Abstract
Tumors actively manipulate the immune response through the production of factors that attract immune cells and subsequently alter their ability to recognize and effectively remove the tumor. While this mechanism for evading the immune system is an important aspect of tumor survival, the factors that serve as primary growth factors for the tumor are less understood. Here we demonstrate a previously unknown mechanism by which breast-cancer cells manipulate tumor-infiltrating myeloid cells to maintain their survival. Tumor-derived interleukin 1α (IL-1α), acting on infiltrating myeloid cells, induced the expression of a critical tumor survival factor, the cytokine TSLP. TSLP promoted the survival of the tumor cells through induction of the expression of the anti-apoptotic molecule Bcl-2. TSLP signaling was also required for metastasis to the lungs. These studies define a novel IL-1α–TSLP-mediated crosstalk between tumor-infiltrating myeloid cells and tumor cells in the control of metastatic breast cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout






Similar content being viewed by others
Change history
18 June 2018
In the version of this article initially published, the far right label along the horizontal axis of the right plot of Fig. 1e (4T1-Tslp–/– 4-6), the middle and right labels along the horizontal axis of the far right plot of Fig. 3d (4T1-Tslpr–/– 4-6 and 4T1-Tslpr–/– 2-3, respectively), and the far right label along the horizontal axis of Fig. 6h (4T1-Tslpr–/– 2-3) were incorrect. The correct labels are as follows: Fig. 1e, 4T1-Tslpr–/– 4-6; Fig. 3d, 4T1-Tslp–/– 2-3 and 4T1-Tslpr–/– 4-6, respectively; and Fig. 6h, 4T1-Tslpr–/– 4-6. Also, Fig. 4e was incorrectly a duplicate of an adjacent panel. The errors have been corrected in the HTML and PDF version of the article.
References
He, R. & Geha, R. S. Thymic stromal lymphopoietin. Ann. NY Acad. Sci. 1183, 13–24 (2010).
Pedroza-Gonzalez, A. et al. Thymic stromal lymphopoietin fosters human breast tumor growth by promoting type 2 inflammation. J. Exp. Med. 208, 479–490 (2011).
De Monte, L. et al. Intratumor T helper type 2 cell infiltrate correlates with cancer-associated fibroblast thymic stromal lymphopoietin production and reduced survival in pancreatic cancer. J. Exp. Med. 208, 469–478 (2011).
Barooei, R., Mahmoudian, R. A., Abbaszadegan, M. R., Mansouri, A. & Gholamin, M. Evaluation of thymic stromal lymphopoietin (TSLP) and its correlation with lymphatic metastasis in human gastric cancer. Med. Oncol. 32, 217 (2015).
Nakajima, S. et al. Induction of thymic stromal lymphopoietin in mesenchymal stem cells by interaction with myeloma cells. Leuk. Lymphoma 55, 2605–2613 (2014).
Vetter, T. et al. Blockade of thymic stromal lymphopoietin (TSLP) receptor inhibits TSLP-driven proliferation and signalling in lymphoblasts from a subset of B-precursor ALL patients. Leuk. Res. 40, 38–43 (2016).
Xie, F. et al. Cervical carcinoma cells stimulate the angiogenesis through TSLP promoting growth and activation of vascular endothelial cells. Am. J. Reprod. Immunol. 70, 69–79 (2013).
Olkhanud, P. B. et al. Thymic stromal lymphopoietin is a key mediator of breast cancer progression. J. Immunol. 186, 5656–5662 (2011).
Ghirelli, C. et al. No evidence for TSLP pathway activity in human breast cancer. Oncoimmunology 5, e1178438 (2016).
Demehri, S. et al. Elevated epidermal thymic stromal lymphopoietin levels establish an antitumor environment in the skin. Cancer Cell 22, 494–505 (2012).
Di Piazza, M., Nowell, C. S., Koch, U., Durham, A. D. & Radtke, F. Loss of cutaneous TSLP-dependent immune responses skews the balance of inflammation from tumor protective to tumor promoting. Cancer Cell 22, 479–493 (2012).
Demehri, S. et al. Thymic stromal lymphopoietin blocks early stages of breast carcinogenesis. J. Clin. Invest. 126, 1458–1470 (2016).
Yue, W. et al. Thymic stromal lymphopoietin (TSLP) inhibits human colon tumor growth by promoting apoptosis of tumor cells. Oncotarget 7, 16840–16854 (2016).
