Abstract
Pancreatic ductal adenocarcinoma (PDA) is characterized by an immune-suppressive tumor microenvironment that renders it largely refractory to immunotherapy. We implemented a multimodal analysis approach to elucidate the immune landscape in PDA. Using a combination of CyTOF, single-cell RNA sequencing and multiplex immunohistochemistry on patient tumors, matched blood and non-malignant samples, we uncovered a complex network of immune-suppressive cellular interactions. These experiments revealed heterogeneous expression of immune checkpoint receptors in individual patients’ T cells and increased markers of CD8+ T cell dysfunction in the advanced disease stage. Tumor-infiltrating CD8+ T cells had an increased proportion of cells expressing an exhausted expression profile that included upregulation of the immune checkpoint TIGIT, a finding that we validated at the protein level. Our findings point to a profound alteration of the immune landscape of tumors, and to patient-specific immune changes that should be taken into account as combination immunotherapy becomes available for pancreatic cancer.
This is a preview of subscription content, access via your institution
Access options
Access Nature and 54 other Nature Portfolio journals
Get Nature+, our best-value online-access subscription
$29.99 / 30 days
cancel any time
Subscribe to this journal
Receive 12 digital issues and online access to articles
$119.00 per year
only $9.92 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
Data availability
All raw data are publicly available without restrictions. All mass cytometry data used for this publication have been deposited in the FlowRepository. All FCS files of tissue (tumor and adjacent/normal) have been uploaded to FlowRepository Experiment FR-FCM-Z2S4 and PBMC files have been uploaded to FlowRepository Experiment FR-FCM-Z2S3 (https://flowrepository.org/id/FR-FCM-Z2S4, https://flowrepository.org/id/FR-FCM-Z2S3). The corresponding file key for the FCS files is in Supplementary Table 6 and refers to Figs. 1 and 7 and Extended Data Figs. 6 and 7. scRNA-seq data with clinical metadata are available from the NIH database of Genotypes and Phenotypes under the accession number phs002071.v1.p1. Deidentified scRNA-seq data are available from the NIH Gene Expression Omnibus database under the accession number GSE155698. All other data supporting the findings of this study are available from the corresponding author upon reasonable request. Source data are provided with this paper.
Code availability
Code is publicly available from GitHub (https://github.com/PascaDiMagliano-Lab/MultimodalMappingPDA-scRNASeq).
References
Royal, R. E. et al. Phase 2 trial of single agent ipilimumab (anti-CTLA-4) for locally advanced or metastatic pancreatic adenocarcinoma. J. Immunother. 33, 828–833 (2010).
Brahmer, J. R. et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N. Engl. J. Med. 366, 2455–2465 (2012).
Stromnes, I. M. et al. T-cell localization, activation, and clonal expansion in human pancreatic ductal adenocarcinoma. Cancer Immunol. Res. 5, 978–991 (2017).
Balli, D. et al. Immune cytolytic activity stratifies molecular subsets of human pancreatic cancer. Clin. Cancer Res. 23, 3129–3138 (2017).
Carstens, J. L. et al. Spatial computation of intratumoral T cells correlates with survival of patients with pancreatic cancer. Nat. Commun. 8, 15095 (2017).
Balachandran, V. P. et al. Identification of unique neoantigen qualities in long-term survivors of pancreatic cancer. Nature 551, 512–516 (2017).
Tsujikawa, T. et al. Quantitative multiplex immunohistochemistry reveals myeloid-inflamed tumor-immune complexity associated with poor prognosis. Cell Rep. 19, 203–217 (2017).
Vonderheide, R. H. The immune revolution: a case for priming, not checkpoint. Cancer Cell 33, 563–569 (2018).
Clark, C. E. et al. Dynamics of the immune reaction to pancreatic cancer from inception to invasion. Cancer Res. 67, 9518–9527 (2007).
