Abstract
Among Italy, Spain, and Japan, the age distributions of COVID-19 mortality show only small variation even though the number of deaths per country shows large variation. To understand the determinant for this situation, we constructed a mathematical model describing the transmission dynamics and natural history of COVID-19 and analyzed the dataset of mortality in Italy, Spain, and Japan. We estimated the parameter which describes the age-dependency of susceptibility by fitting the model to reported data, including the effect of change in contact patterns during the epidemics of COVID-19, and the fraction of symptomatic infections. Our study revealed that if the mortality rate or the fraction of symptomatic infections among all COVID-19 cases does not depend on age, then unrealistically different age-dependencies of susceptibilities against COVID-19 infections between Italy, Japan, and Spain are required to explain the similar age distribution of mortality but different basic reproduction numbers (R0). Variation of susceptibility by age itself cannot explain the robust age distribution in mortality by COVID-19 infections in those three countries, however it does suggest that the age-dependencies of (i) the mortality rate and (ii) the fraction of symptomatic infections among all COVID-19 cases determine the age distribution of mortality by COVID-19.
Similar content being viewed by others
Introduction
Since its emergence, coronavirus disease 2019 (COVID-19) has resulted in a pandemic and has produced a huge number of cases worldwide1. As of May 29, 2020, the number of confirmed cases in Italy was 382.3 (per 100,000 population), with 507.2 in Spain, and 13.2 in Japan1. Of those infected, it has been reported that elderly individuals account for a large portion of fatal cases inducing a large heterogeneity in the age distribution of mortality2,3,4.
The expected value of mortality (the number of deaths, hereafter referred to as mortality) is calculated as the product of the number of cases and the mortality rate among cases (hereafter referred to as morality rate). As the background mechanism of the heterogeneity of mortality by age, the association of two epidemiological factors with mortality can be considered: (i) the age-dependency of susceptibility to infection, which is related to the heterogeneity in the number of cases, and (ii) the age-dependency of severity, which is related to the heterogeneity in the mortality rate, e.g. the rate of becoming a symptomatic, severe, or fatal case among infected individuals. For the first factor, a high susceptibility for infection will generate a larger number of infections and result in an increase in fatal cases. The possibility of heterogeneity in susceptibility by age was pointed out by the analysis of epidemiological data reported from Wuhan, China4,5,6 and from Iceland7. For the second factor, an increase in severity will result in a higher mortality rate and subsequently a rise in the number of fatal cases. This assumption is also reasonable because elder age as well as the existence of comorbidities, which are likely with aging, have been reported as risk factors for severe COVID-19 infections8,9,10,11,12,13. Although not yet shown in relation to severe acute respiratory syndrome coronavirus 2 (SARS Cov-2), which is the causal agent of COVID-19, the presence of age-dependent enhancement of severity has been suggested in SARS coronavirus by the analysis of the innate immune responses in the BALB/c mouse model14,15,16. Additionally, it has been suggested that antibody-dependent enhancement (ADE) can contribute to the formation of the observed age-dependency of severity, as suggested in SARS and Middle East respiratory syndrome (MERS) cases17,18,19,20,21,22.
Interestingly, the age distribution of mortality by COVID-19 (the distribution of the proportion of deaths per age group among all deaths), is similar between Italy, Japan, and Spain, even though the number of deaths are quite different among them23,24,25 (Fig. 1). The reported number of deaths was 3 in 0–9 years old (yo), 0 in 10–19 yo, 11 in 20–29 yo, 58 in 30–39 yo, 257 in 40–49 yo, 1,051 in 50–59 yo, 3,107 in 60–69 yo, and 25,038 in 70 + yo in Italy as of May 13, 2020. In Japan, that was 0 in 0–9 yo, 0 in 10–19 yo, 0 in 20–29 yo, 2 in 30–39 yo, 8 in 40–49 yo, 16 in 50–59 yo, 44 in 60–69 yo, and 330 in over 70 + yo as of May 7, 2020. In Spain, that was 2 in age 0–9 yo, 5 in 10–19 yo, 23 in 20–29 yo, 61 in 30–39 yo, 198 in 40–49 yo, 607 in 50–59 yo, 1669 in 60–69 yo, and 16,253 in over 70 + yo as of May 12, 2020. According to projections by the United Nations26, the population size for 2020 per 1,000,000 was 4.99 in 0–9 yo, 5.73 in 10–19 yo, 6.10 in 20–29 yo, 7.00 in 30–39 yo, 9.02 in 40–49 yo, 9.57 in 50–59 yo, 7.48 in 60–69 yo, and 10.55 in 70 + yo in Italy. In Japan, that was 10.18 in 0–9 yo, 11.27 in 10–19 yo, 12.15 in 20–29 yo, 14.46 in 30–39 yo, 18.47 in 40–49 yo, 16.54 in 50–59 yo, 15.88 in 60–69 yo, and 27.54 in 70 + yo. In Spain, that was 4.23 in 0–9 yo, 4.74 in 10–19 yo, 4.62 in 20–29 yo, 5.90 in 30–39 yo, 7.94 in 40–49 yo, 7.05 in 50–59 yo, 5.34 in 60–69 yo, and 6.94 in 70 + yo. The large difference in the number of deaths between the countries suggests a large difference in their basic reproduction numbers, R0s. An independency between age distribution of mortality by COVID-19 and R0 is suggested. From this independency of age distributions of mortality from R0, it can be expected that the contribution of heterogeneity in susceptibility by age to forming the age distribution of mortality is small. That is because, as we will show in this paper, though the age-dependency of severity will naturally produce a proportional effect on the distribution of mortality and result in the formation of robust distributions, when the age-dependency of susceptibility forms the age distribution of mortality, the age distribution of mortality highly depends on R0 and shows variability.
