Abstract
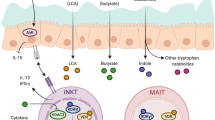
Mucosal-associated invariant T (MAIT) cells are unique innate-like T cells that bridge innate and adaptive immunity. They are activated by conserved bacterial ligands derived from vitamin B biosynthesis and have important roles in defence against bacterial and viral infections. However, they can also have various deleterious and protective functions in autoimmune, inflammatory and metabolic diseases. MAIT cell involvement in a large spectrum of pathological conditions makes them attractive targets for potential therapeutic approaches.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Porcelli, S., Yockey, C. E., Brenner, M. B. & Balk, S. P. Analysis of T cell antigen receptor (TCR) expression by human peripheral blood CD4-8- alpha/beta T cells demonstrates preferential use of several V beta genes and an invariant TCR alpha chain. J. Exp. Med. 178, 1–16 (1993).
Tilloy, F. et al. An invariant T cell receptor alpha chain defines a novel TAP-independent major histocompatibility complex class Ib-restricted alpha/beta T cell subpopulation in mammals. J. Exp. Med. 189, 1907–1921 (1999).
Treiner, E. et al. Selection of evolutionarily conserved mucosal-associated invariant T cells by MR1. Nature 422, 164–169 (2003). This study describes for the first time MAIT cell restriction to the non-polymorphic MHC class I-like protein MR1.
Martin, E. et al. Stepwise development of MAIT cells in mouse and human. PLOS Biol. 7, e54 (2009).
Gold, M. C. et al. Human mucosal associated invariant T cells detect bacterially infected cells. PLOS Biol. 8, e1000407 (2010).
Georgel, P., Radosavljevic, M., Macquin, C. & Bahram, S. The non-conventional MHC class I MR1 molecule controls infection by Klebsiella pneumoniae in mice. Mol. Immunol. 48, 769–775 (2011).
Le Bourhis, L. et al. Antimicrobial activity of mucosal-associated invariant T cells. Nat. Immunol. 11, 701–708 (2010). Gold et al. and Le Bourhis et al. were the first to show human MAIT cell activation by bacteria-infected cells in an MR1-dependent manner.
Kjer-Nielsen, L. et al. MR1 presents microbial vitamin B metabolites to MAIT cells. Nature 491, 717–723 (2012). This is the first study to describe that MR1 binds to vitamin B metabolites derived from the vitamin B 2 pathway or from vitamin B 9 photodegradation.
Corbett, A. J. et al. T cell activation by transitory neo-antigens derived from distinct microbial pathways. Nature 509, 361–365 (2014). This study deciphers the mechanisms by which vitamin B 2 -derived activating ligands for MAIT cells are synthetized.
Eckle, S. B. et al. Recognition of vitamin B precursors and byproducts by mucosal associated invariant T cells. J. Biol. Chem. 290, 30204–30211 (2015).
Dusseaux, M. et al. Human MAIT cells are xenobiotic-resistant, tissue-targeted, CD161hi IL-17-secreting T cells. Blood 117, 1250–1259 (2011).
Fergusson, J. R. et al. CD161int CD8+ T cells: a novel population of highly functional, memory CD8+ T cells enriched within the gut. Mucosal Immunol. 9, 401–413 (2016).
Gibbs, A. et al. MAIT cells reside in the female genital mucosa and are biased towards IL-17 and IL-22 production in response to bacterial stimulation. Mucosal Immunol. 10, 35–45 (2017).
Hinks, T. S. et al. Steroid-induced deficiency of mucosal-associated invariant T cells in the chronic obstructive pulmonary disease lung. implications for nontypeable Haemophilus influenzae infection. Am. J. Respir. Crit. Care Med. 194, 1208–1218 (2016).
Jeffery, H. C. et al. Biliary epithelium and liver B cells exposed to bacteria activate intrahepatic MAIT cells through MR1. J. Hepatol. 64, 1118–1127 (2016).
Magalhaes, I. et al. Mucosal-associated invariant T cell alterations in obese and type 2 diabetic patients. J. Clin. Invest. 125, 1752–1762 (2015). This is the first study to show that MAIT cells in blood and adipose tissue from obese and/or diabetic patients have an activated phenotype and exhibit a strong IL-17 profile.
Schmaler, M. et al. Modulation of bacterial metabolism by the microenvironment controls MAIT cell stimulation. Mucosal Immunol. 11, 1060–1070 (2018).
