Abstract
Metabolic syndrome is characterized by a constellation of comorbidities that predispose individuals to an increased risk of developing cardiovascular pathologies as well as type 2 diabetes mellitus1. The gut microbiota is a new key contributor involved in the onset of obesity-related disorders2. In humans, studies have provided evidence for a negative correlation between Akkermansia muciniphila abundance and overweight, obesity, untreated type 2 diabetes mellitus or hypertension3,4,5,6,7,8. Since the administration of A. muciniphila has never been investigated in humans, we conducted a randomized, double-blind, placebo-controlled pilot study in overweight/obese insulin-resistant volunteers; 40 were enrolled and 32 completed the trial. The primary end points were safety, tolerability and metabolic parameters (that is, insulin resistance, circulating lipids, visceral adiposity and body mass). Secondary outcomes were gut barrier function (that is, plasma lipopolysaccharides) and gut microbiota composition. In this single-center study, we demonstrated that daily oral supplementation of 1010 A. muciniphila bacteria either live or pasteurized for three months was safe and well tolerated. Compared to placebo, pasteurized A. muciniphila improved insulin sensitivity (+28.62 ± 7.02%, P = 0.002), and reduced insulinemia (−34.08 ± 7.12%, P = 0.006) and plasma total cholesterol (−8.68 ± 2.38%, P = 0.02). Pasteurized A. muciniphila supplementation slightly decreased body weight (−2.27 ± 0.92 kg, P = 0.091) compared to the placebo group, and fat mass (−1.37 ± 0.82 kg, P = 0.092) and hip circumference (−2.63 ± 1.14 cm, P = 0.091) compared to baseline. After three months of supplementation, A. muciniphila reduced the levels of the relevant blood markers for liver dysfunction and inflammation while the overall gut microbiome structure was unaffected. In conclusion, this proof-of-concept study (clinical trial no. NCT02637115) shows that the intervention was safe and well tolerated and that supplementation with A. muciniphila improves several metabolic parameters.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout




Similar content being viewed by others
Data availability
The data that support the findings of this study are available upon request. All figures are provided with individual values to have a direct access to the raw data. The 16S sequencing datasets generated during the current study are available from the European Genome-Phenome Archive (https://ega-archive.org/) under accession no. EGAS00001003585.
References
O’Neill, S. & O’Driscoll, L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes. Rev. 16, 1–12 (2015).
Cani, P. D. et al. Microbial regulation of organismal energy homeostasis. Nat. Metab. 1, 34–46 (2019).
Brahe, L. K. et al. Specific gut microbiota features and metabolic markers in postmenopausal women with obesity. Nutr. Diabetes 5, e159 (2015).
Zhang, X. et al. Human gut microbiota changes reveal the progression of glucose intolerance. PLoS ONE 8, e71108 (2013).
Yassour, M. et al. Sub-clinical detection of gut microbial biomarkers of obesity and type 2 diabetes. Genome Med. 8, 17 (2016).
Li, J. et al. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome 5, 14 (2017).
Liu, R. et al. Gut microbiome and serum metabolome alterations in obesity and after weight-loss intervention. Nat. Med. 23, 859–868 (2017).
Dao, M. C. et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436 (2016).
Cani, P. D. & de Vos, W. M. Next-generation beneficial microbes: the case of Akkermansia muciniphila. Front. Microbiol. 8, 1765 (2017).
Everard, A. et al. Cross-talk between Akkermansia muciniphila and intestinal epithelium controls diet-induced obesity. Proc. Natl Acad. Sci. USA 110, 9066–9071 (2013).
Plovier, H. et al. A purified membrane protein from Akkermansia muciniphila or the pasteurized bacterium improves metabolism in obese and diabetic mice. Nat. Med. 23, 107–113 (2017).
Everard, A. et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat. Commun. 10, 457 (2019).
Atkin, S. L. et al. Effect of dipeptidyl peptidase-4 inhibitors on circulating tumor necrosis factor-α concentrations: a systematic review and meta-analysis of controlled trials. J. Diabetes Complicat. 31, 1458–1464 (2017).
Akoumianakis, I. & Antoniades, C. Dipeptidyl peptidase IV inhibitors as novel regulators of vascular disease. Vascul. Pharmacol. 96–98, 1–4 (2017).
Olivares, M. et al. The potential role of the dipeptidyl peptidase-4-like activity from the gut microbiota on the host health. Front. Microbiol. 9, 1900 (2018).
Veronelli, A. et al. White blood cells in obesity and diabetes: effects of weight loss and normalization of glucose metabolism. Diabetes Care 27, 2501–2502 (2004).
