Abstract
Apoptosis is a type of physiological cell death that occurs during development, normal tissue homeostasis, or as a result of different cellular insults. The phenotype of an apoptotic cell is relatively consistent in most cases of apoptosis and involves at least changes in the cell membrane, proteolysis of cytoplasmic and nuclear proteins, and eventual destruction of nuclear DNA. Our laboratory is interested in the reversibility of apoptosis. We have initial evidence that DNA repair is activated early in p53-induced apoptosis and may be involved in its reversibility. The present work further strengthens our proposition that p53-induced apoptosis is reversible. We show that p53 activation induces phosphatidylserine (PS) externalization early in apoptosis, and that these early apoptotic cells with externalized PS can be rescued and proliferate if the apoptotic stimulus is removed. In addition, we show that unscheduled DNA synthesis occurs in early apoptotic cells, and that if DNA repair is inhibited by aphidicolin, apoptosis is accelerated. These results confirm that early p53-induced apoptotic cells can be rescued from the apoptotic program, and that DNA repair can modulate that cell death process.
Similar content being viewed by others
Introduction
Apoptosis was initially defined by Kerr et al1 who suggested that cells dying in this process went through defined morphological changes. Originally defined as ‘shrinkage necrosis’,2 these changes involve chromatin condensation, cytoplasmic and nuclear blebbing, and eventual cellular demise without loss of membrane integrity. This type of death was therefore different from classical necrosis, which results in loss of membrane integrity, leakage of cellular components, and an inflammatory response.3 Since the definition of apoptosis as a novel form of cell death, the field has exploded into a major research area.4
As partial understanding of apoptosis and its mechanisms has developed, we are now able to better define early versus late stages of the process. One of the earliest markers of apoptosis involves the externalization of phosphatidylserine (PS) residues from the inner to the outer leaflet of the cell membrane.5 Cells with PS on their external surfaces may be marked for recognition and possible removal by phagocytes and/or neighbor cells.6 Through the use of a technique employing annexin V, which strongly binds PS, apoptotic cells are now routinely recognized by their PS exposure.
Another early marker of an apoptotic cell is the initial cleavage of genomic DNA into large, 50–300 kb fragments.7 This process has been seen in a number of cell types, such as U937 leukemia cells,8 thymocytes,9 and MOLT-4 human T lymphoblastoid cells.10 It is believed that these larger fragments of DNA are due to cleavage of looped domains at attachment points on the nuclear matrix, and can occur independently of the characteristic internucleosomal 200-base-pair fragmentation visible on agarose gels7 (see below).
One molecule capable of apoptosis induction is the tumor suppressor p53.11,12 p53 is activated by a variety of cellular insults, such as damaged DNA,13,14,15,16 nucleotide depletion,17 hypoxia18 and heat shock.19 The mechanisms of p53-induced apoptosis are still uncertain, but the transcriptional activation of various apoptosis-inducing genes is clearly involved. Such genes include fas,20,21 bax,22,23 KILLER/DR5,24 those involved in reactive oxygen species generation and response,25 and others.26 In addition to its roles in apoptosis induction, p53 is also involved in DNA repair by its transcriptional upregulation of the DNA repair gene GADD4527 and by its physical interaction with the repair proteins XPB and XPD.28,29 In addition, studies on p53-null Li-Fraumeni cells show a reduction in DNA repair capabilities.30,31 However, a possible coordination between DNA repair and apoptosis remains to be elucidated.
It is currently believed that apoptosis induction may be an irreversible process. Initial results from our laboratory have shown that DNA repair is activated early in p53-induced apoptosis, and that early stages may indeed be reversible.32 This current work follows up on these initial findings and provides more evidence that early stages of p53-induced apoptosis are reversible. Using a temperature-sensitive p53 cell line (p53ts), we show that p53 induces PS-flipping in these cells. In addition, PS-positive cells can survive if the apoptotic stimulus is removed. We further strengthen our hypothesis of the importance of DNA repair in this reversibility through unscheduled DNA synthesis assays. Finally, we demonstrate that by inhibiting repair with aphidicolin, p53-induced cells die faster and show earlier signs of apoptosis than non-treated p53-induced cells. Overall, our results show that early stages of p53-induced apoptosis are reversible and that DNA repair is likely involved in this reversibility.
