Abstract
The overall 5-year survival for pancreatic cancer has changed little over the past few decades, and pancreatic cancer is predicted to be the second leading cause of cancer-related mortality in the next decade in Western countries. The past few years, however, have seen improvements in first-line and second-line palliative therapies and considerable progress in increasing survival with adjuvant treatment. The use of biomarkers to help define treatment and the potential of neoadjuvant therapies also offer opportunities to improve outcomes. This Review brings together information on achievements to date, what is working currently and where successes are likely to be achieved in the future. Furthermore, we address the questions of how we should approach the development of pancreatic cancer treatments, including those for patients with metastatic, locally advanced and borderline resectable pancreatic cancer, as well as for patients with resected tumours. In addition to embracing newer strategies comprising genomics, stromal therapies and immunotherapies, conventional approaches using chemotherapy and radiotherapy still offer considerable prospects for greater traction and synergy with evolving concepts.
Key points
-
Pancreatic cancer is currently the fourth leading cause of cancer-associated mortality and is projected to be the second leading cause within the next decade in Western countries.
-
For resectable tumours, surgery followed by adjuvant chemotherapy (gemcitabine plus capecitabine) is the standard of care; median survival in these patients is 26 months, with a 5-year survival of 30%.
-
For borderline resectable and locally advanced, unresectable tumours, neoadjuvant protocols are utilized, with a shift towards chemotherapy rather than radiochemotherapy, although good evidence from randomized controlled trials is lacking.
-
In the metastatic setting, FOLFIRINOX and nab-paclitaxel–gemcitabine are standard treatment options in patients with good performance status; both combinations have shown a survival advantage over previously standard gemcitabine monotherapy.
-
Second-line therapies, notably nanoliposomal irinotecan plus 5-fluorouracil–folinic acid, might prolong survival after first-line gemcitabine failure.
-
Pathway-specific targeted therapies have failed to provide clinically relevant benefits; therapies targeting the stroma as well as immunotherapies hold promise for the future but are currently not standard of care.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout





Similar content being viewed by others
References
Rahib, L. et al. Projecting cancer incidence and deaths to 2030: the unexpected burden of thyroid, liver, and pancreas cancers in the United States. Cancer Res. 74, 2913–2921 (2014).
Ferlay, J. et al. Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int. J. Cancer 136, E359–E386 (2015).
Kleeff, J. et al. Pancreatic cancer. Nat. Rev. Dis. Primers 2, 16022 (2016).
Hartwig, W., Werner, J., Jager, D., Debus, J. & Buchler, M. W. Improvement of surgical results for pancreatic cancer. Lancet Oncol. 14, e476–e485 (2013).
Kalser, M. H. & Ellenberg, S. S. Pancreatic cancer. adjuvant combined radiation and chemotherapy following curative resection. Arch. Surg. 120, 899–903 (1985).
Klinkenbijl, J. H. et al. Adjuvant radiotherapy and 5-fluorouracil after curative resection of cancer of the pancreas and periampullary region: phase III trial of the EORTC gastrointestinal tract cancer cooperative group. Ann. Surg. 230, 776–782; discussion 782–784 (1999).
Neoptolemos, J. P. et al. Adjuvant chemoradiotherapy and chemotherapy in resectable pancreatic cancer: a randomised controlled trial. Lancet 358, 1576–1585 (2001).
Neoptolemos, J. P. et al. A randomized trial of chemoradiotherapy and chemotherapy after resection of pancreatic cancer. N. Engl. J. Med. 350, 1200–1210 (2004).
Regine, W. F. et al. Fluorouracil versus gemcitabine chemotherapy before and after fluorouracil-based chemoradiation following resection of pancreatic adenocarcinoma: a randomized controlled trial. JAMA 299, 1019–1026 (2008).
Oettle, H. et al. Adjuvant chemotherapy with gemcitabine and long-term outcomes among patients with resected pancreatic cancer: the CONKO-001 randomized trial. JAMA 310, 1473–1481 (2013).
Oettle, H. et al. Adjuvant chemotherapy with gemcitabine versus observation in patients undergoing curative-intent resection of pancreatic cancer: a randomized controlled trial. JAMA 297, 267–277 (2007).
Neoptolemos, J. P. et al. Adjuvant chemotherapy with fluorouracil plus folinic acid versus gemcitabine following pancreatic cancer resection: a randomized controlled trial. JAMA 304, 1073–1081 (2010).
Greenhalf, W. et al. Pancreatic cancer hENT1 expression and survival from gemcitabine in patients from the ESPAC-3 trial. J. Natl Cancer Inst. 106, djt347 (2014).
Serdjebi, C., Milano, G. & Ciccolini, J. Role of cytidine deaminase in toxicity and efficacy of nucleosidic analogs. Expert Opin. Drug Metab. Toxicol. 11, 665–672 (2015).