Ohshita, K. et al. Elevated white blood cell count in subjects with impaired glucose tolerance. Diabetes Care 27, 491–496 (2004).
Gu, Y. et al. White blood cells count as an indicator to identify whether obesity leads to increased risk of type 2 diabetes. Diabetes Res. Clin. Pract. 141, 140–147 (2018).
Zhang, H. et al. White blood cell subtypes and risk of type 2 diabetes. J. Diabetes Complicat. 31, 31–37 (2017).
Twig, G. et al. White blood cells count and incidence of type 2 diabetes in young men. Diabetes Care 36, 276–282 (2013).
Shen, J. et al. Low-density lipoprotein receptor signaling mediates the triglyceride-lowering action of Akkermansia muciniphila in genetic-induced hyperlipidemia. Arterioscler. Thromb. Vasc. Biol. 36, 1448–1456 (2016).
Li, J., Lin, S., Vanhoutte, P. M., Woo, C. W. & Xu, A. Akkermansia muciniphila protects against atherosclerosis by preventing metabolic endotoxemia-induced inflammation in Apoe −/− mice. Circulation 133, 2434–2446 (2016).
Ras, R. T. et al. Consumption of plant sterol-enriched foods and effects on plasma plant sterol concentrations—a meta-analysis of randomized controlled studies. Atherosclerosis 230, 336–346 (2013).
Wannamethee, S. G., Shaper, A. G., Lennon, L. & Whincup, P. H. Hepatic enzymes, the metabolic syndrome, and the risk of type 2 diabetes in older men. Diabetes Care 28, 2913–2918 (2005).
Rantala, A. O. et al. Gamma-glutamyl transpeptidase and the metabolic syndrome. J. Intern. Med. 248, 230–238 (2000).
Lim, J. S., Lee, D. H., Park, J. Y., Jin, S. H. & Jacobs, D. R. Jr. A strong interaction between serum γ-glutamyltransferase and obesity on the risk of prevalent type 2 diabetes: results from the Third National Health and Nutrition Examination Survey. Clin. Chem. 53, 1092–1098 (2007).
Fraser, A. et al. Alanine aminotransferase, γ-glutamyltransferase, and incident diabetes: the British Women’s Heart and Health Study and meta-analysis. Diabetes Care 32, 741–750 (2009).
Hänninen, A. et al. Akkermansia muciniphila induces gut microbiota remodelling and controls islet autoimmunity in NOD mice. Gut 67, 1445–1453 (2018).
Grander, C. et al. Recovery of ethanol-induced Akkermansia muciniphila depletion ameliorates alcoholic liver disease. Gut 67, 891–901 (2018).
Wu, W. et al. Protective effect of Akkermansia muciniphila against immune-mediated liver injury in a mouse model. Front. Microbiol. 8, 1804 (2017).
Harte, A. L. et al. Elevated endotoxin levels in non-alcoholic fatty liver disease. J. Inflamm. (Lond.) 7, 15 (2010).
Cani, P. D. et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes 56, 1761–1772 (2007).
Miele, L. et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology 49, 1877–1887 (2009).
Lassenius, M. I. et al. Bacterial endotoxin activity in human serum is associated with dyslipidemia, insulin resistance, obesity, and chronic inflammation. Diabetes Care 34, 1809–1815 (2011).
Allin, K. H. et al. Aberrant intestinal microbiota in individuals with prediabetes. Diabetologia 61, 810–820 (2018).
Wu, H. et al. Metformin alters the gut microbiome of individuals with treatment-naive type 2 diabetes, contributing to the therapeutic effects of the drug. Nat. Med. 23, 850–858 (2017).
Dao, M. C. et al. Akkermansia muciniphila and improved metabolic health during a dietary intervention in obesity: relationship with gut microbiome richness and ecology. Gut 65, 426–436 (2016).
Scherbaum, W. A. et al. Efficacy and tolerability of vildagliptin in drug-naïve patients with type 2 diabetes and mild hyperglycaemia. Diabetes Obes. Metab. 10, 675–682 (2008).
Rosenstock, J., Rigby, S. P., Ford, D. M., Tao, B. & Chou, H. S. The glucose and lipid effects of colesevelam as monotherapy in drug-naïve type 2 diabetes. Horm. Metab. Res. 46, 348–353 (2014).
Kim, S. G. et al. Efficacy and safety of lobeglitazone monotherapy in patients with type 2 diabetes mellitus over 24-weeks: a multicenter, randomized, double-blind, parallel-group, placebo controlled trial. PLoS ONE 9, e92843 (2014).
Werner, M., Tönjes, A., Stumvoll, M., Thiery, J. & Kratzsch, J. Assay-dependent variability of serum insulin levels during oral glucose tolerance test: influence on reference intervals for insulin and on cut-off values for insulin sensitivity indices. Clin. Chem. Lab. Med. 46, 240–246 (2008).