Results
PS is externalized by p53 activation
Initial studies in our laboratory suggest that early stages of p53-induced apoptosis may be reversible, and that DNA repair may play a role in this reversibility.32 To substantiate these results, we have further analyzed p53-induced apoptosis and its reversibility. We have used a temperature-sensitive p53 cell line (p53ts) in which p53 in inactive at 37°C. However, incubation of the p53ts cells at 30°C results in activation of p53, induction of p21, and induction of apoptosis, as measured by gel electrophoresis and TUNEL assay.32 To further our understanding of p53-induced apoptosis in these cells, we wanted next to examine, if possible, earlier stages of apoptosis such as externalization of phosphatidylserine (PS). Using a FITC-labeled Annexin-V antibody (Trevigen, Gaithersburg, MD, USA), which binds tightly to PS that has been externalized to the surface of cells, we analyzed the percentage of apoptotic cells by flow cytometry. As seen in Figure 1a and b, incubation of the p53ts cells at 30°C for 6 h results in a substantial increase in FITC-labeled, annexin-positive apoptotic cells. Quantitation reveals approximately 10% annexin-V positive cells in the p53ts cells at the non-permissive temperature of 37°C. However, after 6 h at the permissive temperature of 30°C, the number of annexin-V positive cells rises to 31% (Figure 1c). Similar analysis in a constitutively mutant p53 construct (p53mut), in which p53 is inactive at both 37°C and 30°C, reveals only 6% annexin-V positive cells at 37°C, with no changes with incubation at 30°C (data not shown). These results show that the p53 activation results in PS flipping and apoptosis within 6 h in the p53ts cells.
P53 induces PS-externalization in p53ts cells. p53ts cells were treated at 37°C (a) or at 30°C (b) for 6 h, then washed and incubated with an FITC-conjugated Annexin-V antibody. Cells were analyzed by flow cytometry. Upper-half of graphs, propidium-iodide-positive; far right, annexin-V-positive. Notice the increase in annexin-V-Positive, PI-negative cells in the 30°C- compared to the 37°C-treated samples (lower right panel). This analysis shows a representative example of at least three experiments. (c) Graph represents the percentage of PS-positive cells at the control temperature of 37°C and at the permissive temperature of 30°C for 6 h. Data represents X±S.E.M. (n=4), P=0.002
PS-positive cells can survive if the apoptotic stimulus is removed
We next analyzed the reversibility of individual, PS-positive apoptotic cells by fluorescent-activated cell sorting (FACS) analysis. Again, p53ts cells were incubated either at 37°C or 30°C for 6 h and incubated with the FITC-labeled annexin-V antibody. Using the MoFlow cell sorter (Cytomation, Inc., Fort Collins, CO, USA), 1500 PS-positive apoptotic cells (PS+, Figure 2b) were removed from the population of cells and sorted into 24-well culture dishes. The dishes were then returned to the nonpermissive temperature (37°C) and allowed to attach and grow over a period of 5 days. In addition, FITC-negative control cells from dishes incubated at 37°C (control, Figure 2a) were sorted to show the number of surviving, non-apoptotic cells that replate and grow during this procedure. As seen in Figure 2c, annexin-V positive, apoptotic cells survive and grow if the apoptotic stimulus (p53) is removed. As PS flipping is a marker of early apoptosis, these results clearly show that early stages of p53-induced apoptosis are reversible.