Dean, L. in Medical Genetics Summaries (ed. Pratt, V. et al.) (National Center for Biotechnology Information, Bethesda, 2012).
Valle, J. W. et al. Optimal duration and timing of adjuvant chemotherapy after definitive surgery for ductal adenocarcinoma of the pancreas: ongoing lessons from the ESPAC-3 study. J. Clin. Oncol. 32, 504–512 (2014).
Sinn, M. et al. CONKO-005: adjuvant chemotherapy with gemcitabine plus erlotinib versus gemcitabine alone in patients after r0 resection of pancreatic cancer: a multicenter randomized phase iii trial. J. Clin. Oncol. 35, 3330–3337 (2017).
Uesaka, K. et al. Adjuvant chemotherapy of S-1 versus gemcitabine for resected pancreatic cancer: a phase 3, open-label, randomised, non-inferiority trial (JASPAC 01). Lancet 388, 248–257 (2016).
Neoptolemos, J. P. et al. Comparison of adjuvant gemcitabine and capecitabine with gemcitabine monotherapy in patients with resected pancreatic cancer (ESPAC-4): a multicentre, open-label, randomised, phase 3 trial. Lancet 389, 1011–1024 (2017).
Cunningham, D. et al. Phase III randomized comparison of gemcitabine versus gemcitabine plus capecitabine in patients with advanced pancreatic cancer. J. Clin. Oncol. 27, 5513–5518 (2009).
Khorana, A. A. et al. Potentially curable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline update. J. Clin. Oncol. 35, 2324–2328 (2017).
Bockhorn, M. et al. Borderline resectable pancreatic cancer: a consensus statement by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 155, 977–988 (2014).
Gillen, S., Schuster, T., Meyer Zum Buschenfelde, C., Friess, H. & Kleeff, J. Preoperative/neoadjuvant therapy in pancreatic cancer: a systematic review and meta-analysis of response and resection percentages. PLoS Med. 7, e1000267 (2010).
Golcher, H. et al. Neoadjuvant chemoradiation therapy with gemcitabine/cisplatin and surgery versus immediate surgery in resectable pancreatic cancer: results of the first prospective randomized phase II trial. Strahlenther. Onkol. 191, 7–16 (2015).
Heinrich, S. et al. Adjuvant gemcitabine versus NEOadjuvant gemcitabine/oxaliplatin plus adjuvant gemcitabine in resectable pancreatic cancer: a randomized multicenter phase III study (NEOPAC study). BMC Cancer 11, 346 (2011).
Tachezy, M. et al. Sequential neoadjuvant chemoradiotherapy (CRT) followed by curative surgery versus primary surgery alone for resectable, non-metastasized pancreatic adenocarcinoma: NEOPA- a randomized multicenter phase III study (NCT01900327, DRKS00003893, ISRCTN82191749). BMC Cancer 14, 411 (2014).
Versteijne, E. et al. Preoperative radiochemotherapy versus immediate surgery for resectable and borderline resectable pancreatic cancer (PREOPANC trial): study protocol for a multicentre randomized controlled trial. Trials 17, 127 (2016).
ISCRTN registry. ESPAC-5F: European Study group for Pancreatic Cancer - Trial 5F. BMC www.isrctn.com/ISRCTN89500674 (2014).
Kwon, W. et al. in 51st Annual Meeting of The Pancreas Club S008 (Chicago, 2017).
Hackert, T. et al. Locally advanced pancreatic cancer: neoadjuvant therapy with Folfirinox results in resectability in 60% of the patients. Ann. Surg. 264, 457–463 (2016).
Conroy, T. et al. Irinotecan plus oxaliplatin and leucovorin-modulated fluorouracil in advanced pancreatic cancer — a groupe tumeurs digestives of the federation nationale des centres de lutte contre le cancer study. J. Clin. Oncol. 23, 1228–1236 (2005).
Rombouts, S. J. et al. Systematic review of resection rates and clinical outcomes after folfirinox-based treatment in patients with locally advanced pancreatic cancer. Ann. Surg. Oncol. 23, 4352–4360 (2016).
Ferrone, C. R. et al. Radiological and surgical implications of neoadjuvant treatment with FOLFIRINOX for locally advanced and borderline resectable pancreatic cancer. Ann. Surg. 261, 12–17 (2015).
Nitsche, U. et al. Resectability after first-line folfirinox in initially unresectable locally advanced pancreatic cancer: a single-center experience. Ann. Surg. Oncol. 22(Suppl. 3), 1212–1220 (2015).
Petrelli, F. et al. FOLFIRINOX-based neoadjuvant therapy in borderline resectable or unresectable pancreatic cancer: a meta-analytical review of published studies. Pancreas 44, 515–521 (2015).