To understand the background of robust age distribution of mortality with varied R0, we constructed a mathematical model describing the transmission dynamics of COVID-19 and analyzed the impact of age-dependent susceptibility on the age distribution of mortality. The heterogeneity in social contacts by age may also contribute to the age distribution of mortality. Our model took into account the heterogeneity in social contacts by age and country, and the effect of behavioral change outside of the household during the outbreak. We also estimated and compared the age-dependent susceptibility in Japan, Italy, and Spain to argue the existence of heterogeneity in susceptibility among age groups.
Results
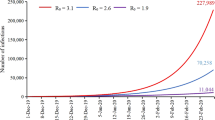
Our result shows variation of susceptibility among age groups measured by the exponent parameter φ can explain the age distribution of mortality by COVID-19 (Fig. 2a). However, the age distribution of mortality formed by the age-dependency of susceptibility is influenced by the value of R0 (Fig. 2b), which cannot explain the similarity in age distributions of mortality among Italy, Japan, and Spain. On the other hand, if susceptibility is constant among age groups, the impact of R0 is quite small on the age distribution of mortality (Fig. 3).
The sensitivity of (a) age-dependency of susceptibility and (b) transmission coefficient β against age distribution of mortality when age-independent mortality was assumed. In panel (a), all parameters except the exponent parameter φ, describing the variation of susceptibility among age groups, were fixed and parameterized as R0 = 2.9 in the setting for Spain. In panel (b), all parameters parameterized as the setting for Spain (φ = 12.3) were fixed except transmission coefficient β.
Assuming that the age-dependency of mortality by COVID-19 is determined by only age-dependent susceptibility (model 1), i.e., the mortality rate does not depend on age, the exponent parameter, φ, describing the variation of susceptibility among age groups for each country, Italy, Japan, and Spain, was estimated as shown in Fig. 4. From the difference of the R0 value and country, the estimated value of φ is largely varied. The impact of reductions in contacts outside of the household on the estimated value of φ was small. The estimate of φ in Italy, assuming a range of R0 = 2.4–3.327,28 was 15.0 (95% CI 14.0–16.0), 16.3 (95% CI 14.9–17.7), and 16.9 (95% CI 15.4–18.4) for 80%, 40%, and no reduction in contacts outside of the household. For Japan, the estimate of φ assuming R0 = 1.729 was 4.2 (95% CI 3.7–4.9), 5.5 (95% CI 4.9–6.3), and 6.1 (95% CI 5.4–6.9) for 80%, 40%, and no reduction in contacts outside of the household. When it comes to Spain, the estimate of φ assuming an R0 = 2.930 was 10.5 (95% CI 10.4–10.6), 11.7 (95% CI 11.6–11.9), and 12.3 (95% CI 12.2–12.5) for 80%, 40%, and no reduction in contacts outside of the household.
