (2014).
Korkaya, H., Liu, S. & Wicha, M. S. Regulation of cancer stem cells by cytokine networks: attacking cancer's inflammatory roots. Clin. Cancer Res. 17, 6125–6129 (2011).
Cui, T. X. et al. Myeloid-derived suppressor cells enhance stemness of cancer cells by inducing microRNA101 and suppressing the corepressor CtBP2. Immunity 39, 611–621 (2013).
Lee, C. G. et al. A rare fraction of drug-resistant follicular lymphoma cancer stem cells interacts with follicular dendritic cells to maintain tumourigenic potential. Br. J. Haematol. 158, 79–90 (2012).
Yang, S. et al. Foxp3+IL-17+ T cells promote development of cancer-initiating cells in colorectal cancer. J. Leukoc. Biol. 89, 85–91 (2011).
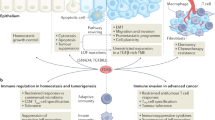
Codd, A. S., Kanaseki, T., Torigo, T. & Tabi, Z. Cancer stem cells as targets for immunotherapy. Immunology 153, 304–314 (2018).
Jinushi, M. Role of cancer stem cell-associated inflammation in creating pro-inflammatory tumorigenic microenvironments. Oncoimmunology 3, e28862 (2014).
Theocharides, A. P. et al. Disruption of SIRPα signaling in macrophages eliminates human acute myeloid leukemia stem cells in xenografts. J. Exp. Med. 209, 1883–1899 (2012).
Liu, L. et al. Anti-CD47 antibody as a targeted therapeutic agent for human lung cancer and cancer stem cells. Front. Immunol. 8, 404 (2017).
Di Tomaso, T. et al. Immunobiological characterization of cancer stem cells isolated from glioblastoma patients. Clin. Cancer Res. 16, 800–813 (2010).
Schatton, T. et al. Modulation of T-cell activation by malignant melanoma initiating cells. Cancer Res. 70, 697–708 (2010).
Todaro, M. et al. Colon cancer stem cells dictate tumor growth and resist cell death by production of interleukin-4. Cell Stem Cell 1, 389–402 (2007).
Wu, A. et al. Glioma cancer stem cells induce immunosuppressive macrophages/microglia. Neuro. Oncol. 12, 1113–1125 (2010).
Zhang, D., Tang, D. G. & Rycaj, K. Cancer stem cells: regulation programs, immunological properties and immunotherapy. Semin. Cancer Biol. 52, 94–106 (2018).
Lee, Y. et al. CD44+ cells in head and neck squamous cell carcinoma suppress T-cell-mediated immunity by selective constitutive and inducible expression of PD-L1. Clin. Cancer Res. 22, 3571–3581 (2016).
Miao, Y. et al. Adaptive immune resistance emerges from tumor-initiating stem cells. Cell 177, 1172–1186.e14 (2019).
Ames, E. et al. NK cells preferentially target tumor cells with a cancer stem cell phenotype. J. Immunol. 195, 4010–4019 (2015).
She, M. et al. Resistance of leukemic stem-like cells in AML cell line KG1a to natural killer cell-mediated cytotoxicity. Cancer Lett. 318, 173–179 (2012).
Wang, B. et al. Metastatic consequences of immune escape from NK cell cytotoxicity by human breast cancer stem cells. Cancer Res. 74, 5746–5757 (2014).
Paczulla, A. M. et al. Absence of NKG2D ligands defines leukaemia stem cells and mediates their immune evasion. Nature 572, 254–259 (2019).
Sia, D. et al. Identification of an immune-specific class of hepatocellular carcinoma, based on molecular features. Gastroenterology 153, 812–826 (2017).
Spranger, S., Bao, R. & Gajewski, T. F. Melanoma-intrinsic β-catenin signalling prevents anti-tumour immunity. Nature 523, 231–235 (2015).
Balli, D., Rech, A. J., Stanger, B. Z. & Vonderheide, R. H. Immune cytolytic activity stratifies molecular subsets of human pancreatic cancer. Clin. Cancer Res. 23, 3129–3138 (2017).
Shen, Q. et al. Notch shapes the innate immunophenotype in breast cancer. Cancer Discov. 7, 1320–1335 (2017).
Sarkar, S. et al. PRKCI promotes immune suppression in ovarian cancer. Genes Dev. 31, 1109–1121 (2017).
Wang, G. et al. Targeting YAP-dependent MDSC infiltration impairs tumor progression. Cancer Discov. 6, 80–95 (2016).
Palaga, T., Miele, L., Golde, T. E. & Osborne, B. A. TCR-mediated Notch signaling regulates proliferation and IFN-γ production in peripheral T cells. J. Immunol. 171, 3019–3024 (2003).
Gattinoni, L., Ji, Y. & Restifo, N. P. Wnt/β-catenin signaling in T-cell immunity and cancer immunotherapy. Clin. Cancer Res. 16, 4695–4701 (2010).
Taha, Z., Janse van Rensburg, H. J. & Yang, X. The Hippo pathway: immunity and cancer. Cancers 10, E94 (2018).