Katz, M. H. et al. Response of borderline resectable pancreatic cancer to neoadjuvant therapy is not reflected by radiographic indicators. Cancer 118, 5749–5756 (2012).
Balaban, E. P., Mangu, P. B. & Yee, N. S. Locally advanced unresectable pancreatic cancer: American Society of Clinical Oncology clinical practice guideline summary. J. Oncol. Pract. 13, 265–269 (2017).
Hurt, C. N. et al. Long-term results and recurrence patterns from SCALOP: a phase II randomised trial of gemcitabine- or capecitabine-based chemoradiation for locally advanced pancreatic cancer. Br. J. Cancer 116, 1264–1270 (2017).
Linecker, M., Pfammatter, T., Kambakamba, P. & DeOliveira, M. L. Ablation strategies for locally advanced pancreatic cancer. Dig. Surg. 33, 351–359 (2016).
Paiella, S. et al. Local ablative strategies for ductal pancreatic cancer (radiofrequency ablation, irreversible electroporation): a review. Gastroenterol. Res. Pract. 2016, 4508376 (2016).
Rombouts, S. J. et al. Systematic review of innovative ablative therapies for the treatment of locally advanced pancreatic cancer. Br. J. Surg. 102, 182–193 (2015).
Sofuni, A. et al. Safety trial of high-intensity focused ultrasound therapy for pancreatic cancer. World J. Gastroenterol. 20, 9570–9577 (2014).
Sung, H. Y. et al. Long-term outcome of high-intensity focused ultrasound in advanced pancreatic cancer. Pancreas 40, 1080–1086 (2011).
Burris, H. A. 3rd et al. Improvements in survival and clinical benefit with gemcitabine as first-line therapy for patients with advanced pancreas cancer: a randomized trial. J. Clin. Oncol. 15, 2403–2413 (1997).
Conroy, T. et al. FOLFIRINOX versus gemcitabine for metastatic pancreatic cancer. N. Engl. J. Med. 364, 1817–1825 (2011).
Von Hoff, D. D. et al. Gemcitabine plus nab-paclitaxel is an active regimen in patients with advanced pancreatic cancer: a phase I/II trial. J. Clin. Oncol. 29, 4548–4554 (2011).
Neesse, A., Michl, P. & Tuveson, D. A. and Ellenrieder, V. nab-Paclitaxel: novel clinical and experimental evidence in pancreatic cancer. Z. Gastroenterol. 52, 360–366 (2014).
Von Hoff, D. D. et al. Increased survival in pancreatic cancer with nab-paclitaxel plus gemcitabine. N. Engl. J. Med. 369, 1691–1703 (2013).
Goldstein, D. et al. nab-Paclitaxel plus gemcitabine for metastatic pancreatic cancer: long-term survival from a phase III trial. J. Natl Cancer Inst. 107, dju413 (2015).
Sohal, D. P. et al. Metastatic pancreatic cancer: American Society of Clinical Oncology clinical practice guideline. J. Clin. Oncol. 34, 2784–2796 (2016).
Hammel, P. et al. Effect of chemoradiotherapy versus chemotherapy on survival in patients with locally advanced pancreatic cancer controlled after 4 months of gemcitabine with or without erlotinib: the lap07 randomized clinical trial. JAMA 315, 1844–1853 (2016).
Nagrial, A. M. et al. Second-line treatment in inoperable pancreatic adenocarcinoma: a systematic review and synthesis of all clinical trials. Crit. Rev. Oncol. Hematol. 96, 483–497 (2015).
Pelzer, U. et al. Best supportive care (BSC) versus oxaliplatin, folinic acid and 5-fluorouracil (OFF) plus BSC in patients for second-line advanced pancreatic cancer: a phase III-study from the German CONKO-study group. Eur. J. Cancer 47, 1676–1681 (2011).
Oettle, H. et al. Second-line oxaliplatin, folinic acid, and fluorouracil versus folinic acid and fluorouracil alone for gemcitabine-refractory pancreatic cancer: outcomes from the CONKO-003 trial. J. Clin. Oncol. 32, 2423–2429 (2014).
Gill, S. et al. PANCREOX: A randomized phase III study of fluorouracil/leucovorin with or without oxaliplatin for second-line advanced pancreatic cancer in patients who have received gemcitabine-based chemotherapy. J. Clin. Oncol. 34, 3914–3920 (2016).
Wang-Gillam, A. et al. Nanoliposomal irinotecan with fluorouracil and folinic acid in metastatic pancreatic cancer after previous gemcitabine-based therapy (NAPOLI-1): a global, randomised, open-label, phase 3 trial. Lancet 387, 545–557 (2016).
Portal, A. et al. Nab-paclitaxel plus gemcitabine for metastatic pancreatic adenocarcinoma after Folfirinox failure: an AGEO prospective multicentre cohort. Br. J. Cancer 113, 989–995 (2015).