Serriari, N. E. et al. Innate mucosal-associated invariant T (MAIT) cells are activated in inflammatory bowel diseases. Clin. Exp. Immunol. 176, 266–274 (2014).
Sobkowiak, M. J. et al. Tissue-resident MAIT cell populations in human oral mucosa exhibit an activated profile and produce IL-17. Eur. J. Immunol. 49, 133–143 (2019).
Reantragoon, R. et al. Antigen-loaded MR1 tetramers define T cell receptor heterogeneity in mucosal-associated invariant T cells. J. Exp. Med. 210, 2305–2320 (2013).
Gutierrez-Arcelus, M. et al. Lymphocyte innateness defined by transcriptional states reflects a balance between proliferation and effector functions. Nat. Commun. 10, 687 (2019).
Salou, M. et al. A common transcriptomic program acquired in the thymus defines tissue residency of MAIT and NKT subsets. J. Exp. Med. 216, 133–151 (2019).
Franciszkiewicz, K. et al. MHC class I-related molecule, MR1, and mucosal-associated invariant T cells. Immunol. Rev. 272, 120–138 (2016).
Rouxel, O. & Lehuen, A. Mucosal-associated invariant T cells in autoimmune and immune-mediated diseases. Immunol. Cell Biol. 96, 618–629 (2018).
Salou, M., Franciszkiewicz, K. & Lantz, O. MAIT cells in infectious diseases. Curr. Opin. Immunol. 48, 7–14 (2017).
Keller, A. N., Corbett, A. J., Wubben, J. M., McCluskey, J. & Rossjohn, J. MAIT cells and MR1-antigen recognition. Curr. Opin. Immunol. 46, 66–74 (2017).
Kurioka, A., Walker, L. J., Klenerman, P. & Willberg, C. B. MAIT cells: new guardians of the liver. Clin. Transl Immunol. 5, e98 (2016).
Ussher, J. E., Willberg, C. B. & Klenerman, P. MAIT cells and viruses. Immunol. Cell Biol. 96, 630–641 (2018).
Karamooz, E., Harriff, M. J. & Lewinsohn, D. M. MR1-dependent antigen presentation. Semin. Cell Dev. Biol. 84, 58–64 (2018).
Gherardin, N. A., McCluskey, J., Rossjohn, J. & Godfrey, D. I. The diverse family of MR1-restricted T cells. J. Immunol. 201, 2862–2871 (2018).
Meermeier, E. W., Harriff, M. J., Karamooz, E. & Lewinsohn, D. M. MAIT cells and microbial immunity. Immunol. Cell Biol. 96, 607–617 (2018).
Magalhaes, I., Kiaf, B. & Lehuen, A. iNKT and MAIT cell alterations in diabetes. Front. Immunol. 6, 341 (2015).
Le Bourhis, L. et al. MAIT cells detect and efficiently lyse bacterially-infected epithelial cells. PLOS Pathog. 9, e1003681 (2013).
Kurioka, A. et al. MAIT cells are licensed through granzyme exchange to kill bacterially sensitized targets. Mucosal Immunol. 8, 429–440 (2015).
Koay, H. F. et al. A three-stage intrathymic development pathway for the mucosal-associated invariant T cell lineage. Nat. Immunol. 17, 1300–1311 (2016).
Kwon, Y. S. et al. Mucosal-associated invariant T cells are numerically and functionally deficient in patients with mycobacterial infection and reflect disease activity. Tuberculosis (Edinb.) 95, 267–274 (2015).
Gade, P. et al. An IFN-gamma-stimulated ATF6-C/EBP-beta-signaling pathway critical for the expression of death associated protein kinase 1 and induction of autophagy. Proc. Natl Acad. Sci. USA 109, 10316–10321 (2012).
Braverman, J. & Stanley, S. A. Nitric oxide modulates macrophage responses to Mycobacterium tuberculosis infection through activation of HIF-1alpha and repression of NF-kappaB. J. Immunol. 199, 1805–1816 (2017).
MacMicking, J. D. et al. Identification of nitric oxide synthase as a protective locus against tuberculosis. Proc. Natl Acad. Sci. USA 94, 5243–5248 (1997).
Salerno-Goncalves, R. et al. Challenge of humans with wild-type Salmonella enterica serovar typhi elicits changes in the activation and homing characteristics of mucosal-associated invariant T cells. Front. Immunol. 8, 398 (2017).