Moncada, R, et al. Integrating microarray-based spatial transcriptomics and single-cell RNA-seq reveals tissue architecture in pancreatic ductal adenocarcinomas. Nat Biotechnol. 38, 333–342 (2020).
Chan-Seng-Yue, M. et al. Transcription phenotypes of pancreatic cancer are driven by genomic events during tumor evolution. Nat. Genet. 52, 231–240 (2020).
Elyada, E. et al. Cross-species single-cell analysis of pancreatic ductal adenocarcinoma reveals antigen-presenting cancer-associated fibroblasts. Cancer Discov. 9, 1102–1123 (2019).
Dominguez, C. X. et al. Single-cell RNA sequencing reveals stromal evolution into LRRC15+ myofibroblasts as a determinant of patient response to cancer immunotherapy. Cancer Discov. 10, 232–253 (2020).
Manieri, N. A., Chiang, E. Y. & Grogan, J. L. TIGIT: a key inhibitor of the cancer immunity cycle. Trends Immunol. 38, 20–28 (2017).
Cohen, M. et al. Lung single-cell signaling interaction map reveals basophil role in macrophage imprinting. Cell 175, 1031–1044.e18 (2018).
Zhang, Q. et al. Landscape and dynamics of single immune cells in hepatocellular carcinoma. Cell 179, 829–845.e20 (2019).
Bendall, S. C. et al. Single-cell mass cytometry of differential immune and drug responses across a human hematopoietic continuum. Science 332, 687–696 (2011).
Nowicka, M. et al. CyTOF workflow: differential discovery in high-throughput high-dimensional cytometry datasets. F1000Res 6, 748 (2017).
Amir, E. D. et al. Development of a comprehensive antibody staining database using a standardized analytics pipeline. Front. Immunol. 10, 1315 (2019).
Zhang, Y. et al. Myeloid cells are required for PD-1/PD-L1 checkpoint activation and the establishment of an immunosuppressive environment in pancreatic cancer. Gut 66, 124–136 (2017).
Panni, R. Z. et al. Agonism of CD11b reprograms innate immunity to sensitize pancreatic cancer to immunotherapies. Sci. Transl. Med. 11, eaau9240 (2019).
Stromnes, I. M. et al. Targeted depletion of an MDSC subset unmasks pancreatic ductal adenocarcinoma to adaptive immunity. Gut 63, 1769–1781 (2014).
Lazarus, J. et al. Spatial and phenotypic immune profiling of metastatic colon cancer. JCI Insight 3, e121932 (2018).
Beatty, G. L. et al. Exclusion of T cells from pancreatic carcinomas in mice is regulated by Ly6ClowF4/80+ extratumoral macrophages. Gastroenterology 149, 201–210 (2015).
Sanford, D. E. et al. Inflammatory monocyte mobilization decreases patient survival in pancreatic cancer: a role for targeting the CCL2/CCR2 axis. Clin. Cancer Res. 19, 3404–3415 (2013).
DeNardo, D. G. & Ruffell, B. Macrophages as regulators of tumour immunity and immunotherapy. Nat. Rev. Immunol. 19, 369–382 (2019).
Nywening, T. M. et al. Targeting both tumour-associated CXCR2+ neutrophils and CCR2+ macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut 67, 1112–1123 (2018).
Zhu, Y. et al. Tissue-resident macrophages in pancreatic ductal adenocarcinoma originate from embryonic hematopoiesis and promote tumor progression. Immunity 47, 323–338.e6 (2017).
Zhu, Y. et al. CSF1/CSF1R blockade reprograms tumor-infiltrating macrophages and improves response to T-cell checkpoint immunotherapy in pancreatic cancer models. Cancer Res. 74, 5057–5069 (2014).
Rosenberg, S. A. Decade in review-cancer immunotherapy: entering the mainstream of cancer treatment. Nat. Rev. Clin. Oncol. 11, 630–632 (2014).