The estimates of φ, assuming that the mortality by COVID-19 infections depends on age but the fraction of infections becoming symptomatic does not depend on age (model 2), were also varied by the value of R0 and by country (Fig. 5, s1 and s2). Employing the same assumptions of R0 value, the estimate of φ in Italy was 5.2 (95% CI 4.7–5.7), 5.9 (95% CI 5.3–6.4), and 6.1 (95% CI 5.5–6.6) for 80%, 40%, and no reduction in contacts outside of the household. For Japan, the estimate of φ was 0.0 (95% CI 0.0–1.0), 0.0 (95% CI 0.0–1.2), and 0.0 (95% CI 0.0–1.4) for 80%, 40%, and no reduction in contacts outside of the household. For Spain, the estimate of φ was 4.1 (95% CI 2.5–5.0), 4.8 (95% CI 2.6–5.8), and 5.1 (95% CI 2.6–6.2) for 80%, 40%, and no reduction in contacts outside of the household.
The estimate of exponent parameter φ describing the variation of susceptibility among age groups using model 2 and assuming that the fraction of infections that becomes symptomatic among all COVID-19 cases is 0.25. True and broken lines represent the maximum likelihood estimates and 95% confidence intervals, respectively.
Discussion
In the present study, we explored the role of susceptibility to COVID-19 in explaining the age distribution of mortality by COVID-19. Interestingly, the age distributions of mortality from COVID-19 are quite similar between Italy, Japan, and Spain (Fig. 1). When comparing the age distributions of mortality, only the comparison between Italy and Spain is significant (p < 0.05 in Wilcoxon rank sum test with Bonferroni correction). On the other hand, the numbers of deaths are quite different (29,525 for Italy, 400 for Japan, 18,818 for Spain). Indeed, R0 values are largely different: 2.4–3.3 for Italy27,28, 1.7 for Japan29, and 2.9 for Spain30. If the variation of mortality by age is determined by only the age-dependency of susceptibility, the age distribution of mortality is affected by R0 as shown in Fig. 2b. However, we observed a similarity in age distributions of mortalities between Italy, Japan, and Spain where their R0s are quite different. Indeed, unrealistically different φs among these three countries are required to explain their age distribution of mortality for both settings, (i) age-independent mortality, and, (ii) the fraction of infections that becomes symptomatic among all COVID-19 cases, fs, does not depend on age. Although we cannot fully reject the existence of age-dependency in susceptibility, our results suggest that it does not largely depend on age, but rather that age-dependency in severity highly contributes to the formation of the observed age distribution in mortality.
The estimates of φs assuming age independency in symptomatic infections were smaller than those that assumed age independency in mortality. This suggests that the age-dependency of the confirmed case fatality rate (cCFR), which can be biased by the age-dependent difference of the fraction of symptomatic infections among all cases, partially explains the age distribution in mortality. Indeed, when we assumed that the fraction of symptomatic infections was not dependent on age, the estimate of φ in Japan was close to zero in all scenarios regarding the fraction of symptomatic infections, meaning that susceptibility is constant among age groups (Fig. 5). Although we observed φs not close to zero in Italy and Spain, this does not mean straightforwardly that susceptibility is age dependent because there is room for an alternative explanation: not susceptibility, but an age-dependent fraction of symptomatic infections can explain this age-dependency. Unfortunately, as we do not yet have detailed data regarding the age-dependent fraction of symptomatic infections and the rate of diagnosis in COVID-19, we cannot conclude which factors (i.e., susceptibility or the fraction of symptomatic infection among all cases) contributed to the observed age-dependency.
Wu et al.4 showed variation of susceptibility to symptomatic infection by age. This susceptibility can be expressed as the product of the susceptibility and the fraction of symptomatic infection among all cases. To accurately understand susceptibility (i.e., without the constraint of the symptom onset), estimates of the age-dependent fraction of symptomatic infections is required.
To understand the mechanism of age-dependency of mortality by COVID-19, an accurate age-dependent mortality rate is required. The data of mortality by COVID-19 infections used in this study might not cover all mortalities by COVID-19 infections. To estimate the age-dependent mortality rate, an accurate estimate of the case fatality rate is required. However, the number of cases, which is the denominator of the case fatality rate, is difficult to estimate for COVID-19 due to changes in the testing rate31,32,33, the change of case definition34, selection biases35, and the delay between the onset of symptoms and death12,36,37,38 as were the cases we experienced in the surveillance of other emerging diseases 39,40. To address this problem, implementation of active epidemiological surveillances, such as a large-scale cohort study including real-time detection of infections, should be considered.
From the modelling perspective on mortality by Covid-19, age-dependency of severity should be carefully taken into consideration. In particular, in the mathematical models of ADE, previous models employed three types of assumptions41, the assumption of: increasing susceptibility to infection42,43, increasing transmissibility once infection occurred42,44,45, and increasing severity and/or mortality associated with infection46. Based on our results and from the biological/epidemiological observations of past SARS and MERS cases, the “increasing severity” assumption should be taken into account when analyzing SARS Cov-2 epidemics.
