Abstract
The developmental programs that generate a broad repertoire of regulatory T cells (Treg cells) able to respond to both self antigens and non-self antigens remain unclear. Here we found that mature Treg cells were generated through two distinct developmental programs involving CD25+ Treg cell progenitors (CD25+ TregP cells) and Foxp3lo Treg cell progenitors (Foxp3lo TregP cells). CD25+ TregP cells showed higher rates of apoptosis and interacted with thymic self antigens with higher affinity than did Foxp3lo TregP cells, and had a T cell antigen receptor repertoire and transcriptome distinct from that of Foxp3lo TregP cells. The development of both CD25+ TregP cells and Foxp3lo TregP cells was controlled by distinct signaling pathways and enhancers. Transcriptomics and histocytometric data suggested that CD25+ TregP cells and Foxp3lo TregP cells arose by coopting negative-selection programs and positive-selection programs, respectively. Treg cells derived from CD25+ TregP cells, but not those derived from Foxp3lo TregP cells, prevented experimental autoimmune encephalitis. Our findings indicate that Treg cells arise through two distinct developmental programs that are both required for a comprehensive Treg cell repertoire capable of establishing immunotolerance.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout








Similar content being viewed by others
Data availability
The data that support the findings of this study are available from the corresponding author upon request. Single-cell RNA-seq data were deposited at Gene Expression Omnibus, with the following accession code: GSE123067.
References
Lio, C. W. & Hsieh, C. S. A two-step process for thymic regulatory T cell development. Immunity 28, 100–111 (2008).
Burchill, M. A. et al. Linked T cell receptor and cytokine signaling govern the development of the regulatory T cell repertoire. Immunity 28, 112–121 (2008).
Mahmud, S. A. et al. Costimulation via the tumor-necrosis factor receptor superfamily couples TCR signal strength to the thymic differentiation of regulatory T cells. Nat. Immunol. 15, 473–481 (2014).
Burchill, M. A., Yang, J., Vogtenhuber, C., Blazar, B. R. & Farrar, M. A. IL-2 receptor β-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J. Immunol. 178, 280–290 (2007).
Yao, Z. et al. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood 109, 4368–4375 (2007).
Tai, X. et al. Foxp3 transcription factor is proapoptotic and lethal to developing regulatory T cells unless counterbalanced by cytokine survival signals. Immunity 38, 1116–1128 (2013).
Hsieh, C. S. et al. Recognition of the peripheral self by naturally arising CD25+CD4+ T cell receptors. Immunity 21, 267–277 (2004).
Fenton, R. G., Marrack, P., Kappler, J. W., Kanagawa, O. & Seidman, J. G. Isotypic exclusion of γδ T cell receptors in transgenic mice bearing a rearranged β-chain gene. Science 241, 1089–1092 (1988).
Jorgensen, J. L., Esser, U., Fazekas de St Groth, B., Reay, P. A. & Davis, M. M. Mapping T-cell receptor-peptide contacts by variant peptide immunization of single-chain transgenics. Nature 355, 224–230 (1992).
Pacholczyk, R., Ignatowicz, H., Kraj, P. & Ignatowicz, L. Origin and T cell receptor diversity of Foxp3+CD4+CD25+ T cells. Immunity 25, 249–259 (2006).
Wong, J. et al. Adaptation of TCR repertoires to self-peptides in regulatory and nonregulatory CD4+ T cells. J. Immunol. 178, 7032–7041 (2007).
Beura, L. K. et al. Normalizing the environment recapitulates adult human immune traits in laboratory mice. Nature 532, 512–516 (2016).
Moran, A. E. et al. T cell receptor signal strength in Treg and iNKT cell development demonstrated by a novel fluorescent reporter mouse. J. Exp. Med. 208, 1279–1289 (2011).
Howie, D. et al. MS4A4B is a GITR-associated membrane adapter, expressed by regulatory T cells, which modulates T cell activation. J. Immunol. 183, 4197–4204 (2009).