Early p53-induced PS-positive apoptotic cells are reversible. p53ts cells were treated at 37°C (a) or at 30°C (b) for 6 h, then washed and incubated with an FITC-conjugated Annexin-V antibody and run through a fluorescent activated cell sorter (FACS). Note the shift to the right in the cells treated at 30°C (b), representing FITC-labeled annexin-V positive apoptotic cells. For sorting, 1500 FITC-negative cells (dashed line ‘control’, (a)) and 1500 apoptotic, FITC-positive cells (dashed line ‘PS+’, (b)) were removed from the population and sorted by FACS. Cells were then plated onto 24-well plates and returned to 37°C for 5 days, fixed in methanol, dried, stained with 0.1% toluidine blue, and photographed using a light microscope at 100X. The cells in (c, top) were the 37°C control-treated cells while those in (c, bottom) are those sorted from the 6 h 30°C-treated cells
Reversible apoptotic cells are not resistant to further apoptosis induction
A hypothesis that may explain the reversibility of apoptotic cells is that those selected cells already have undergone genetic alterations which have led to apoptosis resistance. In order to address this concern, p53ts cells were incubated at 30°C and then allowed to grow, followed by a re-exposure at 30°C for a total of four cycles (Figure 3). As seen in Figure 3a, these cells are still capable of undergoing apoptosis as evident by extensive DNA fragmentation. Therefore, reversible apoptotic cells are not selected due to their resistance to apoptosis induction. As further evidence of p53's continued activity after four cycles at 30°C, the levels of the p53-inducible gene p21 were examined. As shown in Figure 3b, the recovered cells are still capable of p21 induction at the permissive temperature. Therefore, reversibility is not due to inactive p53.
Surviving cells after p53 activation are not resistant to further apoptosis induction. (a) Lanes 3–4: p53ts cells were incubated at 30°C for 12 h, then back to 37°C for 72 h. Cells were then subcultured and grown at 37°C for another 72 h, incubated at either 37°C or 30°C for 12 h for a total of four cycles. DNA from recovered cells (R) was extracted and run on a 1.0% agarose gel and compared to p53ts DNA from 37°C controls (C). (b) p53ts cells were incubated for four cycles at 30°C, as in (a), and protein extracts from both recovered (R) and control (C) cells were examined for p21 levels. β-tubulin is shown as a loading control
DNA repair is activated in p53ts cells treated at the permissive temperature
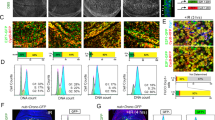
Another early marker of apoptosis is the generation of 50–300 kb fragments in genomic DNA.8,9,10 We hypothesized that PS-positive cells may also have early DNA damage, and that it must be repaired for an early apoptotic cell to survive. Our previous work demonstrated that DNA repair was activated in early stages of p53-induced apoptosis, and now we wanted to follow these results up by analyzing DNA repair in individual cells. To accomplish this, we used a classical unscheduled DNA synthesis assay33 that uses autoradiography to examine 3H-thymidine incorporation into nuclear DNA in the absence of DNA replication. p53ts cells were treated at 37°C or 30°C for 6 h, in the presence or absence of hydroxyurea (HU) or HU and aphidicolin. HU is a DNA replication inhibitor that does not either inhibit DNA repair or induce apoptosis under our experimental conditions, so any 3H-thy incorporation occurring in its presence is the result of DNA repair.34 We also used aphidicolin which is a DNA repair inhibitor.35,36,37 As seen in Figure 4a, without HU or aphidicolin, there is 3H-thy incorporation into nuclei at 37°C. The addition of HU to the cells results in almost complete inhibition of 3H-thy incorporation (Figure 4b), while the addition of HU and aphidicolin together results in 100% inhibition (Figure 4c). In UDS assays done on p53ts cells incubated at 30°C, there is a slight reduction in 3H-thy incorporation when compared to the controls at 37°C (Figure 4d). This is probably due to temperature shift, as both the p53ts and p53mut cell lines have slightly decreased DNA, RNA, and protein synthesis rates, regardless of p53 status.32 In the presence of HU, there is reduced 3H-thy incorporation, but there are cells that contain nuclear grains above background (Figure 4e, arrows). These grains are indicative of DNA repair33 as the addition of HU and aphidicolin results in complete inhibition of 3H-thy incorporation (Figure 4f). Therefore, the fact that repair synthesis is occurring at 30°C but not 37°C suggests that DNA repair is activated in the apoptotic cells.