Tempero, M. A. et al. Pancreatic adenocarcinoma, version 2.2017, nccn clinical practice guidelines in oncology. J. Natl Compr. Canc. Netw. 15, 1028–1061 (2017).
Kindler, H. L. et al. Gemcitabine plus bevacizumab compared with gemcitabine plus placebo in patients with advanced pancreatic cancer: phase III trial of the Cancer and Leukemia Group B (CALGB 80303). J. Clin. Oncol. 28, 3617–3622 (2010).
Rougier, P. et al. Randomised, placebo-controlled, double-blind, parallel-group phase III study evaluating aflibercept in patients receiving first-line treatment with gemcitabine for metastatic pancreatic cancer. Eur. J. Cancer 49, 2633–2642 (2013).
Kindler, H. L. et al. Axitinib plus gemcitabine versus placebo plus gemcitabine in patients with advanced pancreatic adenocarcinoma: a double-blind randomised phase 3 study. Lancet Oncol. 12, 256–262 (2011).
Kindler, H. L. et al. Gemcitabine plus sorafenib in patients with advanced pancreatic cancer: a phase II trial of the University of Chicago Phase II Consortium. Invest. New Drugs 30, 382–386 (2012).
Michl, P. & Gress, T. M. Current concepts and novel targets in advanced pancreatic cancer. Gut 62, 317–326 (2013).
O’Reilly, E. M. et al. A Cancer and Leukemia Group B phase II study of sunitinib malate in patients with previously treated metastatic pancreatic adenocarcinoma (CALGB 80603). Oncologist 15, 1310–1319 (2010).
Fuchs, C. S. et al. A phase 3 randomized, double-blind, placebo-controlled trial of ganitumab or placebo in combination with gemcitabine as first-line therapy for metastatic adenocarcinoma of the pancreas: the GAMMA trial. Ann. Oncol. 26, 921–927 (2015).
Philip, P. A. et al. Dual blockade of epidermal growth factor receptor and insulin-like growth factor receptor-1 signaling in metastatic pancreatic cancer: phase Ib and randomized phase II trial of gemcitabine, erlotinib, and cixutumumab versus gemcitabine plus erlotinib (SWOG S0727). Cancer 120, 2980–2985 (2014).
Deplanque, G. et al. A randomized, placebo-controlled phase III trial of masitinib plus gemcitabine in the treatment of advanced pancreatic cancer. Ann. Oncol. 26, 1194–1200 (2015).
O’Neil, B. H. et al. A phase II/III randomized study to compare the efficacy and safety of rigosertib plus gemcitabine versus gemcitabine alone in patients with previously untreated metastatic pancreatic cancer. Ann. Oncol. 26, 1923–1929 (2015).
Ottaiano, A. et al. Gemcitabine mono-therapy versus gemcitabine plus targeted therapy in advanced pancreatic cancer: a meta-analysis of randomized phase III trials. Acta Oncol. 56, 377–383 (2017).
Moore, M. J. et al. Erlotinib plus gemcitabine compared with gemcitabine alone in patients with advanced pancreatic cancer: a phase III trial of the National Cancer Institute of Canada Clinical Trials Group. J. Clin. Oncol. 25, 1960–1966 (2007).
Potthoff, K. et al. Interdisciplinary management of EGFR-inhibitor-induced skin reactions: a German expert opinion. Ann. Oncol. 22, 524–535 (2011).
Wacker, B. et al. Correlation between development of rash and efficacy in patients treated with the epidermal growth factor receptor tyrosine kinase inhibitor erlotinib in two large phase III studies. Clin. Cancer Res. 13, 3913–3921 (2007).
Kleeff, J. et al. Pancreatic cancer microenvironment. Int. J. Cancer 121, 699–705 (2007).
Hidalgo, M. et al. SPARC expression did not predict efficacy of nab-paclitaxel plus gemcitabine or gemcitabine alone for metastatic pancreatic cancer in an exploratory analysis of the phase III MPACT Trial. Clin. Cancer Res. 21, 4811–4818 (2015).
Provenzano, P. P. et al. Enzymatic targeting of the stroma ablates physical barriers to treatment of pancreatic ductal adenocarcinoma. Cancer Cell 21, 418–429 (2012).
Jacobetz, M. A. et al. Hyaluronan impairs vascular function and drug delivery in a mouse model of pancreatic cancer. Gut 62, 112–120 (2013).
Hingorani, S. R. et al. High response rate and PFS with PEGPH20 added to nab-paclitaxel/gemcitabine in stage IV previously untreated pancreatic cancer patients with high-HA tumors: Interim results of a randomized phase II study. J. Clin. Oncol. 33, 4006 (2015).