We modelled the age-specific susceptibility as a power law function based on the monotonic increase of mortality by COVID-19 over age as seen in Fig. 1. The power law function is widely used to model heterogeneity, e.g., the heterogeneity in risks of sexually transmitted infections47. Although our model for age-specific susceptibility covers a wide variation of monotonic changes, our results might be biased by this formulation if the susceptibility changes over age in non-monotonic fashion.
The increase in width of the confidence interval for the estimate of φ by increasing R0 values were observed in Fig. 5. To explain with the “left-skewed” age-distribution of mortality with high R0, a large φ is required since the higher R0 value decreased the heterogeneity of mortality by age (Fig. 2b) and the large φ increased the heterogeneity of mortality (Fig. 2a). The sensitivity of φ to the age-distribution of mortality becomes smaller when φ is larger (Fig. 2a), the large widths of the confidence intervals for the estimate of φ is necessary to explain the age-distribution of mortality when R0 is high.
In conclusion, the contribution of age-dependency to susceptibility is difficult to use to explain the robust age distribution in mortalities by COVID-19, and it suggests that the age-dependencies of the mortality rate and the fraction of symptomatic infections among all COVID-19 cases determine the age distribution in mortality from COVID-19. Further investigations regarding age-dependency on the fraction of infections becoming symptomatic is required to understand the mechanism behind the mortality by COVID-19 infections.
Materials and methods
Data
We analyzed the number of mortalities caused by COVID-19 in Italy reported on 13th May 2020, Japan reported on 7th May 2020, and Spain reported on 12th May 2020. The data were collected from public data sources in each country23,24,25.
Model
A simple SEIRD model taking into account mixing between age groups (model 1)
To understand the background of robust age distribution of mortality with varied R0, we employed a mathematical model describing transmissions of COVID-19. Clinical observations suggest that both asymptomatic and symptomatic cases are infectious after the latent period48,49, we used a simple age-structured SEIRD (susceptible-exposed-infectious-recovered-dead) model, which can be written as;
where Sn, En, In, Rn and Dn represent the proportion of susceptible, latent, infectious, recovered and dead among the entire population, and the subscript index n denotes age group. We stratified the entire population by into eight groups, n = 1, 2, 3, 4, 5, 6, 7, and 8 for < 10 yo, 10–19 yo, 20–29 yo, 30–39 yo, 40–49 yo, 50–59 yo, 60–69 yo, and 70 + yo. β, kn,m, ε, γ and δ represent a transmission coefficient, an element of the contact matrix between age group n and m, the progression rate from latent to infectious, recovery rate and mortality rate by COVID-19 infections, respectively. σn denotes the susceptibility of age group n. For the sake of simplicity, based on the short study duration of COVID-19 epidemics compared to the length of a human lifespan, births and deaths from causes other than COVID-19 were ignored. To take into account the effect of behavioral changes outside of the household during the outbreak, kn,m is decomposed by a matrix for contacts within household kin,n,m and that for contacts outside the household kout,n,m;
where α denotes the reduced fraction of contacts outside of the household. We modelled age specific susceptibility as
where c is susceptibility among age group 1 and a constant among all age groups, φ denotes the exponent parameter describing the variation of susceptibility among age groups. An increase in φ means an increase in the variation of susceptibility among age groups, and φ = 0 means that susceptibility is equal among all age groups.
SEIRD model taking into account mixing between age groups, asymptomatic/symptomatic, and age-dependency of mortality by COVID-19 (model 2)
Model 1 does not classify the cases into asymptomatic and symptomatic cases explicitly. If the progression of disease is largely different between asymptomatic and symptomatic cases, the estimates using model 1 can be biased. In addition, the age-dependency of mortality by COVID-19 infections is not taken into account. Model 2 takes into account both the different progression of disease between asymptomatic and symptomatic cases and the age-dependency of mortality by COVID-19 infections;
where Is,n and Ia,n represent the proportion of symptomatic and asymptomatic cases among age group n. Other compartments are the same as model 1. fs represents the fraction of symptomatic infections among all COVID-19 cases and δn represents the mortality rate by COVID-19 infection among age group n. \({\gamma }_{s}\) and \({\gamma }_{a}\) denote the recovery rates among symptomatic and asymptomatic cases. Other parameters are the same as model 1.