Borza, D. et al. Influence of assay-dependent variability of serum insulin levels on insulin sensitivity indices. Clin. Chem. Lab. Med. 46, 1655–1656 (2008).
Wallace, T. M., Levy, J. C. & Matthews, D. R. Use and abuse of HOMA modeling. Diabetes Care 27, 1487–1495 (2004).
Dennis, J. M. et al. Precision medicine in type 2 diabetes: clinical markers of insulin resistance are associated with altered short- and long-term glycemic response to DPP-4 inhibitor therapy. Diabetes Care 41, 705–712 (2018).
Vandeputte, D. et al. Quantitative microbiome profiling links gut community variation to microbial load. Nature 551, 507–511 (2017).
Callahan, B. J. et al. DADA2: high-resolution sample inference from Illumina amplicon data. Nat. Methods 13, 581–583 (2016).
Wang, Q., Garrity, G. M., Tiedje, J. M. & Cole, J. R. Naive Bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl. Environ. Microbiol. 73, 5261–5267 (2007).
Oksanen, J. B. et al. vegan: community ecology package. R package version 2.5-3 (2018).
Gloor, G. B. & Reid, G. Compositional analysis: a valid approach to analyze microbiome high-throughput sequencing data. Can. J. Microbiol. 62, 692–703 (2016).
Acknowledgements
We thank A. Barrois for the excellent technical assistance. We are very grateful to M. Buysschaert for his continuous support and helpful criticism during the preparation of this project. We also thank the volunteers who participated in this study. P.D.C. is a senior research associate at Fonds de la Recherche Scientifique (FRS-FNRS). A.E. is a research associated at FRS-FNRS. C. Druart was supported by a FIRST Spin-Off grant from the Walloon Region (no. 1410053). Research in the Wageningen laboratory of W.M.d.V. was partially supported by a European Research Council (ERC) Advanced Grant no. 250172 (Microbes Inside), the SIAM Gravity Grant no. 024.002.002 and the Spinoza Award of the Netherlands Organization for Scientific Research. P.D.C. is the recipient of grants from the FNRS (nos. J.0084.15 and 3.4579.11) and Projet de Recherche (no. T.0138.14) and FRFS-WELBIO grants (no. WELBIO-CR-2017C-02). This work was supported by the Funds Baillet Latour (Grant for Medical Research 2015), a prize of the Banque Transatlantique Belgium and a FIRST Spin-Off grant from the Walloon Region (no. 1410053). P.D.C. is a recipient of the PoC ERC grant 2016 (no. Microbes4U_713547) and ERC Starting Grant 2013 (no. 336452-ENIGMO). P.D.C. and J.R. are recipients of a grant from the FNRS and FWO (EOS program no. 30770923).
Author information
Authors and Affiliations
Contributions
P.D.C. conceived the project. J.-P.T., M.P.H., A.L., D.M., A.E., C. Depommier, C. Druart, H.P., M.V.H., W.M.d.V. and P.D.C. designed the clinical study. P.D.C. supervised the clinical part of the study and W.M.d.V. contributed to the microbial culturing of A. muciniphila. P.D.C., A.E., C. Depommier, C. Druart, M.d.B., J.-P.T., A.L., D.M. and M.P.H. performed the clinical part of the study. N.M.D. contributed to interpretation of the results. S.V.-S., G.F. and J.R. performed the fecal microbiome sequencing and analysis. P.D.C., A.E. and C. Depommier performed the experiments and interpreted all the results. P.D.C., A.E. and C. Depommier generated the figures and tables. P.D.C. and C. Depommier wrote the manuscript. All authors discussed the results and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
A.E., C. Druart, H.P., P.D.C. and W.M.d.V. are inventors of patent applications (nos. PCT/EP2013/073972, PCT/EP2016/071327 and PCT/EP2016/060033 filed with the European Patent Office, Australia, Brazil, Canada, China, the Eurasian Patent Organization, Israel, India, Hong Kong, Japan, South Korea, Mexico, New Zealand and the United States) dealing with the use of A. muciniphila and its components in the context of obesity and related disorders. P.D.C. and W.M.d.V. are cofounders of A-Mansia Biotech S.A.
Additional information
Peer Review Information: Joao Monteiro was the primary editor on this article and managed its editorial process and peer review in collaboration with the rest of the editorial team.
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
Extended Data Fig. 1 Flow chart of the interventional study.
Diagram of the participant selection procedure, which includes the following information: number of individuals enrolled at each step of the study progress; number of individuals included in the final analysis; details of the events that led to a reduction in group size.