Erkan, M. et al. StellaTUM: current consensus and discussion on pancreatic stellate cell research. Gut 61, 172–178 (2012).
Sherman, M. H. et al. Vitamin D receptor-mediated stromal reprogramming suppresses pancreatitis and enhances pancreatic cancer therapy. Cell 159, 80–93 (2014).
Roberts, K. J., Kershner, A. M. & Beachy, P. A. The stromal niche for epithelial stem cells: a template for regeneration and a brake on malignancy. Cancer Cell 32, 404–410 (2017).
Olive, K. P. et al. Inhibition of Hedgehog signaling enhances delivery of chemotherapy in a mouse model of pancreatic cancer. Science 324, 1457–1461 (2009).
Rhim, A. D. et al. Stromal elements act to restrain, rather than support, pancreatic ductal adenocarcinoma. Cancer Cell 25, 735–747 (2014).
Ozdemir, B. C. et al. Depletion of carcinoma-associated fibroblasts and fibrosis induces immunosuppression and accelerates pancreas cancer with reduced survival. Cancer Cell 25, 719–734 (2014).
Van Cutsem, E. et al. MAESTRO: a randomized, double-blind phase III study of evofosfamide (Evo) in combination with gemcitabine (Gem) in previously untreated patients (pts) with metastatic or locally advanced unresectable pancreatic ductal adenocarcinoma (PDAC). J. Clin. Oncol. 34, 4007 (2016).
Hanahan, D. & Weinberg, R. A. Hallmarks of cancer: the next generation. Cell 144, 646–674 (2011).
Farren, M. R. et al. Systemic immune activity predicts overall survival in treatment naive patients with metastatic pancreatic cancer. Clin. Cancer Res. 22, 2565–2574 (2015).
Silva, I. P. & Long, G. V. Systemic therapy in advanced melanoma: integrating targeted therapy and immunotherapy into clinical practice. Curr. Opin. Oncol. 29, 484–492 (2017).
Tang, H., Qiao, J. & Fu, Y. X. Immunotherapy and tumor microenvironment. Cancer Lett. 370, 85–90 (2016).
Limagne, E. et al. Accumulation of MDSC and Th17 cells in patients with metastatic colorectal cancer predict the efficacy of a FOLFOX-bevacizumab drug treatment regimen. Cancer Res. 76, 5241–5252 (2016).
Lepique, A. P., Daghastanli, K. R., Cuccovia, I. M. & Villa, L. L. HPV16 tumor associated macrophages suppress antitumor T cell responses. Clin. Cancer Res. 15, 4391–4400 (2009).
Carmi, Y. et al. Allogeneic IgG combined with dendritic cell stimuli induce antitumour T-cell immunity. Nature 521, 99–104 (2015).
Mills, C. D., Lenz, L. L. & Harris, R. A. A. Breakthrough: macrophage-directed cancer immunotherapy. Cancer Res. 76, 513–516 (2016).
Feig, C. et al. Targeting CXCL12 from FAP-expressing carcinoma-associated fibroblasts synergizes with anti-PD-L1 immunotherapy in pancreatic cancer. Proc. Natl Acad. Sci. USA 110, 20212–20217 (2013).
Spadi, R. et al. Current therapeutic strategies for advanced pancreatic cancer: a review for clinicians. World J. Clin. Oncol. 7, 27–43 (2016).
Melero, I. et al. Therapeutic vaccines for cancer: an overview of clinical trials. Nat. Rev. Clin. Oncol. 11, 509–524 (2014).
Middleton, G. et al. Gemcitabine and capecitabine with or without telomerase peptide vaccine GV1001 in patients with locally advanced or metastatic pancreatic cancer (TeloVac): an open-label, randomised, phase 3 trial. Lancet Oncol. 15, 829–840 (2014).
Palmer, D. H. et al. A prospective, single-arm, phase I/II trial of RAS peptide vaccine TG01/GM-CSF and gemcitabine as adjuvant therapy for patients with resected pancreatic adenocarcinoma [abstract]. J. Clin. Oncol. 35, 4119 (2015).
Dueland, S. et al. 669PTG01/GM-CSF and adjuvant gemcitabine in patients with resected RAS-mutant adenocarcinoma of the pancreas. Ann. Oncol. https://doi.org/10.1093/annonc/mdx369.053 (2017).
Meng, Q. et al. Expansion of tumor-reactive T cells from patients with pancreatic cancer. J. Immunother. 39, 81–89 (2016).
Byrne, K. T., Vonderheide, R. H., Jaffee, E. M. & Armstrong, T. D. Special conference on tumor immunology and immunotherapy: a new chapter. Cancer Immunol. Res. https://doi.org/10.1158/2326-6066.CIR-15-0106 (2015).