Unscheduled DNA synthesis (UDS) assays show that hydroxyurea (HU) inhibits 3H-thy incorporation into p53ts cells incubated at 37°C but not at 30°C. (a–c): p53ts cells were incubated at 37°C in the presence or absence of HU (2.5×10−4 M) or HU+aphidicolin (aph, 3 μM) for 6 h. Cells were then washed, fixed in methanol, and dried, then exposed to emulsion for 5 days. The emulsion was then developed, the cells were stained in 0.1% toluidine blue, and photographed using light microscopy at 1000X. (a) 37°C control; (b) 37°C+HU; (c) 37°C+HU+aph. (d–f): p53ts cells were incubated at 30°C in the presence or absence of HU or HU+aph for 6 h, and the UDS assay was performed as described. (d) 30°C control; (e) 30°C+aph; (f) 30°C+HU+aph. Arrows in e point to cells with 3H-thy incorporation
Quantitation of the number of cells with 3H-thy incorporation reveals that HU inhibits the number of cells with incorporation by 99% in the p53ts cells at 37°C (Figure 5), while the combination of HU and aphidicolin at 37°C results in nearly 100% inhibition. In contrast, at 30°C in the p53ts cells, HU inhibits 3H-thy incorporation by only 88%. The increase in incorporation at 30°C compared to 37°C in the presence of HU shows that DNA repair is occurring in early p53-induced apoptotic cells. Again, the combination of HU and aphidicolin completely inhibits incorporation.
Unscheduled DNA synthesis assay. The labeling index was calculated as the number of cells containing at least five grains above background over the nucleus. Data is expressed as X±S.E.M. (n=100). The percentage of inhibition was determined by comparison with the control samples. For groups 1 versus 2, 3 versus 4, 1 versus 3, and 2 versus 4 P<0.001
Aphidicolin accelerates p53-induced apoptosis
If DNA repair modulates p53-induced apoptosis, we postulated that inhibiting DNA repair should accelerate the death process. We used the classic DNA repair inhibitor aphidicolin to examine this hypothesis. Two methods were used to analyze cell death, the first being a colony formation assay to determine actual cell viability. p53ts cells were treated at 37°C and 30°C for 2, 4, 6 and 12 h, in the presence or absence of aphidicolin. As seen in Figure 6, colony formation remains at control levels up to 6 h at 30°C. Interestingly, after 6 h at 30°C in this cell line, 15% of cells are TUNEL-positive,32 strengthening our hypothesis that early p53-induced apoptotic cells can be reversed. Conversely, inhibition of DNA repair by the addition of aphidicolin results in reduced colony formation within 4 h at 30°C. At 37°C in the presence of aphidicolin, colony formation levels are only slightly inhibited (to approximately 75% of controls after 12 h, not shown). Because aphidicolin accelerates death rates in the p53ts cells, this suggests that DNA repair is involved in regulating p53-induced cell death.
Aphidicolin accelerates death induced in the p53ts cells at 30°C. Two thousand p53ts cells were plated at 37°C and allowed to attach overnight. The cells were then incubated at either 37°C (controls) or at 30°C for 2, 4, 6, or 12 h in the presence or absence of aphidicolin (3 μM). Cells were then returned to 37°C for the remainder of 72 h, fixed in methanol, stained with 0.1% toluidine blue, and all colonies of five or more cells were counted. This technique has been previously described.32 Bars represent percentage of colonies formed (X±S.E.M.) compared to the 37°C controls, which were set at 100%. Comparison between groups 1 versus 2, 1 versus 4, and 1 versus 6 not statistically significant; groups 1 versus 8 P<0.001; 2 versus 3 P=0.146; 4 versus 5 and 6 versus 7 P<0.001; 8 versus 9 P=0.004
In order to further analyze DNA repair inhibition and to confirm that death is induced by an apoptotic mechanism, we analyzed genomic DNA on an agarose gel from p53ts cells treated at 37°C and 30°C, in the presence or absence of aphidicolin. Treatment of p53ts cells with aphidicolin at the nonpermissive temperature of 37°C up to 9 h does not result in any apoptotic DNA fragmentation (Figure 7, lanes 2 and 3). However, addition of aphidicolin results in a slight increase in apoptotic fragmentation by 5 and 6 h at 30°C, whereas there is none seen in cells incubated at 30°C for 5 or 6 h without aphidicolin (compare lanes 7 and 9 with 6 and 8). By 9 h, there is significantly more fragmented DNA in the aphidicolin-treated cells than in cells incubated at 30°C for 9 h alone (lanes 10 and 11). These results, taken together, show that the inhibition of DNA repair accelerates p53-induced apoptosis and strongly suggests a role for DNA repair in the reversibility of early apoptotic phenotypes.