Greene, J. M. et al. MR1-restricted mucosal-associated invariant T (MAIT) cells respond to mycobacterial vaccination and infection in nonhuman primates. Mucosal Immunol. 10, 802–813 (2017).
Grimaldi, D. et al. Specific MAIT cell behaviour among innate-like T lymphocytes in critically ill patients with severe infections. Intensive Care Med. 40, 192–201 (2014).
Chua, W. J. et al. Polyclonal mucosa-associated invariant T cells have unique innate functions in bacterial infection. Infect. Immun. 80, 3256–3267 (2012).
Cui, Y. et al. Mucosal-associated invariant T cell-rich congenic mouse strain allows functional evaluation. J. Clin. Invest. 125, 4171–4185 (2015).
Meierovics, A., Yankelevich, W. J. & Cowley, S. C. MAIT cells are critical for optimal mucosal immune responses during in vivo pulmonary bacterial infection. Proc. Natl Acad. Sci. USA 110, E3119–E3128 (2013).
Meierovics, A. I. & Cowley, S. C. MAIT cells promote inflammatory monocyte differentiation into dendritic cells during pulmonary intracellular infection. J. Exp. Med. 213, 2793–2809 (2016). This study shows that MAIT cells, by producing GM-CSF, induce monocyte polarization into monocyte-derived dendritic cells.
Wang, H. et al. MAIT cells protect against pulmonary Legionella longbeachae infection. Nat. Commun. 9, 3350 (2018).
Chen, Z. et al. Mucosal-associated invariant T cell activation and accumulation after in vivo infection depends on microbial riboflavin synthesis and co-stimulatory signals. Mucosal Immunol. 10, 58–68 (2017).
Jesteadt, E. et al. Interleukin-18 is critical for mucosa-associated invariant T cell gamma interferon responses to francisella species in vitro but not in vivo. Infect. Immun. 86, e00117–18 (2018).
Ussher, J. E. et al. CD161++ CD8+ T cells, including the MAIT cell subset, are specifically activated by IL-12+IL-18 in a TCR-independent manner. Eur. J. Immunol. 44, 195–203 (2014).
Barathan, M. et al. Peripheral loss of CD8+ CD161++ TCRValpha7.2+ mucosal-associated invariant T cells in chronic hepatitis C virus-infected patients. Eur. J. Clin. Invest. 46, 170–180 (2016).
Billerbeck, E. et al. Analysis of CD161 expression on human CD8+ T cells defines a distinct functional subset with tissue-homing properties. Proc. Natl Acad. Sci. USA 107, 3006–3011 (2010).
Boeijen, L. L. et al. Mucosal-associated invariant T cells are more activated in chronic hepatitis B, but not depleted in blood: reversal by antiviral therapy. J. Infect. Dis. 216, 969–976 (2017).
Bolte, F. J. et al. Intra-hepatic depletion of mucosal-associated invariant T cells in hepatitis C virus-induced liver inflammation. Gastroenterology 153, 1392–1403 (2017).
Cosgrove, C. et al. Early and nonreversible decrease of CD161++ /MAIT cells in HIV infection. Blood 121, 951–961 (2013).
Eberhard, J. M. et al. Reduced CD161+ MAIT cell frequencies in HCV and HIV/HCV co-infection: is the liver the heart of the matter? J. Hepatol. 65, 1261–1263 (2016).
Fernandez, C. S. et al. MAIT cells are depleted early but retain functional cytokine expression in HIV infection. Immunol. Cell Biol. 93, 177–188 (2015).
Hengst, J. et al. Nonreversible MAIT cell-dysfunction in chronic hepatitis C virus infection despite successful interferon-free therapy. Eur. J. Immunol. 46, 2204–2210 (2016).
Jo, J. et al. Toll-like receptor 8 agonist and bacteria trigger potent activation of innate immune cells in human liver. PLOS Pathog. 10, e1004210 (2014).
Leeansyah, E. et al. Activation, exhaustion, and persistent decline of the antimicrobial MR1-restricted MAIT-cell population in chronic HIV-1 infection. Blood 121, 1124–1135 (2013).
Leeansyah, E. et al. Arming of MAIT cell cytolytic antimicrobial activity is induced by IL-7 and defective in HIV-1 infection. PLOS Pathog. 11, e1005072 (2015).
Loh, L. et al. Human mucosal-associated invariant T cells contribute to antiviral influenza immunity via IL-18-dependent activation. Proc. Natl Acad. Sci. USA 113, 10133–10138 (2016).