Carlson, C. M. et al. Kruppel-like factor 2 regulates thymocyte and T-cell migration. Nature 442, 299–302 (2006).
Wherry, E. J. et al. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 27, 670–684 (2007).
Li, J. et al. High levels of eomes promote exhaustion of anti-tumor CD8+ T cells. Front. Immunol. 9, 2981 (2018).
Jansen, C. S. et al. An intra-tumoral niche maintains and differentiates stem-like CD8 T cells. Nature 576, 465–470 (2019).
Kondo, T., Takata, H. & Takiguchi, M. Functional expression of chemokine receptor CCR6 on human effector memory CD8+ T cells. Eur. J. Immunol. 37, 54–65 (2007).
Ducimetiere, L., Vermeer, M. & Tugues, S. The interplay between innate lymphoid cells and the tumor microenvironment. Front. Immunol. 10, 2895 (2019).
Arlettaz, L. et al. Expression of inhibitory KIR is confined to CD8+ effector T cells and limits their proliferative capacity. Eur. J. Immunol. 34, 3413–3422 (2004).
McMahon, C. W. et al. Viral and bacterial infections induce expression of multiple NK cell receptors in responding CD8+ T cells. J. Immunol. 169, 1444–1452 (2002).
Smith, S. L. et al. Diversity of peripheral blood human NK cells identified by single-cell RNA sequencing. Blood Adv. 4, 1388–1406 (2020).
Zhang, Y. et al. CD4+ T lymphocyte ablation prevents pancreatic carcinogenesis in mice. Cancer Immunol. Res. 2, 423–435 (2014).
Zhang, Y. et al. Regulatory T-cell depletion alters the tumor microenvironment and accelerates pancreatic carcinogenesis. Cancer Discov. 10, 422–439 (2020).
Jang, J. E. et al. Crosstalk between regulatory T cells and tumor-associated dendritic cells negates anti-tumor immunity in pancreatic cancer. Cell Rep. 20, 558–571 (2017).
Sckisel, G. D. et al. Differential phenotypes of memory CD4 and CD8 T cells in the spleen and peripheral tissues following immunostimulatory therapy. J. Immunother. Cancer 5, 33 (2017).
Bengsch, F. et al. CTLA-4/CD80 pathway regulates T cell infiltration into pancreatic cancer. Cancer Immunol. Immunother. 66, 1609–1617 (2017).
Hegde, S. et al. Dendritic cell paucity leads to dysfunctional immune surveillance in pancreatic cancer. Cancer Cell 37, 289–307.e9 (2020).
Huber, S. et al. IL-22BP is regulated by the inflammasome and modulates tumorigenesis in the intestine. Nature 491, 259–263 (2012).
Lanfranca, M. P. et al. Interleukin 22 signaling regulates acinar cell plasticity to promote pancreatic tumor development in mice. Gastroenterology 158, 1417–1432.e11 (2019).
Collin, M. & Bigley, V. Human dendritic cell subsets: an update. Immunology 154, 3–20 (2018).
Veglia, F. & Gabrilovich, D. I. Dendritic cells in cancer: the role revisited. Curr. Opin. Immunol. 45, 43–51 (2017).
Ramilowski, J. A. et al. A draft network of ligand–receptor-mediated multicellular signalling in human. Nat. Commun. 6, 7866 (2015).
Das, S. et al. Tumor cell-derived IL-1β promotes desmoplasia and immune suppression in pancreatic cancer. Cancer Res. 80, 1088–1101 (2020).
Ohlund, D. et al. Distinct populations of inflammatory fibroblasts and myofibroblasts in pancreatic cancer. J. Exp. Med. 214, 579–596 (2017).
Fourcade, J. et al. CD226 opposes TIGIT to disrupt Tregs in melanoma. JCI Insight 3, e121157 (2018).
Yu, X. et al. The surface protein TIGIT suppresses T cell activation by promoting the generation of mature immunoregulatory dendritic cells. Nat. Immunol. 10, 48–57 (2009).