Buglioni, S. et al. Analysis of the Hippo transducers TAZ and YAP in cervical cancer and its microenvironment. Oncoimmunology 5, e1160187 (2016).
Frese, K. K. & Tuveson, D. A. Maximizing mouse cancer models. Nat. Rev. Cancer 7, 645–658 (2007).
Lee, M. J. et al. Hedgehog pathway inhibitor saridegib (IPI-926) increases lifespan in a mouse medulloblastoma model. Proc. Natl Acad. Sci. USA 109, 7859–7864 (2012).
Xu, Q. et al. Antigen-specific T-cell response from dendritic cell vaccination using cancer stem-like cell-associated antigens. Stem Cells 27, 1734–1740 (2009).
Zhao, L. et al. Targeting CD133high colorectal cancer cells in vitro and in vivo with an asymmetric bispecific antibody. J. Immunother. 38, 217–228 (2015).
Dhodapkar, M. V. & Dhodapkar, K. M. Vaccines targeting cancer stem cells: are they within reach? Cancer J. 17, 397–402 (2011).
Pan, Q. et al. Concise review: targeting cancer stem cells using immunologic approaches. Stem Cells 33, 2085–2092 (2015).
Jordan, C. T. et al. The interleukin-3 receptor alpha chain is a unique marker for human acute myelogenous leukemia stem cells. Leukemia 14, 1777–1784 (2000).
Luo, H. et al. A new strategy using ALDHhigh-CD8+ T cells to inhibit tumorigenesis. PLOS ONE 9, e103193 (2014).
Visus, C. et al. Targeting ALDH(bright) human carcinoma-initiating cells with ALDH1A1-specific CD8+ T cells. Clin. Cancer Res. 17, 6174–6184 (2011).
Maude, S. L. et al. Tisagenlecleucel in children and young adults with B-cell lymphoblastic leukemia. N. Engl. J. Med. 378, 439–448 (2018).
Schuster, S. J. et al. Tisagenlecleucel in adult relapsed or refractory diffuse large B-cell lymphoma. N. Engl. J. Med. 380, 45–56 (2019).
Luo, Y. et al. First-in-man CD123-specific chimeric antigen receptor-modified T cells for the treatment of refractory acute myeloid leukemia. Blood 126, 3778 (2015).
Beard, R. E. et al. Multiple chimeric antigen receptors successfully target chondroitin sulfate proteoglycan 4 in several different cancer histologies and cancer stem cells. J. Immunother. Cancer 2, 25 (2014).
Brown, C. E. et al. Stem-like tumor-initiating cells isolated from IL13Rα2 expressing gliomas are targeted and killed by IL13-zetakine-redirected T Cells. Clin. Cancer Res. 18, 2199–2209 (2012).
Deng, Z., Wu, Y., Ma, W., Zhang, S. & Zhang, Y. Q. Adoptive T-cell therapy of prostate cancer targeting the cancer stem cell antigen EpCAM. BMC Immunol. 16, 1 (2015).
Morgan, R. A. et al. Recognition of glioma stem cells by genetically modified T cells targeting EGFRvIII and development of adoptive cell therapy for glioma. Hum. Gene. Ther. 23, 1043–1053 (2012).
Wang, Y. et al. CD133-directed CAR T cells for advanced metastasis malignancies: a phase I trial. Oncoimmunology 7, e1440169 (2018).
Kubasch, A. S. et al. Anti-CD123 targeted therapy with talacotuzumab in advanced MDS and AML after failing hypomethylating agents — final results of the SAMBA trial. Blood 132, 4045 (2018).
Menke-van der Houven van Oordt, C. W. et al. First-in-human phase I clinical trial of RG7356, an anti-CD44 humanized antibody, in patients with advanced, CD44-expressing solid tumors. Oncotarget 7, 80046–80058 (2016).
Huang, J. et al. Cytokine-induced killer (CIK) cells bound with anti-CD3/anti-CD133 bispecific antibodies target CD133(high) cancer stem cells in vitro and in vivo. Clin. Immunol. 149, 156–168 (2013).
Shi, X. et al. PD-1 blockade enhances the antitumor efficacy of GM-CSF surface-modified bladder cancer stem cells vaccine. Int. J. Cancer 142, 2106–2117 (2018).
Lee, H., Pal, S. K., Reckamp, K., Figlin, R. A. & Yu, H. STAT3: a target to enhance antitumor immune response. Curr. Top Microbiol. Immunol. 344, 41–59 (2011).
Marzec, M. et al. Oncogenic kinase NPM/ALK induces through STAT3 expression of immunosuppressive protein CD274 (PD-L1, B7-H1). Proc. Natl Acad. Sci. USA 105, 20852–20857 (2008).
Ginestier, C. et al. CXCR1 blockade selectively targets human breast cancer stem cells in vitro and in xenografts. J. Clin. Invest. 120, 485–497 (2010).
Jia, D. et al. An autocrine inflammatory forward-feedback loop after chemotherapy withdrawal facilitates the repopulation of drug-resistant breast cancer cells. Cell Death Dis. 8, e2932 (2017).