Paquin-Proulx, D. et al. MAIT cells are reduced in frequency and functionally impaired in human T lymphotropic virus type 1 infection: potential clinical implications. PLOS ONE 12, e0175345 (2017).
Sereti, I. et al. IL-7 administration drives T cell-cycle entry and expansion in HIV-1 infection. Blood 113, 6304–6314 (2009).
Sortino, O. et al. IL-7 treatment supports CD8+ mucosa-associated invariant T cell restoration in HIV-1-infected patients on antiretroviral therapy. AIDS 32, 825–828 (2018).
van Wilgenburg, B. et al. MAIT cells are activated during human viral infections. Nat. Commun. 7, 11653 (2016). This is the first study to show MAIT cell activation upon virus infection in a manner that is TCR independent but dependent on IL-18, IL-12 and IL-15.
Wilgenburg, B. V. et al. MAIT cells contribute to protection against lethal influenza infection in vivo. Nat. Commun. 9, 4706 (2018).
Yong, Y. K. et al. Decrease of CD69 levels on TCR Valpha7.2+CD4+ innate-like lymphocytes is associated with impaired cytotoxic functions in chronic hepatitis B virus-infected patients. Innate Immun. 23, 459–467 (2017).
Hegde, P. et al. Mucosal-associated invariant T cells are a profibrogenic immune cell population in the liver. Nat. Commun. 9, 2146 (2018). This study shows that MAIT cells are profibrogenic in the liver by promoting macrophage inflammatory properties and myofibroblast activation.
Rouxel, O. et al. Cytotoxic and regulatory roles of mucosal-associated invariant T cells in type 1 diabetes. Nat. Immunol. 18, 1321–1331 (2017). The study is the first to describe MAIT cell alterations in patients with type 1 diabetes and the regulatory role of these cells through maintaining gut integrity.
Salou, M. et al. Neuropathologic, phenotypic and functional analyses of mucosal associated invariant T cells in multiple sclerosis. Clin. Immunol. 166–167, 1–11 (2016).
Braudeau, C. et al. Persistent deficiency of circulating mucosal-associated invariant T (MAIT) cells in ANCA-associated vasculitis. J. Autoimmun. 70, 73–79 (2016).
Carolan, E. et al. Altered distribution and increased IL-17 production by mucosal-associated invariant T cells in adult and childhood obesity. J. Immunol. 194, 5775–5780 (2015).
Dunne, M. R. et al. Persistent changes in circulating and intestinal gammadelta T cell subsets, invariant natural killer T cells and mucosal-associated invariant T cells in children and adults with coeliac disease. PLOS ONE 8, e76008 (2013).
Fazekas, B. et al. Alterations in circulating lymphoid cell populations in systemic small vessel vasculitis are non-specific manifestations of renal injury. Clin. Exp. Immunol. 191, 180–188 (2018).
Gerart, S. et al. Human iNKT and MAIT cells exhibit a PLZF-dependent proapoptotic propensity that is counterbalanced by XIAP. Blood 121, 614–623 (2013).
Gracey, E. et al. IL-7 primes IL-17 in mucosal-associated invariant T (MAIT) cells, which contribute to the Th17-axis in ankylosing spondylitis. Ann. Rheum. Dis. 75, 2124–2132 (2016).
Guggino, G. et al. IL-17 polarization of MAIT cells is derived from the activation of two different pathways. Eur. J. Immunol. 47, 2002–2003 (2017).
Haga, K. et al. MAIT cells are activated and accumulated in the inflamed mucosa of ulcerative colitis. J. Gastroenterol. Hepatol. 31, 965–972 (2016).
Hayashi, E. et al. Involvement of mucosal-associated invariant T cells in ankylosing spondylitis. J. Rheumatol 43, 1695–1703 (2016).
Hiejima, E. et al. Reduced numbers and proapoptotic features of mucosal-associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 21, 1529–1540 (2015).
Renand, A. et al. Immune alterations in patients with type 1 autoimmune hepatitis persist upon standard immunosuppressive treatment. Hepatol. Commun. 2, 968–981 (2018).
Riva, A. et al. Mucosa-associated invariant T cells link intestinal immunity with antibacterial immune defects in alcoholic liver disease. Gut 67, 918–930 (2018). This study shows that in patients with alcoholic liver disease, MAIT cell dysfunction is associated with gut dysbiosis.