Xing, Y., Wang, X., Jameson, S. C. & Hogquist, K. A. Late stages of T cell maturation in the thymus involve NF-κB and tonic type I interferon signaling. Nat. Immunol. 17, 565–573 (2016).
Boursalian, T. E., Golob, J., Soper, D. M., Cooper, C. J. & Fink, P. J. Continued maturation of thymic emigrants in the periphery. Nat. Immunol. 5, 418–425 (2004).
McCaughtry, T. M., Wilken, M. S. & Hogquist, K. A. Thymic emigration revisited. J. Exp. Med. 204, 2513–2520 (2007).
Zhan, Y., Bourges, D., Dromey, J. A., Harrison, L. C. & Lew, A. M. The origin of thymic CD4+CD25+ regulatory T cells and their co-stimulatory requirements are determined after elimination of recirculating peripheral CD4+ cells. Int. Immunol. 19, 455–463 (2007).
Paessens, L. C., Singh, S. K., Fernandes, R. J. & van Kooyk, Y. Vascular cell adhesion molecule-1 (VCAM-1) and intercellular adhesion molecule-1 (ICAM-1) provide co-stimulation in positive selection along with survival of selected thymocytes. Mol. Immunol. 45, 42–48 (2008).
Gerner, M. Y., Kastenmuller, W., Ifrim, I., Kabat, J. & Germain, R. N. Histo-cytometry: a method for highly multiplex quantitative tissue imaging analysis applied to dendritic cell subset microanatomy in lymph nodes. Immunity 37, 364–376 (2012).
Fontenot, J. D. et al. Regulatory T cell lineage specification by the forkhead transcription factor foxp3. Immunity 22, 329–341 (2005).
Bettini, M. L. et al. Loss of epigenetic modification driven by the Foxp3 transcription factor leads to regulatory T cell insufficiency. Immunity 36, 717–730 (2012).
Darce, J. et al. An N-terminal mutation of the Foxp3 transcription factor alleviates arthritis but exacerbates diabetes. Immunity 36, 731–741 (2012).
Zheng, Y. et al. Role of conserved non-coding DNA elements in the Foxp3 gene in regulatory T-cell fate. Nature 463, 808–812 (2010).
Feng, Y. et al. A mechanism for expansion of regulatory T-cell repertoire and its role in self-tolerance. Nature 528, 132–136 (2015).
Huang, H. et al. Fine-mapping inflammatory bowel disease loci to single-variant resolution. Nature 547, 173–178 (2017).
Huang, J., Ellinghaus, D., Franke, A., Howie, B. & Li, Y. 1000 Genomes-based imputation identifies novel and refined associations for the Wellcome Trust Case Control Consortium phase 1 Data. Eur. J. Hum. Genet. 20, 801–805 (2012).
Onengut-Gumuscu, S. et al. Fine mapping of type 1 diabetes susceptibility loci and evidence for colocalization of causal variants with lymphoid gene enhancers. Nat. Genet. 47, 381–386 (2015).
Simeonov, D. R. et al. Discovery of stimulation-responsive immune enhancers with CRISPR activation. Nature 549, 111–115 (2017).
Schaeffer, E. M. et al. Tec family kinases modulate thresholds for thymocyte development and selection. J. Exp. Med. 192, 987–1000 (2000).
Huang, W., Jeong, A. R., Kannan, A. K., Huang, L. & August, A. IL-2-inducible T cell kinase tunes T regulatory cell development and is required for suppressive function. J. Immunol. 193, 2267–2272 (2014).
Wu, J. N. et al. Adhesion- and degranulation-promoting adapter protein is required for efficient thymocyte development and selection. J. Immunol. 176, 6681–6689 (2006).
Weinreich, M. A., Odumade, O. A., Jameson, S. C. & Hogquist, K. A. T cells expressing the transcription factor PLZF regulate the development of memory-like CD8+ T cells. Nat. Immunol. 11, 709–716 (2010).
Huang, W., Huang, F., Kannan, A. K., Hu, J. & August, A. ITK tunes IL-4-induced development of innate memory CD8+ T cells in a γδ T and invariant NKT cell-independent manner. J. Leukoc. Biol. 96, 55–63 (2014).