Extended Data Fig. 2 Changes in inflammatory parameters and GLP-1.
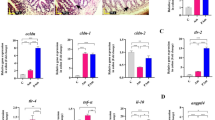
a, Soluble CD40 Ligand. b, Growth-related oncogene (CXCL1). c, MCP1. d, GLP-1. Differential values (mean difference and mean difference from placebo) are expressed as the mean + s.e.m., either as raw data or as percentages. The bars represent the mean change from baseline value per group, with their s.e.m. Mann–Whitney U-tests or unpaired t-tests were performed to compare the differential values of both treated groups versus the placebo group (intergroup changes), according to the distribution. The respective P values are shown in the table below each plot. The lines represent the raw values before and after 3 months of supplementation. The distribution of values within each group for each timing is illustrated by a box-and-whisker plot. In the box plots, the line in the middle of the box is plotted at the median, and the inferior and superior limits of the box correspond to the 25th and the 75th percentiles respectively. Matched-pairs Wilcoxon signed-rank tests or paired t-tests were performed to verify changes from baseline (intragroup changes), according to the distribution; when drawn, the capped line above the group concerned shows the corresponding P value. Changes between 0 and 3 months across the 3 groups were analyzed with Kruskal–Wallis or one-way ANOVA tests according to the distribution; group-wise comparisons were performed using Bonferroni’s and Tukey’s corrections for multiple testing, respectively. Placebo group, n = 11; pasteurized bacteria group, n = 12; live bacteria group, n = 9 for all parameters except for growth-related oncogene: placebo group, n = 7; pasteurized bacteria group, n = 10; live bacteria group, n = 8. All tests were two-tailed.
Extended Data Fig. 3 Changes in fecal microbiome.
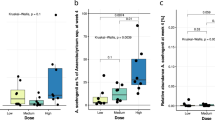
a, Akkermansia muciniphila abundance in feces evaluated by quantitative PCR. Differential values (mean difference and mean difference from placebo) are expressed as the mean ± s.e.m. as raw data. The bars represent the mean change from baseline value per group, with their s.e.m. Mann–Whitney U-tests were performed to compare the differential values of both treated groups versus the placebo group (intergroup changes) according to the distribution. The respective P values are shown in the table below each plot. The lines represent the raw values before and after 3 months of supplementation. The distribution of values within each group for each timing is illustrated by a box-and-whisker plot. In the box plots, the line in the middle of the box is plotted at the median, and the inferior and superior limits of the box correspond to the 25th and the 75th percentiles, respectively. Matched-pairs Wilcoxon signed-rank tests were performed to verify changes from baseline (intragroup changes) according to the distribution. When the difference is significant, a capped line is marked above the group concerned with the corresponding P value. Kruskal–Wallis analyses were used to compare changes between 0 and 3 months across the 3 groups according to the distribution. Placebo group, n = 11; pasteurized bacteria group, n = 12; live bacteria, n = 9. All tests were two-tailed. *P < 0.05. b, Visualization of participants’ fecal microbiota composition at baseline and end point of the intervention. Fecal microbiota dissimilarity between samples is represented by principal coordinates analysis (genus-level Aitchison distance), with six sample groups corresponding to the three different treatment arms at baseline or at end point represented by confidence ellipses (80% confidence interval). Intervention effects are symbolized by the colored arrows, with direction and length corresponding to the shift in group centroid coordinates from baseline to end point for each treatment arm (rescaled ×4 and re-centered at the baseline global centroid). Placebo group, n = 11; pasteurized bacteria group, n = 12; live bacteria, n = 9.
Supplementary information
Supplementary Information
Supplementary Tables 1–5
Rights and permissions
About this article
Cite this article
Depommier, C., Everard, A., Druart, C. et al. Supplementation with Akkermansia muciniphila in overweight and obese human volunteers: a proof-of-concept exploratory study. Nat Med 25, 1096–1103 (2019). https://doi.org/10.1038/s41591-019-0495-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s41591-019-0495-2
This article is cited by
-
Integrative metagenomic analysis reveals distinct gut microbial signatures related to obesity
BMC Microbiology (2024)
-
Non-traumatic osteonecrosis of the femoral head induced by steroid and alcohol exposure is associated with intestinal flora alterations and metabolomic profiles
Journal of Orthopaedic Surgery and Research (2024)
-
Amorphous silica nanoparticles and the human gut microbiota: a relationship with multiple implications
Journal of Nanobiotechnology (2024)
-
16S rRNA sequencing analysis of the oral and fecal microbiota in colorectal cancer positives versus colorectal cancer negatives in Iranian population
Gut Pathogens (2024)
-
The gut-liver axis in hepatobiliary diseases
Inflammation and Regeneration (2024)