Parameterizations
We parameterized ε and γ using values from a previous modelling study of COVID-1948,50,51. The average length of the latent period (i.e., 1/ε) was set to 6.4 days48,50, assuming that the latent period is equal to the incubation period, and the average length of the infectious period (i.e., 1/γ) was 7 days48,51 for model 1. In model 2, to take into account the different infectious period between symptomatic and asymptomatic infections, we set an average length of infectious period among asymptomatic cases (i.e., 1/γa) as 9 days49 and an average length of infectious period among symptomatic cases (i.e., 1/γs) as 7 days. We referred to the contact matrices for Italy, Japan, and Spain from Prem et al.52. β and c were controlled such that the basic reproduction number, R0, becomes arbitral values. R0 was calculated by constructing a next generation matrix53,54 using each country’s demographic data obtained from a public data source26.
In terms of parameterization for mortality rate by COVID-19 infection, a reliable estimate of δn for COVID-19 is difficult to obtain. Due to the uncertainty of the fraction of symptomatic infections per age group, δn is difficult to estimate from observed data, i.e., the confirmed case fatality rate among age group n (cCFRn). Since an estimate of δn is difficult to obtain, we employed two different settings (i) δn is assumed to be a constant among all age groups as assumed in the model 1, i.e., δn = δ for any age group n, or, (ii) δn is calculated from cCFRn assuming that the fraction of symptomatic infections among all COVID-19 cases (fs) is not dependent on age as assumed in model 2.
In the setting for model 1, the value of δ is not required to estimate Dn once the value of R0 is given. We calculated Dn by calculating the proportions of recovered persons per age group among all recovered persons \({R}_{n}(\infty )/\sum_{n}{R}_{n}(\infty )\) instead of \({D}_{n}(\infty )/\sum_{n}{D}_{n}(\infty )\). In our model, shown in Eq. (1–5), \({R}_{n}(\infty )/\sum_{n}{R}_{n}(\infty )\) is determined by the value of R0 completely when all parameter values other than β and δ are fixed, and \({D}_{n}(\infty )/\sum_{n}{D}_{n}(\infty )={R}_{n}(\infty )/\sum_{n}{R}_{n}(\infty )\) if \({\delta }_{n}\ne 0\). The proof can be found in the Supplemental text.
The assumption in model 1, δn is constant among all age groups, may be too strong for the COVID-19 epidemic. To take into account the age-dependency of mortality by COVID-19, δn was calculated from the cCFRn assuming that fs is not dependent with age. For the setting in model 2, assuming three scenarios; fs = 0.05, 0.25, and 0.5, δn for each country were calculated using cCFRn in each country. We obtained δn by solving cCFRn = δn/ (δn + γs).
Fitting
We calculated the proportions of deaths in the age group n among all deaths, Dn (\({=D}_{n}(\infty )/\sum_{n}{D}_{n}(\infty )\)), and fitted them to the observed data in each country. We solved model 1 shown in Eqs. (1–5) and model 2 shown in Eqs. (8–13) numerically, and Dn was calculated after sufficient time was given to finish the epidemics. We estimated φ using a log likelihood function describing the multinomial sampling process of deaths per age group;
Maximum likelihood estimates of φ with given R0 were obtained by maximizing Eq. (14) and the profile likelihood-based confidence intervals were computed.
Data availability
All data collected and analyzed during this study are included in this published article and its Supplementary Information files.
References
World Health Organization. Situation report -130, Coronavirus disease 2019 (COVID-2019) 29 May 2020. https://www.who.int/emergencies/diseases/novel-coronavirus-2019/situation-reports (2020).
Dowd, J. B. et al. Demographic science aids in understanding the spread and fatality rates of COVID-19. Proc. Natl. Acad. Sci. 117, 9696–9698. https://doi.org/10.1073/pnas.2004911117 (2020).
Onder, G., Rezza, G. & Brusaferro, S. Case-fatality rate and characteristics of patients dying in relation to COVID-19 in Italy. JAMA 323, 1775–1776. https://doi.org/10.1001/jama.2020.4683 (2020).
Wu, J. T. et al. Estimating clinical severity of COVID-19 from the transmission dynamics in Wuhan, China. Nat. Med. 26, 506–510. https://doi.org/10.1038/s41591-020-0822-7 (2020).
Lee, P. I., Hu, Y. L., Chen, P. Y., Huang, Y. C. & Hsueh, P. R. Are children less susceptible to COVID-19?. J. Microbiol. Immunol. Infect. 53, 371–372. https://doi.org/10.1016/j.jmii.2020.02.011 (2020).
Zhang, J. et al. Clinical characteristics of 140 patients infected with SARS-CoV-2 in Wuhan, China. Allergy 75, 1730–1741. https://doi.org/10.1111/all.14238 (2020).