Burchill, M. A., Yang, J., Vang, K. B. & Farrar, M. A. Interleukin-2 receptor signaling in regulatory T cell development and homeostasis. Immunol. Lett. 114, 1–8 (2007).
Vang, K. B. et al. IL-2, -7, and -15, but not thymic stromal lymphopoeitin, redundantly govern CD4+Foxp3+ regulatory T cell development. J. Immunol. 181, 3285–3290 (2008).
Watanabe, N. et al. Hassall’s corpuscles instruct dendritic cells to induce CD4+CD25+ regulatory T cells in human thymus. Nature 436, 1181–1185 (2005).
Miller, C. N. et al. Thymic tuft cells promote an IL-4-enriched medulla and shape thymocyte development. Nature 559, 627–631 (2018).
Bornstein, C. et al. Single-cell mapping of the thymic stroma identifies IL-25-producing tuft epithelial cells. Nature 559, 622–626 (2018).
Gerbe, F. et al. Intestinal epithelial tuft cells initiate type 2 mucosal immunity to helminth parasites. Nature 529, 226–230 (2016).
McGeachy, M. J., Stephens, L. A. & Anderton, S. M. Natural recovery and protection from autoimmune encephalomyelitis: contribution of CD4+CD25+ regulatory cells within the central nervous system. J. Immunol. 175, 3025–3032 (2005).
Shahinian, A. et al. Differential T cell costimulatory requirements in CD28-deficient mice. Science 261, 609–612 (1993).
Sha, W. C., Liou, H. C., Tuomanen, E. I. & Baltimore, D. Targeted disruption of the p50 subunit of NF-κB leads to multifocal defects in immune responses. Cell 80, 321–330 (1995).
Liao, X. C. & Littman, D. R. Altered T cell receptor signaling and disrupted T cell development in mice lacking itk. Immunity 3, 757–769 (1995).
Peterson, E. J. et al. Coupling of the TCR to integrin activation by Slap-130/Fyb. Science 293, 2263–2265 (2001).
Sonoda, K. H., Exley, M., Snapper, S., Balk, S. P. & Stein-Streilein, J. CD1-reactive natural killer T cells are required for development of systemic tolerance through an immune-privileged site. J. Exp. Med. 190, 1215–1226 (1999).
Shinkai, Y. et al. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell 68, 855–867 (1992).
Nelson, R. W. et al. T cell receptor cross-reactivity between similar foreign and self peptides influences naive cell population size and autoimmunity. Immunity 42, 95–107 (2015).
Blair-Handon, R., Mueller, K. & Hoogstraten-Miller, S. An alternative method for intrathymic injections in mice. Lab. Anim. (NY) 39, 248–252 (2010).
Ruscher, R., Kummer, R. L., Lee, Y. J., Jameson, S. C. & Hogquist, K. A. CD8αα intraepithelial lymphocytes arise from two main thymic precursors. Nat. Immunol. 18, 771–779 (2017).
Haribhai, D. et al. A requisite role for induced regulatory T cells in tolerance based on expanding antigen receptor diversity. Immunity 35, 109–122 (2011).
Perry, J. S. et al. Distinct contributions of Aire and antigen-presenting-cell subsets to the generation of self-tolerance in the thymus. Immunity 41, 414–426 (2014).
Lin, W. et al. Regulatory T cell development in the absence of functional Foxp3. Nat. Immunol. 8, 359–368 (2007).
McDavid, A. et al. Data exploration, quality control and testing in single-cell qPCR-based gene expression experiments. Bioinformatics 29, 461–467 (2013).
Spanier, J. A., Nashold, F. E., Mayne, C. G., Nelson, C. D. & Hayes, C. E. Vitamin D and estrogen synergy in Vdr-expressing CD4+ T cells is essential to induce Helios+FoxP3+ T cells and prevent autoimmune demyelinating disease. J. Neuroimmunol. 286, 48–58 (2015).