Discussion
Apoptosis is an orderly event that occurs in a reproducible fashion in a number of different cell types, induced by many different agents and physiologic situations. As more information is gained about the regulation and sequence of events in an apoptotic cell, the likelihood of using this knowledge to help diagnose and treat various diseases increases. In the present study, we focus on p53, a key molecule in apoptosis and maintenance of genomic integrity.38 Our results show that early stages of p53-induced apoptosis are reversible if the apoptotic stimulus is removed. In addition, we demonstrate that DNA repair is activated early in p53-induced apoptosis, and the inhibition of DNA repair results in accelerated apoptosis, strongly suggesting a role for DNA repair in the reversibility of early apoptotic phenotypes. Interestingly, this repair is occurring in the absence of a G1 cell cycle arrest, even though p21 is upregulated by incubation at 30°C.32
Previous results in our laboratory suggested that early p53-induced apoptotic phenotypes may be reversible.32 As these results examined reversibility in cell populations, we wanted to determine the reversibility of single apoptotic cells. To do this, we used FACS analysis to sort individual, FITC-labeled PS-positive cells and replated them under non-apoptotic conditions. Our results show that p53 induces PS-flipping in these cells (Figure 1). After these apoptotic cells are removed and replated, and the apoptotic stimulus is removed, a large percentage of sorted cells survive and replicate (Figure 2). This novel finding shows conclusively that early apoptosis induced by p53 is reversible. In addition, these reversible apoptotic cells are not selected as the result of the development of apoptosis resistance or inactivation of p53 (Figure 3). Cells incubated at the permissive temperature, returned to the non-permissive temperature and reincubated again at 30°C over four cycles still show apoptotic DNA fragmentation. Similarly, sorted PS-positive apoptotic cells incubated again at the permissive temperature also undergo apoptotic fragmentation (not shown), proving that the cells that survive initial apoptosis induction are still capable of undergoing apoptosis.
As a possible mechanism for this reversibility, we have previously shown that DNA repair activity is increased early in p53-induced apoptosis.32 In addition to previous work that analyzed repair in a population of cells, we now have analyzed DNA repair in individual p53ts cells in the presence of DNA replication inhibitors. As seen in Figures 4 and 5, the presence of hydroxyurea at the nonpermissive temperature of 37°C results in almost complete inhibition of 3H-thy incorporation. However, there is more incorporation at the permissive temperature of 30°C in the presence of HU, which confirms that the incorporation we are seeing in these cells is due to DNA repair and not replication. The fact that the permissive temperature is inducing TUNEL-positive cells at a time when DNA repair is seen32 supports our contention that DNA repair mechanisms are functional in apoptotic DNA damage. Coincidentally, the percentage of labeled cells in the presence of HU at 30°C after 6 h corresponds very closely to the percentage of TUNEL-positive cells seen at this timepoint (Figure 5 and Geske et al).32 Furthermore, the addition of aphidicolin to the p53ts cells treated at 30°C completely inhibits all 3H-thy incorporation, strongly implicating DNA repair in the early apoptotic cells.
Further proof of the regulatory role of DNA repair in p53-induced apoptosis can be seen by the inhibition of DNA repair in this process. As seen in Figure 6, treating p53ts cells with the DNA repair inhibitor aphidicolin results in accelerated cell death as measured by a clonogenic assay. In addition, apoptotic fragmentation of genomic DNA is seen earlier in cells with activated p53 treated with aphidicolin than in those not treated with the repair inhibitor (Figure 7). These results strongly implicate DNA repair as a mediator of reversibility of early p53-induced apoptotic phenotypes.