Maj, T. et al. Oxidative stress controls regulatory T cell apoptosis and suppressor activity and PD-L1-blockade resistance in tumor. Nat. Immunol. 18, 1332–1341 (2017).
Ligorio, M. et al. Stromal microenvironment shapes the intratumoral architecture of pancreatic cancer. Cell 178, 160–175.e27 (2019).
Stuart, T. & Satija, R. Integrative single-cell analysis. Nat. Rev. Genet. 20, 257–272 (2019).
Xu, J. et al. Peripheral blood T-cell fitness is diminished in patients with pancreatic carcinoma but can be improved with homeostatic cytokines. Cell Mol. Gastroenterol. Hepatol. 8, 656–658.e6 (2019).
Zhang, Q. et al. Blockade of the checkpoint receptor TIGIT prevents NK cell exhaustion and elicits potent anti-tumor immunity. Nat. Immunol. 19, 723–732 (2018).
Solomon, B. L. & Garrido-Laguna, I. TIGIT: a novel immunotherapy target moving from bench to bedside. Cancer Immunol. Immunother. 67, 1659–1667 (2018).
Van Gassen, S. et al. FlowSOM: using self-organizing maps for visualization and interpretation of cytometry data. Cytometry A 87a, 636–645 (2015).
McCarthy, D. J., Chen, Y. & Smyth, G. K. Differential expression analysis of multifactor RNA-Seq experiments with respect to biological variation. Nucleic Acids Res. 40, 4288–4297 (2012).
Robinson, M. D., McCarthy, D. J. & Smyth, G. K. edgeR: a Bioconductor package for differential expression analysis of digital gene expression data. Bioinformatics 26, 139–140 (2010).
RStudio Team RStudio: Integrated Development for R (RStudio, 2015); http://www.rstudio.com/
R Core Development Team R: A Language and Environment for Statistical Computing (R Foundation for Statistical Computing, 2017); https://www.R-project.org/
Krzywinski, M. et al. Circos: an information aesthetic for comparative genomics. Genome Res. 19, 1639–1645 (2009).
Acknowledgements
We thank M. Cochran and T. Wightman at the Flow Cytometry Core at the University of Rochester Medical Center and A. M. Gunawan at the Indiana University Simon Cancer Center Flow Cytometry for support with cell CyTOF acquisition. We thank V. Motta and K. Brown from Fluidigm for assistance with panel design. We thank P. Schnepp and A. Ahmed for assistance with CyTOF experimental design. We thank T. Tamsen and J. Opp from the University of Michigan Advanced Genomics Core. We thank D. Hill and M. Czerwinski for input on designing single-cell analysis pipelines. We thank A. Gilado and I. Amit for expertise in building our pancreatic interactome network. We thank the Tissue Procurement Center at the University of Michigan. We thank E. Stack (formerly with PerkinElmer) for assistance with initial R introduction and basic training using inForm 2.3.0 and earlier versions and staining strategies. We thank P. Turncliff for excellent graphics. We thank J. Spence for the VE-cadherin antibody gift. This project was supported by NIH/NCI grants R01CA151588, R01CA198074 and the American Cancer Society (to M.P.d.M.). This work was also supported by a University of Michigan Cancer Center Support Grant (P30CA046592), including an administrative supplement and by NIH/NCI grant U01CA224145 H.C.C. and M.P.d.M. F.B. was funded by the Association for Academic Surgery Joel J. Roslyn Award. T.L.F. was funded by K08CA201581. S.B.K. was supported by T32-GM113900. N.G.S., V.R.S. and K.D. were supported by T32-CA009676. E.S.C. is supported by the American College of Gastroenterology Clinical Research Award and by T32-DK094775. N.G.S. is a recipient of the American Cancer Society Postdoctoral Award PF-19-096-01 and the Michigan Institute for Clinical and Health Research (MICHR) Postdoctoral Translational Scholars Program fellowship award. A.R. and S.T. were supported by institutional startup funds from the University of Michigan, a gift from Agilent Technologies, NCI grant R37CA214955 and a Research Scholar Grant from the American Cancer Society (RSG-16-005−01). The funders had no role in study design, data collection and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