Mottet, C., Uhlig, H. H. & Powrie, F. Cutting edge: cure of colitis by CD4+CD25+ regulatory T cells. J. Immunol. 170, 3939–3943 (2003).
Acknowledgements
We thank G. Hubbard, A. Rost, A. Meskic, D. Duerre and H. Wiesolek for technical assistance; T. Martin, N. Shah, J. Motl and P. Champoux for cell sorting and maintenance of the Flow Cytometry Core Facility at the University of Minnesota (5P01AI035296); S. Hamilton, M. Pierson and funding from the University of Minnesota academic health center for maintaining the NME mouse facility; P. Fink for providing initial Rag2-GFP thymi; B. Burbach and Y. Shimizu for Adap–/– mice; M. Jenkins for the MOG:I-Ab tetramer; and C. Katerndahl and L. Heltemes-Harris for helpful commentary and reviewing the manuscript. D.L.O. and S.A.M. were supported by an immunology training grant (no. 2T32AI007313). S.A.M. was also supported by an individual predoctoral F30 fellowship from the National Institutes of Health (NIH; no. F30DK096844). J.A.S. was supported by University of Minnesota Medical Foundation grant no. UMF0020624 and NIH grants nos 5U24AI118635 and R01AI106791. Y.Z. was supported by NIH grant no. R01AI107027. U.B. and C.B.W. were supported by grants from the Children’s Hospital of Wisconsin, and C.B.W. was also supported by NIH grant no. R01AI085090-07A1. A.M. and M.S.A were supported by NIH grant no. DP3DK111914-01. A.M. holds a Career Award for Medical Scientists from the Burroughs Wellcome Fund and is an investigator at the Chan Zuckerberg Biohub. M.A. was supported by NIH grant no. R01AI115716. M.S.A. was supported by NIH grant no. R37 AI097457. A.A. was supported by NIH grants nos AI108958, AI120701, AI126814 and AI129422 to A.A. and W.H. W.H. was supported by NIH grant no. AI29422 (to W.H. and A.A.), a Careers in Immunology Fellowship from the American Association of Immunologists, a Faculty Development Award and a competitive research grant from Louisiana State University, and a pilot award from the LSU-Tulane Center for Experimental Infectious Diseases Research funded by NIH grant no. GM110760. M.A.F. was supported by NIH grants nos AI124512, AI113138, AI061165, CA154998, CA151845 and CA185062.
Author information
Authors and Affiliations
Contributions
D.L.O. designed and conducted experiments and wrote the manuscript. S.A.M, L.E.S, J.B.W., J.A.S., D.R.S., R.R., W.H., I.P., C.N.M., C.H., J.C.J, P.A., U.B., R.S.L., C.M.H. and Y.Z. performed some experiments or analyzed data and contributed intellectually to the work. M.A., M.S.A., A.A., A.M., Y.Z., and C.B.W. provided key reagents and/or animals and intellectual contributions. M.A.F. designed experiments, supervised research and assisted in the preparation of this manuscript. All authors read the manuscript and helped with final revisions.
Corresponding author
Ethics declarations
Competing interests
A.M. is a co-founder of Spotlight Therapeutics. A.M. has served as an advisor to Juno Therapeutics and is a member of the scientific advisory board at PACT Pharma. The Marson laboratory has received sponsored research support from Juno Therapeutics, Epinomics and Sanofi, and a gift from Gilead. A.A. has received sponsored research support from 3 M. MAF has received sponsored research support from Merck.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Integrated supplementary information
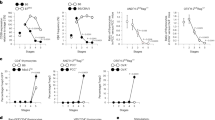
Supplementary Figure 1 Conversion of distinct TregP cell subsets into mature Treg cells in vitro.