Roan, F. et al. The multiple facets of thymic stromal lymphopoietin (TSLP) during allergic inflammation and beyond. J. Leukoc. Biol. 91, 877–886 (2012).
Kashiwagi, M. et al. Direct control of regulatory T cells by keratinocytes. Nat. Immunol. 18, 334–343 (2017).
Miazgowicz, M. M., Elliott, M. S., Debley, J. S. & Ziegler, S. F. Respiratory syncytial virus induces functional thymic stromal lymphopoietin receptor in airway epithelial cells. J. Inflamm. Res. 6, 53–61 (2013).
Liao, B. et al. Interaction of thymic stromal lymphopoietin, IL-33, and their receptors in epithelial cells in eosinophilic chronic rhinosinusitis with nasal polyps. Allergy 70, 1169–1180 (2015).
Reche, P. A. et al. Human thymic stromal lymphopoietin preferentially stimulates myeloid cells. J. Immunol. 167, 336–343 (2001).
Zhang, K. et al. Constitutive and inducible thymic stromal lymphopoietin expression in human airway smooth muscle cells: role in chronic obstructive pulmonary disease. Am. J. Physiol. Lung Cell. Mol. Physiol. 293, L375–L382 (2007).
Soumelis, V. et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat. Immunol. 3, 673–680 (2002).
Kashyap, M., Rochman, Y., Spolski, R., Samsel, L. & Leonard, W. J. Thymic stromal lymphopoietin is produced by dendritic cells. J. Immunol. 187, 1207–1211 (2011).
Spadoni, I., Iliev, I. D., Rossi, G. & Rescigno, M. Dendritic cells produce TSLP that limits the differentiation of Th17 cells, fosters Treg development, and protects against colitis. Mucosal Immunol. 5, 184–193 (2012).
Lee, H. -C., & Ziegler, S. F. Inducible expression of the proallergic cytokine thymic stromal lymphopoietin in airway epithelial cells is controlled by NFKappaB. Proc. Natl Acad. Sci. USA 104, 914–919 (2007).
Di Paolo, N. C. & Shayakhmetov, D. M. Interleukin 1α and the inflammatory process. Nat. Immunol. 17, 906–913 (2016).
Kurtzman, S. H. et al. Cytokines in human breast cancer: IL-1alpha and IL-1beta expression. Oncol. Rep. 6, 65–70 (1999).
Kumar, S. et al. Interleukin-1 alpha promotes tumor growth and cachexia in MCF-7 xenograft model of breast cancer. Am. J. Pathol. 163, 2531–2541 (2003).
Miller, B. E., Roi, L. D., Howard, L. M. & Miller, F. R. Quantitative selectivity of contact-mediated intercellular communication in a metastatic mouse mammary tumor line. Cancer Res. 43, 4102–4107 (1983).
Guy, C. T., Cardiff, R. D. & Muller, W. J. Induction of mammary tumors by expression of polyomavirus middle T oncogene: a transgenic mouse model for metastatic disease. Mol. Cell. Biol. 12, 954–961 (1992).
Stewart, T. J. & Abrams, S. I. Altered immune function during long-term host-tumor interactions can be modulated to retard autochthonous neoplastic growth. J. Immunol. 179, 2851–2859 (2007).
Shi, G. et al. Isolation of rare tumor cells from blood cells with buoyant immuno-microbubbles. PLoS One 8, e58017 (2013).
Cremers, N. et al. CD24 is not required for tumor initiation and growth in murine breast and prostate cancer models. PLoS One 11, e0151468 (2016).
Feng, A.-L. et al. CD16+ monocytes in breast cancer patients: expanded by monocyte chemoattractant protein-1 and may be useful for early diagnosis. Clin. Exp. Immunol. 164, 57–65 (2011).
Ethier, J.-L., Desautels, D., Templeton, A., Shah, P. S. & Amir, E. Prognostic role of neutrophil-to-lymphocyte ratio in breast cancer: a systematic review and meta-analysis. Breast Cancer Res. 19, 2 (2017).
Ginhoux, F. & Jung, S. Monocytes and macrophages: developmental pathways and tissue homeostasis. Nat. Rev. Immunol. 14, 392–404 (2014).
Barry, K. C., Fontana, M. F., Portman, J. L., Dugan, A. S. & Vance, R. E. IL-1α signaling initiates the inflammatory response to virulent Legionella pneumophila in vivo. J. Immunol. 190, 6329–6339 (2013).