Gudbjartsson, D. F. et al. Spread of SARS-CoV-2 in the icelandic population. N. Engl. J. Med. 382, 2302–2315. https://doi.org/10.1056/NEJMoa2006100 (2020).
Bonanad, C. et al. The effect of age on mortality in patients with Covid-19: A meta-analysis with 611,583 subjects. J. Am. Med. Dir. Assoc. 21, 915–918. https://doi.org/10.1016/j.jamda.2020.05.045 (2020).
Guan, W. J. et al. China medical treatment expert group for Covid-19. Clinical characteristics of coronavirus disease 2019 in China. N. Engl. J. Med. 382, 1708–1720. https://doi.org/10.1056/NEJMoa2002032 (2020).
Liu, K., Chen, Y., Lin, R. & Han, K. Clinical features of COVID-19 in elderly patients: A comparison with young and middle-aged patients. J. Infect. 80, e14–e18. https://doi.org/10.1016/j.jinf.2020.03.005 (2020).
Shi, Y. et al. Host susceptibility to severe COVID-19 and establishment of a host risk score: Findings of 487 cases outside Wuhan. Crit. Care 24, 108. https://doi.org/10.1186/s13054-020-2833-7 (2020).
Verity, R. et al. Estimates of the severity of coronavirus disease 2019: A model-based analysis. Lancet Infect. Dis. 20, 667–669. https://doi.org/10.1016/S1473-3099(20)30243-7 (2020).
Zhou, F. et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet 395, 1054–1062. https://doi.org/10.1016/S0140-6736(20)30566-3 (2020).
Baas, T. et al. Genomic analysis reveals age-dependent innate immune responses to severe acute respiratory syndrome coronavirus. J. Virol. 82, 9465–9476. https://doi.org/10.1128/JVI.00489-08 (2008).
Chen, J. et al. Cellular immune responses to severe acute respiratory syndrome coronavirus (SARS-CoV) infection in senescent BALB/c mice: CD4+ T cells are important in control of SARS-CoV infection. J. Virol. 84, 1289–1301. https://doi.org/10.1128/JVI.01281-09 (2010).
Roberts, A. et al. Aged BALB/c mice as a model for increased severity of severe acute respiratory syndrome in elderly humans. J. Virol. 79, 5833–5838. https://doi.org/10.1128/JVI.79.9.5833-5838.2005 (2005).
Arabi, Y. M. et al. Feasibility of using convalescent plasma immunotherapy for MERS-CoV infection, Saudi Arabia. Emerg. Infect. Dis. 22, 1554–1561. https://doi.org/10.3201/eid2209 (2016).
Drosten, C. et al. Transmission of MERS-coronavirus in household contacts. N. Engl. J. Med. 371, 828–835. https://doi.org/10.1056/NEJMoa1405858 (2014).
Tay, M. Z., Poh, C. M., Rénia, L., MacAry, P. A. & Ng, L. F. P. The trinity of COVID-19: Immunity, inflammation and intervention. Nat. Rev. Immunol. 20, 363–374. https://doi.org/10.1038/s41577-020-0311-8 (2020).
Tetro, J. A. Is COVID-19 receiving ADE from other coronaviruses?. Microbes Infect. 22, 72–73. https://doi.org/10.1016/j.micinf.2020.02.006 (2020).
Wan, Y. et al. Molecular mechanism for antibody-dependent enhancement of coronavirus entry. J. Virol. 94, e02015-e2019. https://doi.org/10.1128/JVI.02015-19 (2020).
Yang, Z. et al. Evasion of antibody neutralization in emerging severe acute respiratory syndrome coronaviruses. Proc. Natl. Acad. Sci. 102, 797–801. https://doi.org/10.1073/pnas.0409065102 (2005).
Ministry of Health, Labour and Welfare, Japan. Documents for press release.https://www.mhlw.go.jp/content/10906000/000628510.pdf. (2020).
EpiCentro, Istituto Superiore di Sanità. Infographic available in English - 13 may 2020 update in COVID-19 integrated surveillance: key national data. https://www.epicentro.iss.it/en/coronavirus/sars-cov-2-integrated-surveillance-data. (2020).
Centro de Coordinación de Alertas y Emergencias Sanitarias. Actualización nº 103. Enfermedad por el coronavirus (COVID-19). 12.05.2020. https://www.mscbs.gob.es/profesionales/saludPublica/ccayes/alertasActual/nCov-China/documentos/Actualizacion_103_COVID-19.pdf. (2020).