Sorted Treg cell lineage subsets were stimulated for 3 days in the indicated concentrations of IL-2 and analyzed for the % of cells which converted into (left and middle panel) or remained (right panel) CD25+Foxp3+ mature Treg cells. Data represents 1 experiment, n = 2 (CD25+ TregP cells- untreated, 0.2 Uml–1 IL2; Foxp3lo TregP cells- untreated, 100 Uml–1 IL-2; Treg cells- 0.04 Uml–1 IL-2, 0.2 Uml–1 IL-2, 1 Uml–1 IL-2), n = 1 (Foxp3lo TregP cells- 0.04 Uml–1 IL-2; Treg cells- untreated), n = 3 (CD25+ TregP cells- 0.04 Uml–1 IL-2, 1 Uml–1 IL-2, 100 Uml–1 IL-2; Foxp3lo TregP cells- 0.2 Uml–1 IL-2, 1 Uml–1 IL-2; Treg cells- 100 Uml–1 IL-2) technical replicates. Bars represent mean ± SD.
Supplementary Figure 2 Germ-free and NME mice show no defect in either thymic TregP cell pathway.
a) Representative flow plots of SPF, germ-free reconstituted and germ-free mice thymi and quantification of the percent of each Treg cell lineage subset within CD4+CD73- thymocytes. b) Representative flow plots and quantification of SPF, germ-free reconstituted and germ free mice spleens showing the percent of CD4+ lymphocytes which are Foxp3+. a,b) Data is representative of 1 experiment, n = 6 SPF mice, 5 germ-free reconstituted mice, and 6 germ-free mice. Data was analyzed by one-way ANOVA with Tukey’s multiple comparisons test. c) Representative flow plots of CD4+CD73- thymocytes from SPF mice or mice with normalized microbial experience (NME) and quantification of the percent within each Treg cell lineage population. Data is representative of 2 experiments, n = 3 SPF mice and 5 NME mice. Data was analyzed by two-sided unpaired t test. All bars represent mean ± SD. *P<0.05, **P<0.005, ns- not significant.
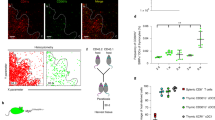
Supplementary Figure 3 Single-cell RNA-seq of thymic Treg cell lineage.
a) Violin plots (left) or feature plots (right) displaying single-cell expression for either Foxp3-GFP reporter (top) or Il2ra (bottom) for each cluster from the single-cell RNA-seq data set presented in Fig. 2e,f. b) Data from an independent repeat of 10x Genomics single-cell RNA-seq. Heatmap displays the top 10 differentially regulated genes in each cluster from the data set presented in Fig. 2e,f. c) Violin plots displaying single-cell expression for Bcl2l11 (left) and Nr4a1 (right) for each cluster from the single-cell RNA-seq data set presenting in Fig. 2e,f. a-c) Data is representative from 3 independent experiments, n = 3 mice.
Supplementary Figure 4 Maturation analysis of thymic Treg cell populations.
a) Thymocytes of the indicated subsets were analyzed for expression of HSA and Qa-2. Gates are drawn to demonstrate the frequency of cells within each maturation state. Data is representative of 1 experiment, n = 2 mice. b) Thymocytes of the indicated subsets were analyzed for CD69 and MHC-I expression. Gates are drawn to demonstrate the frequency of cells within each maturation state. Data is representative of 1 experiment, n = 3 mice.
Supplementary Figure 5 Frequency of contaminating recirculating cells in thymic Treg cell lineage subsets.
Thymocytes of the indicated subsets were analyzed for RAG2-GFP expression. Gates were drawn to determine the frequency of RAG2-GFP- (recirculating) and RAG2-GFP+ (newly developing) fractions of cells within each subset. Displayed are concatenated data from 3 thymi. Results are representative of 7 experiments, n = 9 mice.
Supplementary Figure 6 Enhancer deletions do not cause reduced levels of Foxp3 or CD25.
a) Quantification of Foxp3-gMFI in mature, CD73+ thymic Treg cells in WT, Foxp3-GFPKIN or Foxp3 Cns3-/- mice. All data points are normalized to the Foxp3-GFPKIN average within each experiment. Data is representative of 4 experiments, n = 8 wild-type mice, 14 GFPKIN mice and 14 Cns3-/- mice. Data was analyzed by a one-way ANOVA with Tukey’s multiple comparisons test. b) Quantification of CD25-gMFI in CD73- thymic Treg cells in WT or EDEL (Il2ra CaRE4-/-) mice in the non-obese diabetic (NOD) background. Data is representative of 3 experiments, n = 10 wild-type NOD mice and 10 EDEL NOD mice. Data was analyzed by a two-sided Mann-Whitney test. a,b) Bars represent mean ± SD. *P<0.05, ns- not significant.