One of the earliest measures of apoptosis induction is the externalization of PS from the inner to the outer leaflet of the cell membrane. PS-flipping is a method by which apoptotic cells may be recognized and removed by phagocytic cells.5,6 Although necrotic cells also expose PS on their cell surfaces,39 PS-flipping and its recognition by the lipid-binding protein annexin-V is a common and efficient measure of apoptotic cells in a population. This assay also uses the nuclear stain propidium iodide, which can be used to detect necrotic cells in flow cytometry experiments. By selecting only FITC-labeled annexin-V positive cells, FACS analysis can separate apoptotic, PS-positive cells from normal, healthy cells as well as necrotic cells. Our results showing surviving annexin-V positive cells therefore unequivocally demonstrate that single PS-positive apoptotic cells can survive if the apoptotic stimulus is removed. As later stages of apoptosis, including those with 200 bp fragmented DNA and activated caspases, may also have externalized PS, we propose that only early-stage PS-positive cells may be reversible. These results are consistent with those of Hammill et al who also showed that annexin-positive B lymphoma cells could survive if the apoptotic stimulus was removed.40 In addition to their results, we also show that DNA repair is involved in this reversibility.
What is the role of DNA repair in the reversal of an early apoptotic cell? As previously mentioned, studies have shown that many cells have early, larger genomic DNA fragmentation patterns (50–300 kb) that precede 200 basepair fragment formation.8,9,10 In previous studies, we have seen TUNEL-positive p53ts cells by 6 h of incubation at 30°C even though clonogenic survival at this timepoint is still at control levels.32 In addition, as seen in our current work, PS-positive cells are reversible at this timepoint (Figure 2). As past studies have shown, these DNA fragmentation patterns are recognizable by the TUNEL assay.10 We suggest that these earlier DNA breaks coincide with PS-flipping in these cells, and that these breaks can be repaired. Further studies are required to determine whether this is the case.
Once a cell has begun to undergo apoptosis, there are a number of events that must be inhibited in order to allow it to recover. In the case of p53-induced apoptosis, p53 itself be inactivated and its apoptotic functions must cease. Perhaps the central mechanism for p53 inactivation is by mdm-2, a p53-induced gene that binds to and inactivates p53 function.41 mdm-2 promotes the ubiquitination and degradation of p53 with the aid of the p300/CBP transcriptional coactivator.42,43 Therefore, upregulation of mdm2 by p53 may be necessary to reduce p53 activity and return the cell to its normal state. In addition, after DNA repair, replication, transcription, and translation must regain their normal functions so that the cell can be operational again.
Our theory suggests that in order for p53-induced apoptosis to occur, DNA repair mechanisms have to be eventually overcome. At what point can DNA repair no longer rescue an apoptotic cell? It appears likely that once DNA has been broken down into 200 basepair fragments, this amount of damage may be too extensive for the repair mechanisms. In addition, caspase activation, namely the executioner caspases, results in destruction of many critical cellular proteins, including the repair proteins poly-(ADP) ribose polymerase (PARP) and the DNA-dependent protein kinase (DNA-PK).44 Since caspases are essential for cleavage of the inhibitor of the caspase-activated DNase, which leads to oligonucleosomal-length DNA fragmentation,45,46,47 the activation of executioner-phase caspases may also signal the point of no return in p53-induced apoptosis. Caspase-3 is activated in p53ts cells and reaches maximal activity by 12 h at 30°C, a timepoint where cell viability begins to decline.32
The question remains, why would p53-induced apoptosis be a reversible process? In addition, are other types of apoptosis also reversible, or is this unique for the p53-dependent process? Cells may begin early stages of apoptosis immediately upon different cellular insults. For example, upon DNA damage, p53 may induce apoptosis and DNA repair programs simultaneously. If DNA repair is successful on the damaged DNA, early apoptotic phenotypes may also be reversed and the cell could survive. However, if the amount of DNA damage is too extensive for repair mechanisms to handle, then apoptotic processes have already been initiated and the cell can quickly die before any further damage could induce transformed or neoplastic phenotypes. As p53 is a mediator of both processes, it may be required for the reversal of all types of apoptosis induction.