M.P.d.M. directed the study. T.L.F., F.B. and H.C.C. assisted with study design and analysis. M.P.d.M., N.G.S., E.S.C. and S.B.K. developed the study concept and were responsible for the study design. J.L., L.D., V.I.-N. and S.B. stained and analyzed the mfIHC. T.L.F. coordinated the mfIHC. V.G., E.S.C., E.-J.W., R.K., A. Schulman., A. Sondhi, A.P., R.L., J.Y., A.P., H.N., C.C., M.A.A. and H.S. procured human tissue and assisted with clinical data collection. V.G. and E.S.C. maintained the database of human biospecimens. N.G.S., E.S.C., S.B.K., D.P., J.M., C.E. and F.L. processed the samples for CyTOF. E.S.C., V.R.S., J.M., C.E., D.P. and L.H. processed samples for scRNA-seq. F.B. developed an R pipeline for CyTOF analysis. E.D.A. analyzed the CyTOF PBMC data. A.R., S.T. and A.K.Y.C. developed the bioinformatics analysis pipelines and carried out the interactome analysis. V.R.S., N.G.S., S.B.K. and K.D. analyzed the scRNA-seq data. N.G.S., S.B.K. and E.S.C. analyzed the CyTOF data. V.S., C.A.L., B.L.A., A.R., H.S., W.Z., H.C.C., F.B., T.L.F., C.C., K.D. and E.D.A. provided study guidance and feedback on the manuscript. N.G.S., E.S.C., S.B.K. and M.P.d.M. wrote the manuscript, which was then edited by all of the co-authors. All of the co-authors approved the final version of the manuscript before submission.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Extended data
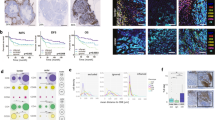
Extended Data Fig. 1 CyTOF and multiplex fluorescent immunohistochemistry (mfIHC) mapping can be readily performed on patient tumor samples and show a heterogeneous immune infiltration in human pancreatic cancer.
a, Patient breakdown and tumor characteristics of CyTOF performed on 8 adj/norm pancreas and 10 PDA tumor samples (surgical (7) vs. fine needle biopsy (FNB) (3)). b, Representative H&E stains of samples DS20191258 (Adj/Norm), DS20191299 (PDA tumor from surgical resection), and DS20191324 (PDA tumor from fine needle biopsy). c, The ConsensusClusterPlus and FlowSOM R packages were used to define the initial 22 clusters identified in the tissue CyTOF samples. d, Final heatmap demonstrating marker expression used to define cell populations. e, Manual gating of CD3-CD8A-CD45+CD56+ NK cells in adjacent/normal and PDA tissue samples, n=5 adjacent/normal tissue samples and n=4 PDA tissue samples. Two-sided Student’s t-test was performed to compare between groups and asterisk indicates a p value of less than 0.05 was considered significant. For manual gating of NK cells n=5 for adj/norm and n=4 for PDA patient samples. f, mfIHC composite image of PDA (left). Phenotype map with the following basic phenotypes at their x and y coordinates: T cell (green), epithelial cells (pink), APCs (orange), other cells (grey) (right). 71 individual PDA and 34 individual chronic pancreatitis subjects were examined in this analysis. g, Relative cellular composition by quantitation of mfIHC of representative surgical PDA tissue of additional patients DS20181166 (PDA tumor from distal pancreatectomy), DS20181141 (PDA tumor from distal pancreatectomy). h, Corresponding mfIHC images of DS20181166, and DS20181141.
