Abstract
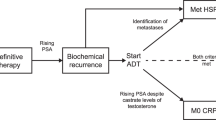
Metastatic prostate cancer is associated with considerable morbidity and mortality. Standard treatment for non-metastatic prostate cancer, to prevent metastatic progression, is androgen deprivation therapy (ADT); however, many patients will eventually develop castration-resistant prostate cancer (CRPC), which can prove challenging to treat. Between the stages of non-metastatic androgen-sensitive disease and metastatic CRPC is an intermediate disease state that has been termed non-metastatic CRPC (nmCRPC), which is a heterogeneous, man-made disease stage that occurs after a patient who has no radiological evidence of metastasis shows evidence of cancer progression even after ADT. Awareness of nmCRPC has risen owing to an increased use of ADT and its eventual failure. Men with nmCRPC are at a high risk of progression to mCRPC, with historically few options to halt this process. However, in the past two decades, multiple therapies have been investigated for the treatment of nmCRPC, including endothelin receptor antagonists and bone-targeted therapies, but none has changed the standard of care. In the past decade, the efficacy of androgen receptor pathway-targeting modalities has been investigated. Three novel nonsteroidal antiandrogen agents for treating high-risk nmCRPC have been investigated; the PROSPER, SPARTAN and ARAMIS trials were phase III, randomized, placebo-controlled clinical trials that investigated the efficacy and safety of enzalutamide, apalutamide and darolutamide, respectively. All three therapeutics showed statistically significant improvements in metastasis-free survival, progression to antineoplastic therapy was lengthened and at final analysis, overall survival was significantly improved. The comparative efficacy and safety of all three agents has not yet been investigated in a comprehensive clinical trial, but approval of these medications by the FDA and other regulatory agencies means that providers now have three effective therapeutic options to augment ADT for patients with nmCRPC.
Key points
-
Non-metastatic castration-resistant prostate cancer (nmCRPC) is a heterogeneous disease classification. This disease stage occurs after a patient with prostate cancer who has no radiological evidence of metastasis shows evidence of cancer progression even after androgen deprivation therapy.
-
Multiple therapies have been investigated for treating nmCRPC, including endothelin receptor antagonists and bone-targeted therapies. Within the past decade, the efficacy of androgen receptor pathway-targeting modalities has been investigated.
-
Three phase III, randomized, placebo-controlled, clinical trials (PROSPER, SPARTAN and ARAMIS) have shown significant metastasis-free survival and overall survival benefits of enzalutamide, apalutamide and darolutamide, respectively.
-
These novel nonsteroidal antiandrogen agents have been approved by governmental agencies, such as the FDA, for nmCRPC, and apalutamide and enzalutamide have been included in international guidelines, such as those from the AUA and EAU.
-
To our knowledge, no head-to-head comparison of all three therapeutic agents exists to compare their efficacy and safety; however, all three have shown comparable efficacy in separate trials.
-
Patient selection can be made through shared decision-making between patients and physicians based on differing adverse effect profiles and other differing parameters between the three therapeutics.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout

Similar content being viewed by others
References
Pernar, C. H., Ebot, E. M., Wilson, K. M. & Mucci, L. A. The epidemiology of prostate cancer. Cold Spring Harb. Perspect. Med. 10, 63–89 (2018).
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 70, 7–30 (2020).
American Cancer Society. Cancer facts & figures 2018 (American Cancer Society, 2019).
Dickinson, J. et al. Trends in prostate cancer incidence and mortality in Canada during the era of prostate-specific antigen screening. CMAJ Open 4, E73–E79 (2016).
Hodges, C. Studies on prostatic cancer I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res. 1, 293–297 (1941).
Huggins, C. & Hodges, C. V. Studies on prostatic cancer. I. The effect of castration, of estrogen and androgen injection on serum phosphatases in metastatic carcinoma of the prostate. CA Cancer J. Clin. 22, 232–240 (1972).
Loriot, Y. et al. Management of non-metastatic castrate-resistant prostate cancer: a systematic review. Cancer Treat. Rev. 70, 223–231 (2018).
Scher, H. I. et al. Trial design and objectives for castration-resistant prostate cancer: updated recommendations from the prostate cancer clinical trials working group 3. J. Clin. Oncol. 34, 1402–1418 (2016).