United Nations. Annual Population Indicators in Standard Projections. https://population.un.org/wpp/Download/Standard/Interpolated/ (2020).
Zhuang, Z. et al. Preliminary estimates of the reproduction number of the coronavirus disease (COVID-19) outbreak in Republic of Korea and Italy by 5 March 2020. Int. J. Infect. Dis. 95, 308–310. https://doi.org/10.1016/j.ijid.2020.04.044 (2020).
D’Arienzo, M. & Coniglio, A. Assessment of the SARS-CoV-2 basic reproduction number, R0, based on the early phase of COVID-19 outbreak in Italy. Biosaf. Health https://doi.org/10.1016/j.bsheal.2020.03.004 (2020).
Expert Meeting on the Novel Coronavirus Disease Control. The minutes of the fifth meeting. 2020 March 2.https://www.kantei.go.jp/jp/singi/novel_coronavirus/senmonkakaigi/sidai_r020302.pdf. (2020).
Caicedo-Ochoa, Y., Rebellón-Sánchez, D. E., Peñaloza-Rallón, M., Cortés-Motta, H. F. & Méndez-Fandiño, Y. R. Effective reproductive number estimation for initial stage of COVID-19 pandemic in Latin American Countries. Int. J. Infect. Dis. 95, 316–318. https://doi.org/10.1016/j.ijid.2020.04.069 (2020).
Gostic, K., Gomez, A. C. R., Kucharski, A. J. & Lloyd-Smith, J. O. Effectiveness of traveller screening for emerging pathogens is shaped by epidemiology and natural history of infection. Elife 4, e05564. https://doi.org/10.7554/eLife.05564 (2020).
Gostic, K., Gomez, A. C. R., Mummah, R. O., Kucharski, A. J. & Lloyd-Smith, J. O. Estimated effectiveness of symptom and risk screening to prevent the spread of COVID-19. Elife 9, e55570. https://doi.org/10.7554/eLife.55570 (2020).
Omori, R., Mizumoto, K. & Chowell, G. Changes in testing rates could mask the novel coronavirus disease (COVID-19) growth rate. Int. J. Infect. Dis. 94, 116–118. https://doi.org/10.1016/j.ijid.2020.04.021 (2020).
Tsang, T. K. et al. Effect of changing case definitions for COVID-19 on the epidemic curve and transmission parameters in mainland China: A modelling study. Lancet Public Health 5, e289–e296. https://doi.org/10.1016/S2468-2667(20)30089-X (2020).
Bar-on, Y. M., Flamholz, A., Phillips, R. & Milo, R. SARSCoV-2 (COVID-19) by the numbers. Elife 9, e57309. https://doi.org/10.7554/eLife.57309 (2020).
Linton, N. M. et al. Incubation period and other epidemiological characteristics of 2019 novel coronavirus infections with right truncation: A statistical analysis of publicly available case data. J. Clin. Med. 9, 538. https://doi.org/10.3390/jcm9020538 (2020).
Shim, E., Tariq, A., Choi, W., Lee, Y. & Chowell, G. Transmission potential and severity of COVID-19 in South Korea. Int. J. Infect. Dis. 93, 339–344. https://doi.org/10.1016/j.ijid.2020.03.031 (2020).
Sun, K., Chen, J. & Viboud, C. Early epidemiological analysis of the coronavirus disease 2019 outbreak based on crowdsourced data: a population-level observational study. Lancet Digital Health 2, e201–e208. https://doi.org/10.1016/S2589-7500(20)30026-1 (2020).
Ghani, A. C. et al. Methods for estimating the case fatality ratio for a novel, emerging infectious disease. Am. J. Epidemiol. 162, 479–486. https://doi.org/10.1093/aje/kwi230 (2005).
Garske, T. et al. Assessing the severity of the novel influenza A/H1N1 pandemic. BMJ 339, b2840. https://doi.org/10.1136/bmj.b2840 (2008).
Woodall, H. & Adams, B. Partial cross-enhancement in models for dengue epidemiology. J. Theor. Biol. 351, 67–73. https://doi.org/10.1016/j.jtbi.2014.02.016 (2014).
Recker, M. et al. Immunological serotype interactions and their effect on the epidemiological pattern of dengue. Proc. Biol. Sci. 276, 2541–2548. https://doi.org/10.1098/rspb.2009.0331 (2009).
Tang, B., Huo, X., Xiao, Y., Ruan, S. & Wu, J. A conceptual model for optimizing vaccine coverage to reduce vector-borne infections in the presence of antibody-dependent enhancement. Theor. Biol. Med. Model 15, 13. https://doi.org/10.1186/s12976-018-0085-x (2018).