Supplementary Figure 7 Treg cells derived from IL2 and IL4 exhibit distinct phenotypes.
a,b) Flow plots of the indicated TregP cell subsets following 3 days of stimulation with the indicated cytokines. c,d) Quantification of the gMFI of CD25 or Foxp3 within mature Treg cells (CD25+Foxp3+) generated from the indicated cytokine conditions. Data was analyzed by two-sided Kruskal-Wallis test. Data represents 3 experiments, n = 9 (CD25+ TregP cells- 1 Uml–1 IL2, 1 ng/mL IL4), n = 8 (CD25+ TregP cells- 100 ng/mL IL4, 1 Uml–1 IL2 + 1 ng/mL IL4, 1 Uml–1 IL2 + 100 ng/mL IL4; Foxp3lo TregP cells- 1 Uml–1 IL2, 1 Uml–1 IL2 + 1 ng/mL IL4, 1 Uml–1 IL2 + 100 ng/mL IL4), n = 7 (Foxp3lo TregP cells- 1 ng/mL IL4, 100 ng/mL IL4) or 1 experiment, n = 3 (CD25+ TregP cells- 0.5 ng/mL IL4, 1 Uml–1 IL2 + 0.5 ng/mL IL4; Foxp3lo TregP cells- 1 Uml–1 IL2 + 0.5 ng/mL IL4), n = 2 (Foxp3lo TregP cells- 0.5 ng/mL IL4) replicates. Bars represent mean ± SD. *P<0.05; **P<0.005; ***P<0.0001.
Supplementary Figure 8 Treg cells derived from Foxp3lo TregP cells protect against transfer induced colitis.
Data depicts % starting weight from Foxp3lo TregP cell transfer over the time-course of colitis experiment. Graph represents 2 experiments, n = 4 mice per group. Data was analyzed by two-sided multiple t test with Holm-Sidak method. *adjusted p value<0.05. Bars represent mean ± SEM.
Supplementary information
Supplementary Figures 1-8
Supplementary Table 1 and Supplementary Note
Supplementary Table 2
Differentially expressed genes between CD25+ TregP and Foxp3lo TregP cells from single-cell RNA-seq data sets. This table displays differentially expressed genes between CD25+ and Foxp3lo TregP cells in 2 independent single-cell RNA-seq data sets. The list is annotated by protein type and if it is a known gene correlated with negative selection
Supplementary Video 1: Kinetics of Treg cell development through RAG2-GFP expression
The video displays expression of CD25 and Foxp3 for cells falling within different bins of RAG2-GFP expression, from high RAG2-GFP to RAG2-GFP low (RAG2-GFP negative cells were excluded here). Data is from 3 concatenated thymi and is representative of 6 additional independent experiments
Bioinformatics code
Code used to analyze single cell RNA-seq data
Rights and permissions
About this article
Cite this article
Owen, D.L., Mahmud, S.A., Sjaastad, L.E. et al. Thymic regulatory T cells arise via two distinct developmental programs. Nat Immunol 20, 195–205 (2019). https://doi.org/10.1038/s41590-018-0289-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41590-018-0289-6
This article is cited by
-
Breaking tolerance: the autoimmune aspect of atherosclerosis
Nature Reviews Immunology (2024)
-
Oleic acid availability impacts thymocyte preprogramming and subsequent peripheral Treg cell differentiation
Nature Immunology (2024)
-
New insights into the stemness of adoptively transferred T cells by γc family cytokines
Cell Communication and Signaling (2023)
-
Foxp3 orchestrates reorganization of chromatin architecture to establish regulatory T cell identity
Nature Communications (2023)
-
How autoreactive thymocytes differentiate into regulatory versus effector CD4+ T cells after avoiding clonal deletion
Nature Immunology (2023)