Extended Data Fig. 2 Single Cell RNA Sequencing of PDA tissue reveals heterogeneous cellular composition and expression of immune checkpoints.
a, Single cell RNA sequencing tissue sample breakdown (n = 3 Adj/Norm tissue, n=10 PDA tissue from fine needle biopsy, and n=6 PDA tissue from surgical resection), patient clinical data, and tumor characteristics (grade and stage) (Left panel). Breakdown of sequenced PBMC samples with corresponding patient clinical data (Right panel). b, UMAP of the merged tissue colored by Patient ID prior to batch correction (Left panel) and post batch correction (Right panel). c, UMAP of 3 individual adjacent/normal samples and d, 16 PDA tissues. We distinguished two epithelial populations: tumor cells and acinar cells. In the non-epithelial compartment, we identified fibroblasts, pericytes, CD8+ T cells, CD4+ T cells, Tregs, NK cells, B cells, plasma cells, mast cells, macrophages, granulocytes, dendritic cells, endothelial cells, and a small endocrine population.
Extended Data Fig. 3 Single Cell RNA Sequencing of PDA PBMCs reveals heterogeneous cellular composition and expression of immune checkpoints.
a, Merged UMAP plots of PBMCs from 4 healthy donors and 16 PDA patients (total of 70,113 cells). CD8 T cells (green), CD4 T cells (light green), NK cells (purple), pDCs (blue), Granulocyte (light orange), Monocyte (orange), B cells (yellow), Plasma cells (light yellow). b, Dot plot analysis of key markers to define the 8 identified cell populations. Color of dot represents average expression, while the size of the dot represents percent expression. Dot plot represents merged healthy (n=4) and PDA (n=16) patient gene expression of PBMC lineage markers. c, Average expression of immune checkpoint ligands and receptors in the identified cell populations in merged blood samples. d, Average expression of immune checkpoint receptors on CD8+ T cells in merged PBMCs. e, Average expression of differentially expressed genes in CD8+ T cells comparing healthy (black) to PDA (grey) PBMCs. Disease stage is plotted on the left.
Extended Data Fig. 4 Single cell RNA sequencing reveals 3 CD8+ T cell populations: effector, exhausted, and memory CD8+ T Cells.
a, Feature plots of immune checkpoints (PDCD1, LAG3, TIGIT, HAVCR2), activation markers (IFNG, GZMB), and exhaustion markers (GZMK, EOMES) in CD8+ T cells. b, Number of effector (pink), exhausted (green), and memory (blue) CD8+ T cells captured in each individual tissue sample by scRNA seq. c, Average scaled expression heatmap of highly enriched genes by potential effector, exhausted, and memory cell populations. (n = 3 Adj/Norm tissue and n= 16 PDA tissue for panels a–c).
Extended Data Fig. 5 Single cell RNA sequencing of myeloid subsets in human pancreatic cancer.
a, Violin Plots illustrating comparison of immune checkpoint ligands in myeloid clusters in PDA vs. adjacent normal/pancreas samples. b, Average expression heatmap of checkpoint ligands in merged macrophages (all cells expressing CD68 within the myeloid population) and c, merged granulocytes (all cells expressing FCGR3B within the myeloid population). Left panels denote disease state (adjacent/normal vs. PDA tissue) and stage. d, Map of all putative ligand receptor differential interactions that are upregulated in 16 PDA compared to 3 adjacent/normal pancreas. The line color denotes cellular source of the ligand, and putative interactions were visualized in Cytoscape® V3.7.1. e, Dot plot analysis showing expression of adenosine receptors in PDA tumor cell types. Red indicates high expression, blue low expression, and the size of the dot is relative to the percent that marker is expressed. Dot plot represent n=16 PDA patient gene expression of adenosine receptors.