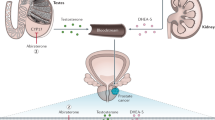
Roy, A. K. et al. Regulation of androgen action. Vitam. Hormones 55, 309–352 (1999).
Mangelsdorf, D. J. et al. The nuclear receptor superfamily: the second decade. Cell 83, 835–839 (1995).
Crawford, E. D. et al. Androgen receptor targeted treatments of prostate cancer: 35 years of progress with antiandrogens. J. Urol. 200, 956–966 (2018).
Culig, Z. & Santer, F. R. Androgen receptor signaling in prostate cancer. Cancer Metastasis Rev. 33, 413–427 (2014).
Waltering, K. K., Urbanucci, A. & Visakorpi, T. Androgen receptor (AR) aberrations in castration-resistant prostate cancer. Mol. Cell. Endocrinol. 360, 38–43 (2012).
Ahmed, A., Ali, S. & Sarkar, F. H. Advances in androgen receptor targeted therapy for prostate cancer. J. Cell Physiol. 229, 271–276 (2014).
Beer, T. M. et al. Enzalutamide in metastatic prostate cancer before chemotherapy. N. Engl. J. Med. 371, 424–433 (2014).
Chi, K. N. et al. Apalutamide for metastatic, castration-sensitive prostate cancer. N. Engl. J. Med. 381, 13–24 (2019).
Hong, J. H. & Kim, I. Y. Nonmetastatic castration-resistant prostate cancer. Korean J. Urol. 55, 153–160 (2014).
Fizazi, K. et al. Darolutamide in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 380, 1235–1246 (2019).
Hussain, M. et al. Enzalutamide in men with nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 378, 2465–2474 (2018).
Smith, M. R. et al. Apalutamide treatment and metastasis-free survival in prostate cancer. N. Engl. J. Med. 378, 1408–1418 (2018).
El-Amm, J. & Aragon-Ching, J. B. The current landscape of treatment in non-metastatic castration-resistant prostate cancer. Clin. Med. Insights Oncol. 13, 1179554919833927 (2019).
Mateo, J. et al. Managing nonmetastatic castration-resistant prostate cancer. Eur. Urol. 75, 285–293 (2019).
Saad, F., Bögemann, M., Suzuki, K. & Shore, N. Treatment of nonmetastatic castration-resistant prostate cancer: focus on second-generation androgen receptor inhibitors. Prostate Cancer Prostatic Dis. https://doi.org/10.1038/s41391-020-00310-3 (2021).
Scher, H. I., Solo, K., Valant, J., Todd, M. B. & Mehra, M. Prevalence of prostate cancer clinical states and mortality in the United States: estimates using a dynamic progression model. PLoS One 10, e0139440 (2015).
Wade, C. A. & Kyprianou, N. Profiling prostate cancer therapeutic resistance. Int. J. Mol. Sci. 19, 904 (2018).
Halabi, S. et al. Meta-analysis evaluating the impact of site of metastasis on overall survival in men with castration-resistant prostate cancer. J. Clin. Oncol. 34, 1652–1659 (2016).
Oefelein, M. G. et al. Clinical predictors of androgen-independent prostate cancer and survival in the prostate-specific antigen era. Urology 60, 120–124 (2002).
Hussain, M. et al. Absolute prostate-specific antigen value after androgen deprivation is a strong independent predictor of survival in new metastatic prostate cancer: data from Southwest Oncology Group Trial 9346 (INT-0162). J. Clin. Oncol. 24, 3984–3990 (2006).
Ma, J. et al. Prediagnostic body-mass index, plasma C-peptide concentration, and prostate cancer-specific mortality in men with prostate cancer: a long-term survival analysis. Lancet Oncol. 9, 1039–1047 (2008).
Moreira, D. M. et al. Predictors of time to metastasis in castration-resistant prostate cancer. Urology 96, 171–176 (2016).
Kohaar, I., Petrovics, G. & Srivastava, S. A rich array of prostate cancer molecular biomarkers: opportunities and challenges. Int. J. Mol. Sci. 20, 1813 (2019).
de Bono, J. S. et al. Circulating tumor cells predict survival benefit from treatment in metastatic castration-resistant prostate cancer. Clin. Cancer Res. 14, 6302–6309 (2008).
Thalgott, M. et al. Detection of circulating tumor cells in different stages of prostate cancer. J. Cancer Res. Clin. Oncol. 139, 755–763 (2013).