Starck, L., Popp, K., Pircher, H. & Uckert, W. Immunotherapy with TCR-redirected T cells: comparison of TCR-transduced and TCR-engineered hematopoietic stem cell-derived T cells. J. Immunol. 192, 206–213 (2014).
Morgan, R. A. et al. Cancer regression and neurological toxicity following anti-MAGE-A3 TCR gene therapy. J. Immunother. 36, 133–151 (2013).
Maus, M. V. & June, C. H. Making better chimeric antigen receptors for adoptive t-cell therapy. Clin. Cancer Res. 22, 1875–1884 (2016).
Sathyanarayanan, V. & Neelapu, S. S. Cancer immunotherapy: strategies for personalization and combinatorial approaches. Mol. Oncol. 9, 2043–2053 (2015).
Alrifai, D., Sarker, D. & Maher, J. Prospects for adoptive immunotherapy of pancreatic cancer using chimeric antigen receptor-engineered T-cells. Immunopharmacol. Immunotoxicol. 38, 50–60 (2016).
David, J. M., Dominguez, C., Hamilton, D. H. & Palena, C. The IL-8/IL-8R axis: a double agent in tumor immune resistance. Vaccines 4, E22 (2016).
Weed, D. T. et al. Tadalafil reduces myeloid-derived suppressor cells and regulatory T cells and promotes tumor immunity in patients with head and neck squamous cell carcinoma. Clin. Cancer Res. 21, 39–48 (2015).
Wesolowski, R., Markowitz, J. & Carson, W. E. 3rd. Myeloid derived suppressor cells - a new therapeutic target in the treatment of cancer. J. Immunother. Cancer 1, 10 (2013).
Noman, M. Z. et al. Tumor-promoting effects of myeloid-derived suppressor cells are potentiated by hypoxia-induced expression of miR-210. Cancer Res. 75, 3771–3787 (2015).
Bigelow, E. et al. Immunohistochemical staining of B7-H1 (PD-L1) on paraffin-embedded slides of pancreatic adenocarcinoma tissue. J. Vis. Exp. 71, 4059 (2013).
Noy, R. & Pollard, J. W. Tumor-associated macrophages: from mechanisms to therapy. Immunity 41, 49–61 (2014).
Wolchok, J. D. & Saenger, Y. The mechanism of anti-CTLA-4 activity and the negative regulation of T-cell activation. Oncologist 13 (Suppl. 4), 2–9 (2008).
Yeung, A. W., Terentis, A. C., King, N. J. & Thomas, S. R. Role of indoleamine 2,3-dioxygenase in health and disease. Clin. Sci. 129, 601–672 (2015).
Bessede, A. et al. Aryl hydrocarbon receptor control of a disease tolerance defence pathway. Nature 511, 184–190 (2014).
Wolchok, J. D. et al. Development of ipilimumab: a novel immunotherapeutic approach for the treatment of advanced melanoma. Ann. NY Acad. Sci. 1291, 1–13 (2013).
Zhai, L. et al. Molecular pathways: targeting ido1 and other tryptophan dioxygenases for cancer immunotherapy. Clin. Cancer Res. 21, 5427–5433 (2015).
Voo, K. S. et al. Antibodies targeting human OX40 expand effector T cells and block inducible and natural regulatory T cell function. J. Immunol. 191, 3641–3650 (2013).
Bauer, C. et al. Prevailing over T cell exhaustion: new developments in the immunotherapy of pancreatic cancer. Cancer Lett. 381, 259–268 (2016).
Beatty, G. L. et al. CD40 agonists alter tumor stroma and show efficacy against pancreatic carcinoma in mice and humans. Science 331, 1612–1616 (2011).
Ryter, S. W. & Choi, A. M. Targeting heme oxygenase-1 and carbon monoxide for therapeutic modulation of inflammation. Transl Res. 167, 7–34 (2016).
Manrique, S. Z. et al. Definitive activation of endogenous antitumor immunity by repetitive cycles of cyclophosphamide with interspersed Toll-like receptor agonists. Oncotarget 7, 42919–42942 (2016).
Vaz, J. & Andersson, R. Intervention on toll-like receptors in pancreatic cancer. World J. Gastroenterol. 20, 5808–5817 (2014).
Fearon, D. T. The carcinoma-associated fibroblast expressing fibroblast activation protein and escape from immune surveillance. Cancer Immunol. Res. 2, 187–193 (2014).
Overman, M. J. et al. Nivolumab in patients with metastatic DNA mismatch repair-deficient or microsatellite instability-high colorectal cancer (CheckMate 142): an open-label, multicentre, phase 2 study. Lancet. Oncol. 18, 1182–1191 (2017).
Hofmann, L. et al. Cutaneous, gastrointestinal, hepatic, endocrine, and renal side-effects of anti-PD-1 therapy. Eur. J. Cancer 60, 190–209 (2016).