Li, Z. & Kang, Y. Emerging therapeutic targets in metastatic progression: a focus on breast cancer. Pharmacol. Ther. 161, 79–96 (2016).
Scully, O. J., Bay, B. H., Yip, G. & Yu, Y. Breast cancer metastasis. Cancer Genomics Proteomics 9, 311–320 (2012).
Taranova, A. G. et al. Allergic pulmonary inflammation promotes the recruitment of circulating tumor cells to the lung. Cancer Res. 68, 8582–8589 (2008).
West, E. E., Kashyap, M. & Leonard, W. J. TSLP: a key regulator of asthma pathogenesis. Drug Discov. Today Dis. Mech. 9, e83–e88 (2012).
Zhou, B. et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat. Immunol. 6, 1047–1053 (2005).
Grivennikov, S. I., Greten, F. R. & Karin, M. Immunity, inflammation, and cancer. Cell 140, 883–899 (2010).
Rochman, Y. & Leonard, W. J. The role of thymic stromal lymphopoietin in CD8+ T cell homeostasis. J. Immunol. 181, 7699–7705 (2008).
Scheeren, F. A. et al. Thymic stromal lymphopoietin induces early human B-cell proliferation and differentiation. Eur. J. Immunol. 40, 955–965 (2010).
Xie, F. et al. The infiltration and functional regulation of eosinophils induced by TSLP promote the proliferation of cervical cancer cell. Cancer Lett. 364, 106–117 (2015).
Cipolat, S., Hoste, E., Natsuga, K., Quist, S. R. & Watt, F. M. Epidermal barrier defects link atopic dermatitis with altered skin cancer susceptibility. eLife 3, e01888 (2014).
Gabrilovich, D. I., Ostrand-Rosenberg, S. & Bronte, V. Coordinated regulation of myeloid cells by tumours. Nat. Rev. Immunol. 12, 253–268 (2012).
Han, H. et al. Thymic stromal lymphopoietin (TSLP)-mediated dermal inflammation aggravates experimental asthma. Mucosal. Immunol 5, 342–351 (2012).
Larson, R. P. et al. Dibutyl phthalate-induced thymic stromal lymphopoietin is required for Th2 contact hypersensitivity responses. J. Immunol. 184, 2974–2984 (2010).
Lau, L., Gray, E. E., Brunette, R. L. & Stetson, D. B. DNA tumor virus oncogenes antagonize the cGAS-STING DNA-sensing pathway. Science 350, 568–571 (2015).
Acknowledgements
We thank G. Kaber for assitance in generating 4T1-GFP cell lines; D.B. Stetson and E. Gray (University of Washington) for CRISPR vectors and their protocol; J. B. Tan, F. Roan, A. Sheih, S. de Jesus Carrion and S. Ong for aid in randomizing samples for blinded experiments; S. Abrams (Roswell Park Cancer Institute) for MTAG mice on the C57BL/6 background and the AT3 cell line; P. Y. Johnson for assistance in histology; all the staff of the Virginia Mason BRITE program and BRI clinical core for human sample recruitment and preparation; all the staff of BRI Flow Cytometry Core Lab for maintaining flow-cytometry machines; D. J. Campbell, J. Hamerman and T. N. Wight for discussions; and R. Penn for administrative support. Supported by the US National Institute of Health (R01-CA182783 to S.F.Z.).
Author information
Authors and Affiliations
Contributions
E.L.K. designed and performed experiments; and E.L.K. and S.F.Z. wrote and edited the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
Supplementary Figure 1 Non-tumor breast tissue does not express TSLPR
a, Left panel: mRNA expression, examined by qPCR, of Tslp and other genes were tested in 4T1-M1 (non-targeting gRNA M1 in CRISPR-Cas9-GFP) and 4T1-Tslp-/- 2-3 cells and were normalized to those in 4T1 cells to confirm the depletion of Tslp but intact of other genes in 4T1-Tslp-/- 2-3 and no difference between 4T1 and 4T1-M1. n=3 per group. Right panel: TSLP protein expression in supernatant of 4T1-Tslp-/- 2-3, 4T1-M1, and 4T1 cells in vitro culture for 2 days, examined by ELISA. n=3per group. b, Immunohistochemistry staining of TSLPR on adjacent non-tumor breast tissue (top) and on breast tumors collected (bottom) from breast cancer patients. A summary of breast cancer stage, tumor types, age, and gender from all recruited patients. Scale bar, 100 μm. Breast tumor tissue, n=12; adjacent non-tumor breast tissue, n=3. c, TSLPR and IL7R mRNA expression relative to expression of GAPDH, examined by qPCR, in MDA-MB-468 and MCF10A cells. n=3 per group. d, Left panel: mRNA expression of Tslpr and other genes in 4T1-Tslpr-/- 4-6 and 4T1-M1 normalized to 4T1, examined by qPCR. n=3. Right panel: A representative histogram of surface TSLPR expression in 4T1-Tslpr-/- 4–6, compared 4T1-M1 and 4T1 cells, examined by flow cytometry. IC: isotype control. Each symbol in (b) represents an individual patient and in (a, c, d left) represents individual cell culture. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (a, d) are representative of two independent experiments.