Di Nunno, V. et al. Recent advances in liquid biopsy in patients with castration resistant prostate cancer. Front. Oncol. 8, 397–397 (2018).
Chalfin, H. J. et al. Prostate cancer disseminated tumor cells are rarely detected in the bone marrow of patients with localized disease undergoing radical prostatectomy across multiple rare cell detection platforms. J. Urol. 199, 1494–1501 (2018).
Taneja, S. S. Imaging in the diagnosis and management of prostate cancer. Rev. Urol. 6, 101–113 (2004).
Maurer, T. et al. Diagnostic efficacy of 68gallium-PSMA positron emission tomography compared to conventional imaging for lymph node staging of 130 consecutive patients with intermediate to high risk prostate cancer. J. Urol. 195, 1436–1443 (2016).
Pyka, T. et al. Comparison of bone scintigraphy and 68 Ga-PSMA PET for skeletal staging in prostate cancer. Eur. J. Nucl. Med. Mol. Imaging 43, 2114–2121 (2016).
Stattin, P. et al. Prostate cancer mortality in areas with high and low prostate cancer incidence. J. Natl Cancer Inst. 106, dju007 (2014).
Gravis, G. et al. Androgen deprivation therapy (ADT) plus docetaxel versus ADT alone in metastatic non castrate prostate cancer: impact of metastatic burden and long-term survival analysis of the randomized phase 3 GETUG-AFU15 trial. Eur. Urol. 70, 256–262 (2016).
Xie, W. et al. Metastasis-free survival is a strong surrogate of overall survival in localized prostate cancer. J. Clin. Oncol. 35, 3097 (2017).
Nakayama, M. et al. Association of early PSA decline and time to PSA progression in abiraterone acetate-treated metastatic castration-resistant prostate cancer; a post-hoc analysis of Japanese phase 2 trials. BMC Urol. 16, 27 (2016).
Pinover, W. H., Horwitz, E. M., Hanlon, A. L., Uzzo, R. G. & Hanks, G. E. Validation of a treatment policy for patients with prostate specific antigen failure after three-dimensional conformal prostate radiation therapy. Cancer 97, 1127–1133 (2003).
Smith, M. R., Cook, R., Lee, K. A. & Nelson, J. B. Disease and host characteristics as predictors of time to first bone metastasis and death in men with progressive castration-resistant nonmetastatic prostate cancer. Cancer 117, 2077–2085 (2011).
Duchesne, G. M. et al. Timing of androgen-deprivation therapy in patients with prostate cancer with a rising PSA (TROG 03.06 and VCOG PR 01-03 [TOAD]): a randomised, multicentre, non-blinded, phase 3 trial. Lancet Oncol. 17, 727–737 (2016).
Jung, M. E. et al. Structure-activity relationship for thiohydantoin androgen receptor antagonists for castration-resistant prostate cancer (CRPC). J. Med. Chem. 53, 2779–2796 (2010).
Tran, C. et al. Development of a second-generation antiandrogen for treatment of advanced prostate cancer. Science 324, 787–790 (2009).
Penson, D. F. et al. Enzalutamide versus bicalutamide in castration-resistant prostate cancer: the STRIVE trial. J. Clin. Oncol. 34, 2098–2106 (2016).
Clegg, N. J. et al. ARN-509: a novel antiandrogen for prostate cancer treatment. Cancer Res. 72, 1494–1503 (2012).
Fujita, K. & Nonomura, N. Role of androgen receptor in prostate cancer: a review. World J. Mens Health 37, 288–295 (2019).
Smith, M. R. et al. Phase 2 study of the safety and antitumor activity of apalutamide (ARN-509), a potent androgen receptor antagonist, in the high-risk nonmetastatic castration-resistant prostate cancer cohort. Eur. Urol. 70, 963–970 (2016).
Leibowitz-Amit, R. & Joshua, A. M. Targeting the androgen receptor in the management of castration-resistant prostate cancer: rationale, progress, and future directions. Curr. Oncol. 19, S22–S31 (2012).
Moilanen, A. M. et al. Discovery of ODM-201, a new-generation androgen receptor inhibitor targeting resistance mechanisms to androgen signaling-directed prostate cancer therapies. Sci. Rep. 5, 12007 (2015).