Brunet, L. R., Hagemann, T., Andrew, G., Mudan, S. & Marabelle, A. Have lessons from past failures brought us closer to the success of immunotherapy in metastatic pancreatic cancer? Oncoimmunology 5, e1112942 (2016).
Mackall, C. L. et al. Distinctions between CD8 + and CD4 + T-cell regenerative pathways result in prolonged T-cell subset imbalance after intensive chemotherapy. Blood 89, 3700–3707 (1997).
Schiavoni, G. et al. Cyclophosphamide induces type I interferon and augments the number of CD44(hi) T lymphocytes in mice: implications for strategies of chemoimmunotherapy of cancer. Blood 95, 2024–2030 (2000).
Middleton, G. et al. Immunobiological effects of gemcitabine and capecitabine combination chemotherapy in advanced pancreatic ductal adenocarcinoma. Br. J. Cancer 114, 510–518 (2016).
Prasanna, A., Ahmed, M. M., Mohiuddin, M. & Coleman, C. N. Exploiting sensitization windows of opportunity in hyper and hypo-fractionated radiation therapy. J. Thorac. Dis. 6, 287–302 (2014).
Biankin, A. V., Piantadosi, S. & Hollingsworth, S. J. Patient-centric trials for therapeutic development in precision oncology. Nature 526, 361–370 (2015).
Boj, S. F. et al. Organoid models of human and mouse ductal pancreatic cancer. Cell 160, 324–338 (2015).
Chantrill, L. A. et al. Precision medicine for advanced pancreas cancer: the individualized molecular pancreatic cancer therapy (IMPaCT) trial. Clin. Cancer Res. 21, 2029–2037 (2015).
Hurwitz, H. I. et al. Randomized, double-blind, phase II study of Ruxolitinib or placebo in combination with capecitabine in patients with metastatic pancreatic cancer for whom therapy with gemcitabine has failed. J. Clin. Oncol. 33, 4039–4047 (2015).
Waddell, N. et al. Whole genomes redefine the mutational landscape of pancreatic cancer. Nature 518, 495–501 (2015).
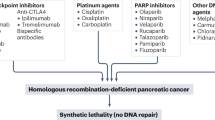
Bendell, J. et al. Phase I study of olaparib plus gemcitabine in patients with advanced solid tumours and comparison with gemcitabine alone in patients with locally advanced/metastatic pancreatic cancer. Ann. Oncol. 26, 804–811 (2015).
Kaufman, B. et al. Olaparib monotherapy in patients with advanced cancer and a germline BRCA1/2 mutation. J. Clin. Oncol. 33, 244–250 (2015).
Witkiewicz, A. K. et al. Whole-exome sequencing of pancreatic cancer defines genetic diversity and therapeutic targets. Nat. Commun. 6, 6744 (2015).
Bailey, P. et al. Genomic analyses identify molecular subtypes of pancreatic cancer. Nature 531, 47–52 (2016).
Chung, V. M. et al. SWOG S1115: randomized phase ii trial of selumetinib (AZD6244; ARRY 142886) hydrogen sulfate (NSC-748727) and MK-2206 (NSC-749607) versus mFOLFOX in pretreated patients (Pts) with metastatic pancreatic cancer [abstract]. ASCO Meet. Abstr. 33, 4119 (2015).
Ge, F. et al. S-1 as monotherapy or in combination with leucovorin as second-line treatment in gemcitabine-refractory advanced pancreatic cancer: a randomized, open-label, multicenter, phase II study. Oncologist 19, 1133–1134 (2014).
Wu, Z. et al. Phase II study of lapatinib and capecitabine in second-line treatment for metastatic pancreatic cancer. Cancer Chemother. Pharmacol. 76, 1309–1314 (2015).
Dragovich, T. et al. Phase II trial of vatalanib in patients with advanced or metastatic pancreatic adenocarcinoma after first-line gemcitabine therapy (PCRT O4-001). Cancer Chemother. Pharmacol. 74, 379–387 (2014).
Soares, H. P. et al. A phase II study of capecitabine plus docetaxel in gemcitabine-pretreated metastatic pancreatic cancer patients: CapTere. Cancer Chemother. Pharmacol. 73, 839–845 (2014).
Saif, M. W. et al. First-in-human phase II trial of the botanical formulation PHY906 with capecitabine as second-line therapy in patients with advanced pancreatic cancer. Cancer Chemother. Pharmacol. 73, 373–380 (2014).
Acknowledgements
The work of J.K. and J.P.N. is supported by the European Cooperation in Science and Technology action BM1204, “EUPancreas: An integrated European platform for pancreas cancer research: from basic science to clinical and public health interventions for a rare disease”.
Author information
Authors and Affiliations
Contributions
The authors contributed equally to the Review.