Toussirot, E., Laheurte, C., Gaugler, B., Gabriel, D. & Saas, P. Increased IL-22- and IL-17A-producing mucosal-associated invariant T cells in the peripheral blood of patients with ankylosing spondylitis. Front. Immunol. 9, 1610 (2018).
von Seth, E. et al. Primary sclerosing cholangitis leads to dysfunction and loss of MAIT cells. Eur. J. Immunol. 48, 1997–2004 (2018).
Wang, J. J., Macardle, C., Weedon, H., Beroukas, D. & Banovic, T. Mucosal-associated invariant T cells are reduced and functionally immature in the peripheral blood of primary Sjogren’s syndrome patients. Eur. J. Immunol. 46, 2444–2453 (2016).
Bottcher, K. et al. MAIT cells are chronically activated in patients with autoimmune liver disease and promote profibrogenic hepatic stellate cell activation. Hepatology 68, 172–186 (2018).
Chiba, A. et al. Activation status of mucosal-associated invariant T cells reflects disease activity and pathology of systemic lupus erythematosus. Arthritis Res. Ther. 19, 58 (2017).
Cho, Y. N. et al. Mucosal-associated invariant T cell deficiency in systemic lupus erythematosus. J. Immunol. 193, 3891–3901 (2014).
Hinks, T. S. et al. Innate and adaptive T cells in asthmatic patients: relationship to severity and disease mechanisms. J. Allergy Clin. Immunol. 136, 323–333 (2015).
Kim, M. et al. TNFalpha and IL-1beta in the synovial fluid facilitate mucosal-associated invariant T (MAIT) cell migration. Cytokine 99, 91–98 (2017).
Lezmi, G. et al. Circulating IL-17-producing mucosal-associated invariant T cells (MAIT) are associated with symptoms in children with asthma. Clin. Immunol. 188, 7–11 (2018).
Li, Y. et al. Mucosal-associated invariant T cells improve nonalcoholic fatty liver disease through regulating macrophage polarization. Front. Immunol. 9, 1994 (2018).
Touch, S. et al. Mucosal-associated invariant T (MAIT) cells are depleted and prone to apoptosis in cardiometabolic disorders. FASEB J. 32, fj201800052RR (2018).
Miyazaki, Y., Miyake, S., Chiba, A., Lantz, O. & Yamamura, T. Mucosal-associated invariant T cells regulate Th1 response in multiple sclerosis. Int. Immunol. 23, 529–535 (2011).
Illes, Z., Shimamura, M., Newcombe, J., Oka, N. & Yamamura, T. Accumulation of Valpha7.2-Jalpha33 invariant T cells in human autoimmune inflammatory lesions in the nervous system. Int. Immunol. 16, 223–230 (2004).
Li, J. et al. The frequency of mucosal-associated invariant T cells is selectively increased in dermatitis herpetiformis. Australas. J. Dermatol. 58, 200–204 (2017).
Teunissen, M. B. M. et al. The IL-17A-producing CD8+ T cell population in psoriatic lesional skin comprises mucosa-associated invariant T cells and conventional T cells. J. Invest. Dermatol. 134, 2898–2907 (2014).
Willing, A. et al. CD8+ MAIT cells infiltrate into the CNS and alterations in their blood frequencies correlate with IL-18 serum levels in multiple sclerosis. Eur. J. Immunol. 44, 3119–3128 (2014).
Abrahamsson, S. V. et al. Non-myeloablative autologous haematopoietic stem cell transplantation expands regulatory cells and depletes IL-17 producing mucosal-associated invariant T cells in multiple sclerosis. Brain 136, 2888–2903 (2013).
Nicoletti, F. et al. Increased serum levels of interleukin-18 in patients with multiple sclerosis. Neurology 57, 342–344 (2001).
Ifergan, I. et al. Central nervous system recruitment of effector memory CD8+ T lymphocytes during neuroinflammation is dependent on alpha4 integrin. Brain 134, 3560–3577 (2011).
Ito, T., Carson, W. F.t., Cavassani, K. A., Connett, J. M. & Kunkel, S. L. CCR6 as a mediator of immunity in the lung and gut. Exp. Cell Res. 317, 613–619 (2011).
Ganz, T. Defensins: antimicrobial peptides of innate immunity. Nat. Rev. Immunol. 3, 710–720 (2003).
Miani, M. et al. Gut microbiota-stimulated innate lymphoid cells support beta-defensin 14 expression in pancreatic endocrine cells, preventing autoimmune diabetes. Cell Metab. 28, 557–572 (2018).