Supplementary Figure 2 T cells in the primary tumor in TSLP-KO mice
a, Gating strategy for tumor-infiltrating T cells in the D28 primary tumor in 4T1 tumor-bearing mice. b, Frequencies of total T, CD4+ T, and CD8+ T cells in the primary tumor at day 28 post tumor transplantation, examined by flow cytometry. n=9 per group. c, Frequencies of PD-1+ in non-Treg CD4+ or CD8+ T cells in the primary tumor at day 28 post tumor transplantation, examined by flow cytometry. n=9 per group. Gating strategy for sorting 4T1-GFP tumor cells (CD45- EpCAM+GFP+) from the day 28 primary tumor in 4T1-GFP tumor-bearing mice (d) or for sorting tumor cells in the primary tumor in 20-week-old MTAG mice (CD45-EpCAM+CD24+) (e). Fixable viability dye: FVD. Each symbol in (b, c) represents an individual mouse. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals These results are pooled from 3 independent experiments.
Supplementary Figure 3 TSLP stimulation on murine or human breast tumor cell line increases their survival by up-regulating Bcl-2 expression
Frequencies of dying (Annexin V+) AT3 cells (a) and Bcl-2 expression (b) when treated with TSLP for 2 days in vitro, examined by flow cytometry. n=4 per group. c, Gating strategy for dying tumor cells (CD45-EpCAM+GFP+fixable viability dye+) in the primary tumor of 4T1-GFP tumor-bearing mice. d, Representative images of cleaved caspase 3 expression in the primary breast tumors in wild-type (WT) or TSLP-KO tumor-bearing mice post 28 days 4T1 cell injection. WT, n=4; KO, n=3. Scale bar, 50 μm. e, qPCR analysis of mRNA expression of Bcl2 and Bcl2l1 in sorted tumor cells from 20-week-old MTAG or MTAG-TSLP-KO mice at . MTAG, n=6; MTAG-TSLP-KO, n=4. Expression values normalized to Gapdh expression. Frequencies Flow cytometric analysis of dying cells (Annexin V+) (f) (n=4 per group) and qPCR analysis of Bcl2 mRNA expression (g) in MDA-MB-468 or MCF10A cells treated with TSLP for 2 days in vitro culture (n=3 per group). h, Frequency of proliferating tumor cells (Ki67+) in vitro (n=3 per group) or in vivo (h middle n=4 per group; h right n=3 per group) when transplanted into mice for 28 days. Fixable viability dye: FVD. Each symbol in (d, e, h middle, h right) represents an individual mouse and in (a, b, f, g, h left) represents individual cell culture. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (a, b, f, g, h) are representative of two independent experiments and (d, e) are pooled from two experiments.