Extended Data Fig. 6 CyTOF analysis of PBMCs from healthy, chronic pancreatitis, and PDA patients.
a, Patient breakdown and characteristics of CyTOF performed on patient blood samples (n = 16 healthy patients, n = 10 chronic pancreatitis patients, and n=36 PDA patients). b, t-SNE analysis of CyTOF of all merged PBMC samples with granulocytes (CD66b+). c, t-SNE analysis of CyTOF of all merged PBMC samples without granulocytes. Key marker t-SNE feature plots of CD3 (total T cells), CD4 (Helper CD4+ T cells), CD8 (Cytotoxic T cells), CD19 (B cells), CD11b (Myeloid cells), CCR2, PDL-1, and CD68 (Macrophage marker). d, Bar plots of relative cell type abundance (B cell, CD4+/CD8+ T cell, CD4+ T cell, CD4-/CD8- T cell, Dendritic cell, CD14+/CD16+ Monocyte, CD14+/CD16- Monocyte, and CD14- CD16+ Monocyte) from CyTOF of PBMCs of healthy, chronic pancreatitis, and PDA patients. e, Quantification of unbiased analysis (Astrolabe pipeline) of PBMC immune populations in n=16 healthy patients, n=36 PDA patients, and n=10 chronic pancreatitis patients. Two-sided Student’s t-tests were performed to compare between groups and a p value of <0.05 was considered significant. f, Relative CyTOF marker expression in CD8+ T Cells from PDA tumor tissue. g, PCA analysis of PBMCs at different disease states. Healthy (neon blue), PDA (red), and Chronic Pancreatitis (green).
Extended Data Fig. 7 Immunofluorescence of immune checkpoints in pancreatic tumors.
a, Individual channels of immunofluorescent staining of patient tissues with antibodies specific for TIGIT/CD8A, TIGIT/FOXP3, PVR/Pan-cytokeratin, PVR/CD163, PVR/Vimentin, and PVR/VE-cadherin. Three individual patient tumors were examined independently per staining analysis. b, Manual gating of PD-L1+ CD68+ macrophages in normal adjacent (n=8) and PDA (n=10) tissue. Two-sided Student’s t-test was performed, and asterisk indicates a p value of <0.05, which was considered statistically significant. Representative individual CyTOF biaxial density plots from normal adjacent and PDA tissue of a matched patient (19–262) of PD-L1 expression in CD68+ macrophages (as a percentage of total CD11b+ cells).
Supplementary information
Supplementary Information
Supplementary Tables 1–6.
Source data
Source Data Fig. 1
Source numerical data.
Source Data Fig. 3
Source numerical data.
Source Data Fig. 7
Source numerical data.
Source Data Extended Data Fig. 1
Source numerical data.
Source Data Extended Data Fig. 4
Source numerical data.
Source Data Extended Data Fig. 6
Source numerical data.
Source Data Extended Data Fig. 7
Source numerical data.
Rights and permissions
About this article
Cite this article
Steele, N.G., Carpenter, E.S., Kemp, S.B. et al. Multimodal mapping of the tumor and peripheral blood immune landscape in human pancreatic cancer. Nat Cancer 1, 1097–1112 (2020). https://doi.org/10.1038/s43018-020-00121-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/s43018-020-00121-4
This article is cited by
-
Immune characteristics of dedifferentiated retroperitoneal liposarcomas and the reliability of regional samples in evaluating their tumor immune microenvironments
World Journal of Surgical Oncology (2024)
-
Tumor immune microenvironment-based therapies in pancreatic ductal adenocarcinoma: time to update the concept
Journal of Experimental & Clinical Cancer Research (2024)
-
Characterizing the impacts of dataset imbalance on single-cell data integration
Nature Biotechnology (2024)
-
HMGA2 alleviates ferroptosis by promoting GPX4 expression in pancreatic cancer cells
Cell Death & Disease (2024)
-
Plasticity-induced repression of Irf6 underlies acquired resistance to cancer immunotherapy in pancreatic ductal adenocarcinoma
Nature Communications (2024)