Godfrey, D. I., Uldrich, A. P., McCluskey, J., Rossjohn, J. & Moody, D. B. The burgeoning family of unconventional T cells. Nat. Immunol. 16, 1114–1123 (2015).
Novak, J., Dobrovolny, J., Novakova, L. & Kozak, T. The decrease in number and change in phenotype of mucosal-associated invariant T cells in the elderly and differences in men and women of reproductive age. Scand. J. Immunol. 80, 271–275 (2014).
Walker, L. J., Tharmalingam, H. & Klenerman, P. The rise and fall of MAIT cells with age. Scand. J. Immunol. 80, 462–463 (2014).
Hanson, E. D. et al. Exercise increases mucosal-associated invariant T cell cytokine expression but not activation or homing markers. Med. Sci. Sports Exerc. 51, 379–388 (2019).
Keller, A. N. et al. Drugs and drug-like molecules can modulate the function of mucosal-associated invariant T cells. Nat. Immunol. 18, 402–411 (2017).
Annibali, V. et al. CD161highCD8+T cells bear pathogenetic potential in multiple sclerosis. Brain 134, 542–554 (2011).
Hinks, T. S. Reduced numbers and proapoptotic features of mucosal-associated invariant T cells as a characteristic finding in patients with inflammatory bowel disease. Inflamm. Bowel Dis. 21, E30 (2015).
Dias, J., Leeansyah, E. & Sandberg, J. K. Multiple layers of heterogeneity and subset diversity in human MAIT cell responses to distinct microorganisms and to innate cytokines. Proc. Natl Acad. Sci. USA 114, E5434–E5443 (2017).
Dias, J. et al. The CD4−CD8− MAIT cell subpopulation is a functionally distinct subset developmentally related to the main CD8+ MAIT cell pool. Proc. Natl Acad. Sci. USA 115, E11513–E11522 (2018).
Rahimpour, A. et al. Identification of phenotypically and functionally heterogeneous mouse mucosal-associated invariant T cells using MR1 tetramers. J. Exp. Med. 212, 1095–1108 (2015). This is the first study to provide exhaustive MAIT cell phenotypes and cytokine production in different tissues of C57BL/6 and BALB/c mice.
Marwaha, A. K., Leung, N. J., McMurchy, A. N. & Levings, M. K. TH17 cells in autoimmunity and immunodeficiency: protective or pathogenic? Front. Immunol. 3, 129 (2012).
Chiba, A. et al. Mucosal-associated invariant T cells promote inflammation and exacerbate disease in murine models of arthritis. Arthritis Rheum. 64, 153–161 (2012).
Willing, A., Jager, J., Reinhardt, S., Kursawe, N. & Friese, M. A. Production of IL-17 by MAIT cells is increased in multiple sclerosis and is associated with IL-7 receptor expression. J. Immunol. 200, 974–982 (2018).
Ciccia, F. et al. Type 3 innate lymphoid cells producing IL-17 and IL-22 are expanded in the gut, in the peripheral blood, synovial fluid and bone marrow of patients with ankylosing spondylitis. Ann. Rheum. Dis. 74, 1739–1747 (2015).
Rihl, M. et al. Identification of interleukin-7 as a candidate disease mediator in spondylarthritis. Arthritis Rheum. 58, 3430–3435 (2008).
International Genetics of Ankylosing Spondylitis Consortium (IGAS). Identification of multiple risk variants for ankylosing spondylitis through high-density genotyping of immune-related loci. Nat. Genet. 45, 730–738 (2013).
Fabbrini, E. et al. Association between specific adipose tissue CD4+ T cell populations and insulin resistance in obese individuals. Gastroenterology 145, 366–374 (2013).
Zuniga, L. A. et al. IL-17 regulates adipogenesis, glucose homeostasis, and obesity. J. Immunol. 185, 6947–6959 (2010).
Kuric, E. et al. No evidence for presence of mucosal-associated invariant T cells in the insulitic lesions in patients recently diagnosed with type 1 diabetes. Am. J. Pathol. 188, 1744–1748 (2018).
Ruijing, X. et al. Jα33+ MAIT cells play a protective role in TNBS induced intestinal inflammation. Hepatogastroenterology 59, 762–767 (2012).
Croxford, J. L., Miyake, S., Huang, Y. Y., Shimamura, M. & Yamamura, T. Invariant Vα19i T cells regulate autoimmune inflammation. Nat. Immunol. 7, 987–994 (2006). This is the first report to describe MAIT cell inhibition of autoimmune disease in the context of experimental autoimmune encephalomyelitis.