In attempting to repair apoptotic DNA, repair processes may actually increase the possibility of mutations due to the fact that DNA repair polymerases are not always 100% accurate.48 Due to the nature of apoptotic DNA breaks, it is likely that some genetic information may be lost in the repair of double-stranded breaks. In a large proportion of DNA, those genetic changes may not matter. But in the case of repairing critical genes, the repair of strand breaks may result in mutations and even neoplastic transformation. Further research on the repair of individual genes in apoptosis is necessary to elucidate these answers.
Ultimately, increased understanding of apoptotic mechanisms is necessary to impact diagnosis and therapy. Is it possible that the reversibility of early stages of p53-induced apoptosis is critical in disease treatment? It is clear that antineoplastic apoptosis-inducing drugs must be delivered at doses large enough to kill cells and get past the point of no return. In addition, inhibition of DNA repair when inducing apoptosis in neoplastic cells could be synergistic. The results presented in this work suggest that this may be the case.
Our data suggest the following model: DNA damage results in the activation of p53, which induces both early stages of apoptosis (PS-flipping and early 50–300 kb DNA breaks) and DNA repair simultaneously. If the damaged DNA is repaired, then the apoptotic functions of p53 are switched off, probably by p53 degradation, and the cell survives. However, if repair is not successful or if DNA damage is too extensive, apoptotic functions such as executioner-phase caspase activation and oligonucleosomal DNA fragmentation occur, along with the inhibition of repair processes, and the cell ultimately dies.
Materials and Methods
Cell culture
MOD cells were originally obtained from a mouse mammary carcinoma49 and contain a truncated p53 mRNA and nonfunctional p53 protein. We stably transfected these cells with either a temperature-sensitive p53 plasmid (p53ts) or a constitutively mutant p53 (p53mut). The p53ts plasmid results in a valine substituted for alanine at amino acid #135 of the mouse protein, while the p53mut construct contains a cysteine to phenylalanine substitution at amino acid #132. Both plasmids are a chimera of mouse p53 cDNA and genomic DNA under the transcriptional control of a Harvey sarcoma virus long terminal repeat.51 Although the MOD parent cell line contains no p53, we felt the p53mut cell line was a better control for the p53ts cells in that there is only a single amino acid difference between the two cell lines. Both cell lines exhibit the same growth rate. These plasmids were cotransfected with a neo-resistance vector at a ratio of 20 : 1 (p53 vector : neoR vector) and cells are maintained in DMEM/F12 media supplemented with EGF (12.5 ng/ml), insulin (10 μg/ml), 1% serum, and 200 μg/ml Geneticin (Gibco/BRL). For unscheduled DNA synthesis assays, DMEM supplemented with the above nutrients was used in place of DMEM/F12. Activation of p53 in the p53ts cells occurs at 30°C.32
Annexin-V assay
Cells were incubated at 37°C or 30°C for 6 h. They were then removed from dishes by dispase and centrifuged, and the pellet was then washed with PBS (37°C) and the cells were spun again. After the PBS wash, cells were resuspended in a modified 1× binding buffer (10 mM HEPES, pH 7.4, 0.15 M NaCl, 5 mM KCl, 1 mM MgCl2, 2.5 mM CaCl2, MEM amino acids solution (1×), MEM non-essential amino acids solution (1×), 0.5% glucose). The reaction was then performed for 15 min at room temperature and contained the following: 10 μl 10× binding buffer (Trevigen, same as modified binding buffer except 1.8 mM CaCl2 without amino acids solution or glucose), 10 μl 50 μg/ml propidium iodide, 5 μl Annexin-V-FITC conjugate, and 5×105 cells in 50 μl. After the reaction, 400 μl of modified 1× binding buffer was added to the tube, and analysis was performed. To determine the per cent of Annexin-V-positive cells, flow cytometry was done and the number of FITC-positive, PI-negative cells was obtained. For cell sorting, the MoFlow cell sorter (Cytomation, Inc., Fort Collins, CO, USA) was used to remove FITC-positive cells, or FITC-negative control cells. One thousand five hundred cells (FITC-+ or FITC-negative) were sorted onto 24-well culture dishes, and the cells were returned to 37°C for 5 days. Cells were then fixed for 30 s in 100% methanol, dried, and stained with 0.1% toluidine blue and photographed.