Supplementary Figure 4 Expansion of neutrophils and Ly6Chi monocytes in 4T1 tumor-bearing mice
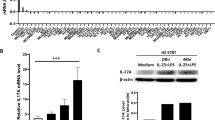
a, Gating strategies for neutrophils (CD45+CD3ε−CD11b+B220-Ly6G+SiglecF-CD11c-MHCII-), Ly6Chi monocytes (CD45+CD3ε-CD11b+B220-Ly6G-SiglecF-Ly6C+CD11c-MHCII-), and TAMs (CD45+CD3ε-CD11b+B220-Ly6G-SiglecF-Ly6C-CD11c+MHCII+) in the primary breast tumor. Total number of neutrophils (b) and Ly6Chi monocytes (c) in the blood of tumor free and 4T1 tumor-bearing mice at day 0, 7, 14, 21 and 28 post tumor injection. d, Percentage of different hematopoietic cell populations in blood in naïve or tumor-bearing mice (Tumor-bearing mice, n=7 ; tumor-free mice, n=4). e, qPCR analysis of Tslp mRNA expression in sorted splenic Ly6Chi monocytes (Tumor-free WT, n=3; Tumor-bearing WT mice, n=6; Tumor-bearing TSLP-KO mice, n=3) or neutrophilsTumor-free WT, n=5; Tumor-bearing WT mice, n=6; Tumor-bearing TSLP-KO mice, n=3) from tumor-free wild-type (WT) or in day 28 4T1 tumor-bearing wild-type or TSLP-KO mice. Expression values normalized to Gapdh expression. f, qPCR analysis of Tslp mRNA expression from splenic neutrophils in 20-week-old tumor-free or MTAG mice. Expression values normalized to Gapdh expression. n=5 per group. g, Gating strategies for classical monocytes in human peripheral blood mononuclear cells . h, A summary of information for recruited breast cancer patients control healthy donors. i, Percentage of Annexin V+ 4T1-M1 and 4T1-Tslpr-/- 4-6 cells co-cultured with neutrophils or Ly6Chi monocytes for 2 days in vitro, examined by flow cytometry. Cell number ratio of 4T1 to neutrophils or Ly6Chi monocytes was about 2 at the time of starting co-culturing. Experimental design was shown in the upper panel. n=3 per group. Fixable viability dye: FVD. Each symbol in (b, c, e, f, i) represents an individual mouse or human donor. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (f) are pooled from two experiments and the rests are representative of two independent experiments.
Supplementary Figure 5 IL-1α deficient 4T1 cell survival in vitro culture
a, Left panel: Il1a and other gene mRNA expression, examined by qPCR, in 4T1-M1 and two clones of 4T1-Il1a-/- cells normalized to 4T1. n=3. Right panel: Representative histogram plots of intracellular IL-1α protein expression in 4T1 cells in vitro or in vivo after 4T1-M1 or two clones of 4T1-Il1a-/- cell injecting to WT mice for 28 days, examined by flow cytometry. b, Percentage of Annexin V+ 4T1-M1 and 4T1-Il1a-/- cells in vitro culture for 2 days, examined by flow cytometry. n=4 per group. Each symbol in (b) represents an individual mouse and in (a left) represents individual cell culture. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results are representative of two independent experiments.
Supplementary Figure 6 TSLP from non-tumor derived sources is also critical for breast tumor metastasis in lungs
a, Counts of tumor metastases in lungs at day 19 in wild-type (WT) or TSLP-KO mice injected with 4T1 cells i.v. n=3/group. b, Primary tumor volume in SPC-TSLP tumor-bearing mice post 0-28 day 4T1 injection. Right: tumor weights at day 28 (WT day 21, n=7; SPC-TSLP day 21, n=8; WT day 28, n=6, SPC-TSP day 28, n=7). c, Primary tumor volume in WT tumor-bearing mice treated with anti-TSLP antibodies in lungs post 0-28 day 4T1 injection. Right: tumor weights at day 28. n=6/group. d, A schematic summary of IL-1α and TSLP regulating breast tumor progression in vivo. Each symbol in (a-c) represents an individual mouse. Data are represented as mean ± s.e.m. Statistical analysis by unpaired, two-tailed t test with 95 % confidence intervals. Results in (b) are pooled data and (a, c) are representative of two independent experiments.
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1-6
Rights and permissions
About this article
Cite this article
Kuan, E.L., Ziegler, S.F. A tumor–myeloid cell axis, mediated via the cytokines IL-1α and TSLP, promotes the progression of breast cancer. Nat Immunol 19, 366–374 (2018). https://doi.org/10.1038/s41590-018-0066-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0066-6
This article is cited by
-
Interferon-γ in the tumor microenvironment promotes the expression of B7H4 in colorectal cancer cells, thereby inhibiting cytotoxic T cells
Scientific Reports (2024)
-
Relevance of Thymic Stromal Lymphopoietin on the Pathogenesis of Glioblastoma: Role of the Neutrophil
Cellular and Molecular Neurobiology (2024)
-
Role of thymic stromal lymphopoietin in allergy and beyond
Nature Reviews Immunology (2023)
-
Epithelial cell-derived cytokine TSLP activates regulatory T cells by enhancing fatty acid uptake
Scientific Reports (2023)
-
Comprehensive evaluation of breast cancer immunotherapy and tumor microenvironment characterization based on interleukin genes-related risk model
Scientific Reports (2022)