Fizazi, K. et al. Activity and safety of ODM-201 in patients with progressive metastatic castration-resistant prostate cancer (ARADES): an open-label phase 1 dose-escalation and randomised phase 2 dose expansion trial. Lancet Oncol. 15, 975–985 (2014).
Zurth, C. et al. Blood-brain barrier penetration of [14C]darolutamide compared with [14C]enzalutamide in rats using whole body autoradiography. J. Clin. Oncol. 36 (Suppl. 6), 345 (2018).
Zurth, C., Sandmann, S., Trummel, D., Seidel, D. & Gieschen, H. Higher blood–brain barrier penetration of [14C]apalutamide and [14C]enzalutamide compared to [14C]darolutamide in rats using whole-body autoradiography. J. Clin. Oncol. 37 (Suppl. 7), 156 (2019).
Huggins, C. Effect of orchiectomy and irradiation on cancer of the prostate. Ann. Surg. 115, 1192–1200 (1942).
Denmeade, S. R. & Isaacs, J. T. A history of prostate cancer treatment. Nat. Rev. Cancer 2, 389–396 (2002).
Taylor, C. D., Elson, P. & Trump, D. L. Importance of continued testicular suppression in hormone-refractory prostate cancer. J. Clin. Oncol. 11, 2167–2172 (1993).
Gilligan, T. & Kantoff, P. W. Chemotherapy for prostate cancer. Urology 60, 94–100 (2002).
Newling, D. W. The management of hormone refractory prostate cancer. Eur. Urol. 29 (Suppl 2), 69–74 (1996).
Rice, M. A., Malhotra, S. V. & Stoyanova, T. Second-generation antiandrogens: from discovery to standard of care in castration resistant prostate cancer. Front. Oncol. 9, 801 (2019).
Masiello, D., Cheng, S., Bubley, G. J., Lu, M. L. & Balk, S. P. Bicalutamide functions as an androgen receptor antagonist by assembly of a transcriptionally inactive receptor. J. Biol. Chem. 277, 26321–26326 (2002).
Schellhammer, P. F. et al. Clinical benefits of bicalutamide compared with flutamide in combined androgen blockade for patients with advanced prostatic carcinoma: final report of a double-blind, randomized, multicenter trial. Casodex Combination Study Group. Urology 50, 330–336 (1997).
Sarosdy, M. F. Which is the optimal antiandrogen for use in combined androgen blockade of advanced prostate cancer? The transition from a first- to second-generation antiandrogen. Anticancer. Drugs 10, 791–796 (1999).
Hotte, S. J. & Saad, F. Current management of castrate-resistant prostate cancer. Curr. Oncol. 17, S72 (2010).
Patel, V., Liaw, B. & Oh, W. The role of ketoconazole in current prostate cancer care. Nat. Rev. Urol. 15, 643–651 (2018).
Thakur, A., Roy, A., Ghosh, A., Chhabra, M. & Banerjee, S. Abiraterone acetate in the treatment of prostate cancer. Biomed. Pharmacother. 101, 211–218 (2018).
Klaassen, Z., Wallis, C. J. D. & Fleshner, N. E. Abiraterone acetate for nonmetastatic castration-resistant prostate cancer — the forgotten dance partner? JAMA Oncol. 5, 144–145 (2019).
Ryan, C. J. et al. The IMAAGEN study: effect of abiraterone acetate and prednisone on prostate specific antigen and radiographic disease progression in patients with nonmetastatic castration resistant prostate cancer. J. Urol. 200, 344–352 (2018).
Dhillon, S. Zoledronic acid (Reclast®, Aclasta®): a review in osteoporosis. Drugs 76, 1683–1697 (2016).
Smith, M. R. et al. Natural history of rising serum prostate-specific antigen in men with castrate nonmetastatic prostate cancer. J. Clin. Oncol. 23, 2918–2925 (2005).
Nelson, J. B. et al. Identification of endothelin-1 in the pathophysiology of metastatic adenocarcinoma of the prostate. Nat. Med. 1, 944–949 (1995).
Nelson, J. B. et al. Phase 3, randomized, controlled trial of atrasentan in patients with nonmetastatic, hormone-refractory prostate cancer. Cancer 113, 2478–2487 (2008).
Miller, K. et al. Phase III, randomized, placebo-controlled study of once-daily oral zibotentan (ZD4054) in patients with non-metastatic castration-resistant prostate cancer. Prostate Cancer Prostatic Dis. 16, 187–192 (2013).