Corresponding authors
Ethics declarations
Competing interests
J.P.N. reports grants from AstraZeneca, Cancer Research UK, Clovis Oncology and Ventana, Immunovia, KAEL & GemVax (Korea), Pharma Nord and Taiho Pharma (Japan); payment for lectures from Amgen and Mylan; paid consultancy from Astellas, Boehringer Ingelheim Pharma GmbH & Co KG, Erytech, KAEL & GemVax, Novartis Pharma AG, Redhill Biopharma and Tragovax; and educational travel grants from NuCana, all of which were not related to the submitted work. J.P.N. was a UK National Institute for Health Research (NIHR) senior investigator. D.H.P. reports grants from Cancer Research UK, the NIHR and NuCana and paid consultancy from NuCana. E.C. and W.G. report grants from Cancer Research UK, Immunova, the NIHR and the European Union. J.K. and P.M. declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Glossary
- Hand–foot syndrome
-
A condition that can occur after chemotherapy in which there is redness, swelling, numbness and/or skin peeling on the palms of the hands and the soles of the feet.
- FOLFIRINOX
-
A therapy combination including folinic acid, 5-fluoruracil, irinotecan and oxaliplatin.
- Irreversible electroporation
-
(IRE). A nonthermal ablation technique that uses short (microsecond) pulses of high voltage electrical current to create permanent, lethal nanopores in the cell membrane.
- Radiofrequency ablation
-
(RFA). An ablation technique that uses heat generated from medium frequency alternating current.
- Stereotactic body radiation
-
(SBRT). A specialized type of external beam radiation therapy that uses focused radiation beams to precisely target a tumour that is well defined by detailed imaging scans.
- High-intensity focused ultrasound
-
(HIFU). A procedure that uses an acoustic lens to concentrate multiple intersecting beams of ultrasound on a target.
- Nanoliposomal irinotecan
-
An artificial, nanosized liposomal delivery system for irinotecan that is designed to keep irinotecan in circulation for longer than free irinotecan.
- Cancer-associated fibroblasts
-
(CAFs). Fibroblasts within the tumour microenvironment that promote tumorigenic features by initiating the remodelling of extracellular matrix or by secreting cytokines.
- T cell checkpoints
-
Molecules in the immune system that can either turn up (via co-stimulatory pathways) or turn down (via inhibitory pathways) immune responses
- Regulatory T cells
-
(Treg cells). A subpopulation of T cells that modulate immune system function, maintain self-tolerance and prevent autoimmune disease.
- Myeloid-derived suppressor cells
-
A heterogeneous group of immune cells of myeloid lineage (derived from bone marrow stem cells), they are strongly immunosuppressive rather than immunostimulatory.
- Effector T cells
-
A subpopulation of T cells that have an important role in executing immune functions, including releasing T cell cytokines.
- Antigen-presenting cells
-
(APCs). Cells that display antigens complexed with major histocompatibility complexes on their cell surfaces.
- Suicide cassettes
-
Components of vector DNA consisting of a suicide gene and regulatory sequence to be expressed by a transfected cell, which cause the transfected cell to undergo apoptosis.
- Kynurenine
-
A metabolite of tryptophan produced by indoleamine 2,3-dioxygenase (IDO) and tryptophan 2,3-dioxygenase (TDO).
- Neo-antigen
-
A new antigen expressed exclusively by tumour cells that is generated by the progressive mutational process that drives cancer evolution.
- Abscopal effects
-
A phenomenon whereby local radiotherapy causes regression of not only the targeted tumour but also distant tumours.
- Intensity-modulated radiation therapy
-
A high-precision conformal radiotherapy approach to deliver precise radiation doses to a malignant tumour or specific areas within the tumour.
Rights and permissions
About this article
Cite this article
Neoptolemos, J.P., Kleeff, J., Michl, P. et al. Therapeutic developments in pancreatic cancer: current and future perspectives. Nat Rev Gastroenterol Hepatol 15, 333–348 (2018). https://doi.org/10.1038/s41575-018-0005-x
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41575-018-0005-x
This article is cited by
-
NUSAP1 promotes pancreatic ductal adenocarcinoma progression by drives the epithelial-mesenchymal transition and reduces AMPK phosphorylation
BMC Cancer (2024)
-
Recent advances in the potential role of RNA N4-acetylcytidine in cancer progression
Cell Communication and Signaling (2024)
-
Construction and validation of a nomogram for cancer specific survival of postoperative pancreatic cancer based on the SEER and China database
BMC Gastroenterology (2024)
-
EPYC functions as a novel prognostic biomarker for pancreatic cancer
Scientific Reports (2024)
-
Polyunsaturated fatty acids-induced ferroptosis suppresses pancreatic cancer growth
Scientific Reports (2024)