Ferguson, N., Anderson, R. & Gupta, S. The effect of antibody-dependent enhancement on the transmission dynamics and persistence of multiple-strain pathogens. Proc. Natl. Acad. Sci. 96, 790–794. https://doi.org/10.1073/pnas.96.2.790 (1999).
Ferguson, N. & Andreasen, V. The influence of different forms of cross-protective immunity on the population dynamics of antigenically diverse populations. In Mathematical Approaches for Emerging and Reemerging Infectious Diseases: Models, Methods and Theory. The IMA Volumes Mathematics and its Applications (eds Castillo-Chavez, C. et al.) 157–169 (Springer, Berlin, 2002).
Kawaguchi, I., Sasaki, A. & Boots, M. Why are dengue virus serotypes so distantly related? Enhancement and limiting serotype similarity between dengue virus strains. Proc. Biol. Sci. 270, 2241–2247. https://doi.org/10.1098/rspb.2003.2440 (2003).
Awad, S. F. & Abu-Raddad, L. J. Could there have been substantial declines in sexual risk behavior across sub-Saharan Africa in the mid-1990s?. Epidmics 8, 9–17. https://doi.org/10.1016/j.epidem.2014.06.001 (2014).
Prem, K. et al. The effect of control strategies to reduce social mixing on outcomes of the COVID-19 epidemic in Wuhan, China: A modelling study. Lancet Public Health 5, e261–e270. https://doi.org/10.1016/S2468-2667(20)30073-6 (2020).
Sakurai, A. et al. Natural history of asymptomatic SARS-CoV-2 infection. N. Engl. J. Med. https://doi.org/10.1056/NEJMc2013020 (2020).
Backer, J. A., Klinkenberg, D. & Wallinga, J. Incubation period of 2019 novel coronavirus (2019-nCoV) infections among travellers from Wuhan, China. Eur. Surv. 25, 2000062. https://doi.org/10.2807/1560-7917.ES.2020.25.5.2000062 (2020).
Woelfel, R. et al. Clinical presentation and virological assessment of hospitalized cases of coronavirus disease 2019 in a travel-associated transmission cluster. medRxiv https://doi.org/10.1101/2020.03.05.20030502 (2020).
Prem, K., Cook, A. R. & Jit, M. Projecting social contact matrices in 152 countries using contact surveys and demographic data. PLoS Comput. Biol. 13, e1005697. https://doi.org/10.1371/journal.pcbi.1005697 (2017).
Diekmann, O. & Heesterbeek, J. A. P. Mathematical epidemiology of infectious diseases: Model building, analysis and interpretation (Wiley, New York, 2000).
Mossong, J. et al. Social contacts and mixing patterns relevant to the spread of infectious diseases. PLoS Med. 5, e74. https://doi.org/10.1371/journal.pmed.0050074 (2008).
Acknowledgements
The authors gratefully acknowledge the manuscript language review of Dr. Heidi L. Gurung. R.O. acknowledges support from the Japan Society for the Promotion of Science (JSPS) (website: https://www.jsps.go.jp/english/index.html) Grant-in-Aid for Young Scientists 19K20393. Y.N. was supported by JSPS Grant-in-Aid for Young Scientists (B) 16K20976. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
R.O. conceived the study and developed the mathematical model. R.O., R.M. and Y.N. performed the analyses. R.M. collected data. R.O., R.M. and Y.N. contributed to the interpretation of the results and drafting of the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Omori, R., Matsuyama, R. & Nakata, Y. The age distribution of mortality from novel coronavirus disease (COVID-19) suggests no large difference of susceptibility by age. Sci Rep 10, 16642 (2020). https://doi.org/10.1038/s41598-020-73777-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73777-8
This article is cited by
-
Regional opening strategies with commuter testing and containment of new SARS-CoV-2 variants in Germany
BMC Infectious Diseases (2022)
-
COVID-19 pandemic vaccination strategies of early 2021 based on behavioral differences between residents of Tokyo and Osaka, Japan
Archives of Public Health (2022)
-
Post-lockdown changes of age-specific susceptibility and its correlation with adherence to social distancing measures
Scientific Reports (2022)
-
Measuring concerns about the COVID-19 vaccine among Japanese internet users through search queries
Scientific Reports (2022)
-
Learning accurate personalized survival models for predicting hospital discharge and mortality of COVID-19 patients
Scientific Reports (2022)
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.