Song, F. et al. Expression of the neutrophil chemokine KC in the colon of mice with enterocolitis and by intestinal epithelial cell lines: effects of flora and proinflammatory cytokines. J. Immunol. 162, 2275–2280 (1999).
Kaneko, M., Akiyama, Y., Takimoto, H. & Kumazawa, Y. Mechanism of up-regulation of immunoglobulin A production in the intestine of mice unresponsive to lipopolysaccharide. Immunology 116, 64–70 (2005).
Chandra, S. et al. Development of asthma in inner-city children: possible roles of MAIT cells and variation in the home environment. J. Immunol. 200, 1995–2003 (2018).
Bach, J. F. The hygiene hypothesis in autoimmunity: the role of pathogens and commensals. Nat. Rev. Immunol. 18, 105–120 (2018).
Shimamura, M. et al. Regulation of immunological disorders by invariant Valpha19-Jalpha33 TCR-bearing cells. Immunobiology 216, 374–378 (2011).
Harley, I. T. et al. IL-17 signaling accelerates the progression of nonalcoholic fatty liver disease in mice. Hepatology 59, 1830–1839 (2014).
Marra, F. & Lotersztajn, S. Pathophysiology of NASH: perspectives for a targeted treatment. Curr. Pharm. Des. 19, 5250–5269 (2013).
Ling, L. et al. Circulating and tumor-infiltrating mucosal associated invariant T (MAIT) cells in colorectal cancer patients. Sci. Rep. 6, 20358 (2016).
Zabijak, L. et al. Increased tumor infiltration by mucosal-associated invariant T cells correlates with poor survival in colorectal cancer patients. Cancer Immunol. Immunother. 64, 1601–1608 (2015).
Won, E. J. et al. Clinical relevance of circulating mucosal-associated invariant T cell levels and their anti-cancer activity in patients with mucosal-associated cancer. Oncotarget 7, 76274–76290 (2016).
Duan, M. et al. Activated and exhausted MAIT cells foster disease progression and indicate poor outcome in hepatocellular carcinoma. Clin. Cancer Res. 25, 3304–3316 (2019). This study describes major MAIT cell alterations in hepatocellular carcinoma and the poor clinical outcome associated with high tumour MAIT cell infiltration.
Sundstrom, P. et al. Human mucosa-associated invariant T cells accumulate in colon adenocarcinomas but produce reduced amounts of IFN-gamma. J. Immunol. 195, 3472–3481 (2015).
Shaler, C. R. et al. Mucosa-associated invariant T cells infiltrate hepatic metastases in patients with colorectal carcinoma but are rendered dysfunctional within and adjacent to tumor microenvironment. Cancer Immunol. Immunother. 66, 1563–1575 (2017).
Kawaguchi, K. et al. Influence of post-transplant mucosal-associated invariant T cell recovery on the development of acute graft-versus-host disease in allogeneic bone marrow transplantation. Int. J. Hematol. 108, 66–75 (2018).
Bhattacharyya, A. et al. Graft-derived reconstitution of mucosal-associated invariant T cells after allogeneic hematopoietic cell transplantation. Biol. Blood Marrow Transplant. 24, 242–251 (2018).
Varelias, A. et al. Recipient mucosal-associated invariant T cells control GVHD within the colon. J. Clin. Invest. 128, 1919–1936 (2018).
Mondot, S., Boudinot, P. & Lantz, O. MAIT, MR1, microbes and riboflavin: a paradigm for the co-evolution of invariant TCRs and restricting MHCI-like molecules? Immunogenetics 68, 537–548 (2016).
Garner, L. C., Klenerman, P. & Provine, N. M. Insights into mucosal-associated invariant T cell biology from studies of invariant natural killer T cells. Front. Immunol. 9, 1478 (2018).
Seino, K. & Taniguchi, M. Functionally distinct NKT cell subsets and subtypes. J. Exp. Med. 202, 1623–1626 (2005).
Kain, L. et al. The identification of the endogenous ligands of natural killer T cells reveals the presence of mammalian alpha-linked glycosylceramides. Immunity 41, 543–554 (2014).
Tastan, C. et al. Tuning of human MAIT cell activation by commensal bacteria species and MR1-dependent T cell presentation. Mucosal Immunol. 11, 1591–1605 (2018).