McClung, M. R. et al. Denosumab in postmenopausal women with low bone mineral density. N. Engl. J. Med. 354, 821–831 (2006).
Smith, M. R. et al. Denosumab and bone metastasis-free survival in men with nonmetastatic castration-resistant prostate cancer: exploratory analyses by baseline prostate-specific antigen doubling time. J. Clin. Oncol. 31, 3800–3806 (2013).
Sternberg, C. N. et al. Enzalutamide and survival in nonmetastatic, castration-resistant prostate cancer. N. Engl. J. Med. 382, 2197–2206 (2020).
Smith, M. R. et al. Apalutamide and overall survival in prostate cancer. Eur. Urol. 30, 1813–1820 (2020).
Small, E. J. et al. Final survival results from SPARTAN, a phase III study of apalutamide (APA) versus placebo (PBO) in patients (pts) with nonmetastatic castration-resistant prostate cancer (nmCRPC). J. Clin. Oncol. 38, 5516–5516 (2020).
Fizazi, K. et al. Nonmetastatic, castration-resistant prostate cancer and survival with darolutamide. N. Engl. J. Med. 383, 1040–1049 (2020).
Wallis, C. J. D. et al. Advanced androgen blockage in nonmetastatic castration-resistant prostate cancer: an indirect comparison of apalutamide and enzalutamide. Eur. Urol. Oncol. 1, 238–241 (2018).
Tombal, B. et al. Patient-reported outcomes following enzalutamide or placebo in men with non-metastatic, castration-resistant prostate cancer (PROSPER): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 20, 556–569 (2019).
Saad, F. et al. Effect of apalutamide on health-related quality of life in patients with non-metastatic castration-resistant prostate cancer: an analysis of the SPARTAN randomised, placebo-controlled, phase 3 trial. Lancet Oncol. 19, 1404–1416 (2018).
Gillessen, S. et al. Management of patients with advanced prostate cancer: report of the advanced prostate cancer consensus conference 2019. Eur. Urol. 77, 508–547 (2020).
Unger, J. M., Cook, E., Tai, E. & Bleyer, A. The role of clinical trial participation in cancer research: barriers, evidence, and strategies. Am. Soc. Clin. Oncol. Educ. Book 35, 185–198 (2016).
El-Amm, J. & Aragon-Ching, J. B. The current landscape of treatment in non-metastatic castration-resistant prostate cancer. Clin. Med. Insights Oncol. 13, 1179554919833927 (2019).
Higano, C. Enzalutamide, apalutamide, or darolutamide: are apples or bananas best for patients? Nat. Rev. Urol. 16, 335–336 (2019).
Sung, W. W., Choi, H. C., Luk, P. H. & So, T. H. A cost-effectiveness analysis of systemic therapy for metastatic hormone-sensitive prostate cancer. Front. Oncol. 11, 144 (2021).
Author information
Authors and Affiliations
Contributions
The authors contributed equally to all aspects of the article.
Corresponding author
Ethics declarations
Competing interests
The authors declare consultancy fees and honoraria as well as institution research funding from Astellas, Bayer and Janssen.
Additional information
Peer review information
Nature Reviews Urology thanks V. Conteduca, D. Quinn and T. Friedlander for their contribution to the peer review of this work.
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Lokeshwar, S.D., Klaassen, Z. & Saad, F. Treatment and trials in non-metastatic castration-resistant prostate cancer. Nat Rev Urol 18, 433–442 (2021). https://doi.org/10.1038/s41585-021-00470-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41585-021-00470-4
This article is cited by
-
The nerve growth factor-delivered signals in prostate cancer and its associated microenvironment: when the dialogue replaces the monologue
Cell & Bioscience (2023)
-
Cost–Utility Analysis of Darolutamide Combined with Androgen Deprivation Therapy for Patients with High-Risk Non-Metastatic Castration-Resistant Prostate Cancer in China
Advances in Therapy (2023)
-
Targeting PI3K/Akt signaling in prostate cancer therapy
Journal of Cell Communication and Signaling (2023)
-
Personalizing approaches to the management of metastatic hormone sensitive prostate cancer: role of advanced imaging, genetics and therapeutics
World Journal of Urology (2023)
-
Second generation androgen receptor antagonists and challenges in prostate cancer treatment
Cell Death & Disease (2022)