Kim, S. Y. et al. Role of the IL-6-JAK1-STAT3-Oct-4 pathway in the conversion of non-stem cancer cells into cancer stem-like cells. Cell. Signal. 25, 961–969 (2013).
Majeti, R. et al. CD47 is an adverse prognostic factor and therapeutic antibody target on human acute myeloid leukemia stem cells. Cell 138, 286–299 (2009).
Cioffi, M. et al. Inhibition of CD47 effectively targets pancreatic cancer stem cells via dual mechanisms. Clin. Cancer Res. 21, 2325–2337 (2015).
Sikic, B. I. et al. First-in-human, first-in-class phase I trial of the anti-CD47 antibody Hu5F9-G4 in patients with advanced cancers. J. Clin. Oncol. 37, 946–953 (2019).
Adeegbe, D. O. et al. Synergistic immunostimulatory effects and therapeutic benefit of combined histone deacetylase and bromodomain inhibition in non-small cell lung cancer. Cancer Discov. 7, 852–867 (2017).
Deangelo, D. J. et al. A phase I clinical trial of the Notch inhibitor MK-0752 in patients with T-cell acute lymphoblastic leukemia/lymphoma (T-ALL) and other leukemias. J. Clin. Oncol. 24, 6585 (2006).
Piha-Paul, S. A. et al. Results of a phase 1 trial combining ridaforolimus and MK-0752 in patients with advanced solid tumours. Eur. J. Cancer 51, 1865–1873 (2015).
Locatelli, M. A. et al. Phase I study of the gamma secretase inhibitor PF-03084014 in combination with docetaxel in patients with advanced triple-negative breast cancer. Oncotarget 8, 2320–2328 (2017).
Pant, S. et al. A first-in-human phase I study of the oral Notch inhibitor, LY900009, in patients with advanced cancer. Eur. J. Cancer 56, 1–9 (2016).
De Jesus-Acosta, A. et al. A phase II study of the γ secretase inhibitor RO4929097 in patients with previously treated metastatic pancreatic adenocarcinoma. Invest. New Drugs 32, 739–745 (2014).
Strosberg, J. R. et al. A phase II study of RO4929097 in metastatic colorectal cancer. Eur. J. Cancer 48, 997–1003 (2012).
Tolcher, A. W. et al. Phase I study of RO4929097, a γ secretase inhibitor of Notch signaling, in patients with refractory metastatic or locally advanced solid tumors. J. Clin. Oncol. 30, 2348–2353 (2012).
Richter, S. et al. A phase I study of the oral γ secretase inhibitor R04929097 in combination with gemcitabine in patients with advanced solid tumors (PHL-078/CTEP 8575). Invest. New Drugs 32, 243–249 (2014).
Diaz-Padilla, I. et al. A phase Ib combination study of RO4929097, a γ-secretase inhibitor, and temsirolimus in patients with advanced solid tumors. Invest. New Drugs 31, 1182–1191 (2013).
Falchook, G. S. et al. Phase I study of MEDI0639 in patients with advanced solid tumors. J. Clin. Oncol. 33, 3024 (2015).
Kim, E. J. et al. Pilot clinical trial of Hedgehog pathway inhibitor GDC-0449 (vismodegib) in combination with gemcitabine in patients with metastatic pancreatic adenocarcinoma. Clin. Cancer Res. 20, 5937–5945 (2014).
Sloan, A. E. et al. Targeting glioma initiating cells in GBM: ABTC-0904, a randomized phase 0/II study targeting the sonic Hedgehog-signaling pathway. Neuro-Oncology 16, iii46 (2014).
Lee, M. et al. A phase II study of itraconazole in biochemically recurrent prostate cancer. J. Clin. Oncol. 36, 362 (2018).
Author information
Authors and Affiliations
Contributions
All authors contributed substantially to researching the data for the article, discussions of content, writing the article and review/editing of the manuscript before submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Peer review information
Nature Reviews Clinical Oncology thanks M. Katoh, M. Wicha and the other, anonymous, reviewer for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
About this article
Cite this article
Clara, J.A., Monge, C., Yang, Y. et al. Targeting signalling pathways and the immune microenvironment of cancer stem cells — a clinical update. Nat Rev Clin Oncol 17, 204–232 (2020). https://doi.org/10.1038/s41571-019-0293-2
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41571-019-0293-2
This article is cited by
-
PARD3 drives tumorigenesis through activating Sonic Hedgehog signalling in tumour-initiating cells in liver cancer
Journal of Experimental & Clinical Cancer Research (2024)
-
Critical appraisal of the piRNA-PIWI axis in cancer and cancer stem cells
Biomarker Research (2024)
-
Small cells – big issues: biological implications and preclinical advancements in small cell lung cancer
Molecular Cancer (2024)
-
Targeting the macrophage immunocheckpoint: a novel insight into solid tumor immunotherapy
Cell Communication and Signaling (2024)
-
Crosstalk between colorectal CSCs and immune cells in tumorigenesis, and strategies for targeting colorectal CSCs
Experimental Hematology & Oncology (2024)