Lepore, M. et al. Functionally diverse human T cells recognize non-microbial antigens presented by MR1. eLife 6, e24476 (2017).
Opazo, M. C. et al. Intestinal microbiota influences non-intestinal related autoimmune diseases. Front. Microbiol. 9, 432 (2018).
Kamada, N., Seo, S. U., Chen, G. Y. & Nunez, G. Role of the gut microbiota in immunity and inflammatory disease. Nat. Rev. Immunol. 13, 321–335 (2013).
Kho, Z. Y. & Lal, S. K. The human gut microbiome - a potential controller of wellness and disease. Front. Microbiol. 9, 1835 (2018).
Voillet, V. et al. Human MAIT cells exit peripheral tissues and recirculate via lymph in steady state conditions. JCI Insight 3, 98487 (2018).
Sugimoto, C. et al. Mucosal-associated invariant T cell is a potential marker to distinguish fibromyalgia syndrome from arthritis. PLOS ONE 10, e0121124 (2015).
Gherardin, N. A. et al. Human blood MAIT cell subsets defined using MR1 tetramers. Immunol. Cell Biol. 96, 507–525 (2018).
Nicol, B. et al. An intermediate level of CD161 expression defines a novel activated, inflammatory, and pathogenic subset of CD8+ T cells involved in multiple sclerosis. J. Autoimmun. 88, 61–74 (2018).
Cheuk, S. et al. CD49a expression defines tissue-resident CD8+ T cells poised for cytotoxic function in human skin. Immunity 46, 287–300 (2017).
Jiang, J. et al. Mucosal-associated invariant T cells from patients with tuberculosis exhibit impaired immune response. J. Infect. 72, 338–352 (2016).
Cliff, J. M. et al. Cellular immune function in myalgic encephalomyelitis/chronic fatigue syndrome (ME/CFS). Front. Immunol. 10, 796 (2019).
Ishimori, A. et al. Circulating activated innate lymphoid cells and mucosal-associated invariant T cells are associated with airflow limitation in patients with asthma. Allergol. Int. 66, 302–309 (2017).
Peterfalvi, A. et al. Invariant Vα7.2-Jα33 TCR is expressed in human kidney and brain tumors indicating infiltration by mucosal-associated invariant T (MAIT) cells. Int. Immunol. 20, 1517–1525 (2008).
Zumwalde, N. A., Haag, J. D., Gould, M. N. & Gumperz, J. E. Mucosal associated invariant T cells from human breast ducts mediate a Th17-skewed response to bacterially exposed breast carcinoma cells. Breast Cancer Res. 20, 111 (2018).
Acknowledgements
The authors are grateful to U. Rogner for critical reading of the manuscript. This work was supported by grants from INSERM, CNRS, Laboratoire d’Excellence consortium Inflamex (grant number ANR-11-IDEX-0005-02) and the Fondation pour la Recherche Médicale (FRM grant numbers DEQ20140329520, to A.L., and DEQ20150331726, to S.L.), EFSD/JDRF/Lilly and EFSD/Lilly (to A.L. and A.T.), Fondation Francophone pour la recherche sur le Diabète (to A.L.), an Aide aux Jeunes Diabétiques fellowship to I.N., and Agence Nationale de la Recherche (ANR OBEMAIT, Provide and Diab1MAIT grants to A.L.).
Reviewer information
Nature Reviews Immunology thanks P. Klenerman and J. Sandberg for their contribution to the peer review of this work.
Author information
Authors and Affiliations
Contributions
All authors contributed to writing the review. A.T. and I.N. contributed equally.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Toubal, A., Nel, I., Lotersztajn, S. et al. Mucosal-associated invariant T cells and disease. Nat Rev Immunol 19, 643–657 (2019). https://doi.org/10.1038/s41577-019-0191-y
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41577-019-0191-y
This article is cited by
-
Mitochondrial and metabolic dysfunction of peripheral immune cells in multiple sclerosis
Journal of Neuroinflammation (2024)
-
Mucosal-associated invariant T cells in infectious diseases of respiratory system: recent advancements and applications
Journal of Inflammation (2024)
-
MAIT cell inhibition promotes liver fibrosis regression via macrophage phenotype reprogramming
Nature Communications (2023)
-
Hepatic inflammatory responses in liver fibrosis
Nature Reviews Gastroenterology & Hepatology (2023)
-
Locally sourced: site-specific immune barriers to metastasis
Nature Reviews Immunology (2023)