UDS assay
Cells were incubated in DMEM at 37°C or 30°C for 6 h, with or without hydroxyurea (2.5×10−3 M) or aphidicolin (3 μM) in 4-well slide chambers (Lab-Tek). For the last hour of each incubation, 3H-thymidine was added to each well (10 μCi/ml). After the 6 h incubations, a rinse in DMEM continued cold thymidine (0.1 μM) and cold cytidine (0.01 μM) was continued at either 37°C or 30°C, with or without hydroxyurea or aphidicolin, for an additional hour. Media was poured off, and cells were fixed for 30 s in 100% methanol. Wells were removed from the slide chambers, and the slides were rinsed in five containers of PBS/BSA (5 g/L BSA, pH 7.4), and dried overnight. Slides were then immersed in Amersham EM-1 emulsion at 43°C, dried, and stored in the dark for 4 days. The slides were then developed, fixed, dried, and stained in hematoxylin. Cells were considered positive if five grains or more above background appeared over the nucleus, while background was determined by analysis of the number of grains in an area with no cells corresponding to the size of one cell.
Colony formation assay
Two thousand cells were plated on 35 mm diameter culture dishes and allowed to attach overnight at 37°C. Dishes were either left at 37°C for 72 h (control) or put at 30°C for 2, 4, 6, or 12 h with or without aphidicolin (3 μM), then returned to 37°C for the remainder of 72 h. Cells were fixed for 30 s in methanol, dried, and stained with 0.1% toluidine blue. Colonies counted as positive contained five or more cells. The number of colonies obtained in the 72-h 37°C dishes was set at 100% and compared to the 30°C-treated dishes. In addition, to control for colonies formed by initial plating, the number of colonies were counted 18 h after plating, and results showed that only 10% of the number of colonies were formed at this stage compared to the 37°C controls. Therefore, this background does not affect final colony formation percentages.
DNA fragmentation analysis
Cells were incubated at either 37°C or 30°C for indicated times, with or without aphidicolin (3 μM). Media was then removed and digestion buffer was added (0.1 M NaCl, 10 mM Tris pH 8, 25 mM EDTA, 0.5% SDS, 0.3 mg/ml proteinase K). The cells were scraped off the plates with a rubber policeman and incubated at 50°C overnight, followed by a phenol/chloroform extraction and a chloroform extraction. DNA was then treated with RNase (20 μg/ml) for 1 h at 37°C, followed by another phenol/chloroform and chloroform extraction, and purified by ethanol precipitation. The concentration of DNA was determined by OD260 measurement, and 25 μg of DNA/lane was run on a 1.0% agarose gel (Trevigen) in a 0.25% Orange G loading buffer, stained with ethidium bromide, visualized on a UV light box, and photographed. The molecular weight marker used was pBR322 cut by HpaII, which results in bands at 622, 527, 404, and 309 base pairs, and a series of bands between 242 and 180 base pairs.
Western blots
Cells were incubated at either 37°C or 30°C for indicated times, the media removed, and RIPA buffer was added (50 mM Tris, 150 mM NaCl, 2 mM EDTA, 50 mM NaF, 1% Triton X-100, 1% Na-deoxycholate, 0.1% SDS, 1 mM DTT, 5 mM Na-orthovanadate, 1 μg/ml aprotinin, 0.5 μg/ml leupeptin). Extracts were then scraped off the plates, boiled for 5 min, and spun at 10,000 r.p.m. for 30 min. Twenty μg/lane (as determined by Bradford assay) was run on a 12% polyacrylamide gel in a loading buffer of 2% SDS, 10% glycerol, 60 mM Tris pH 6.8, and 2% β-mercaptoethanol, transferred to PVDF membrane, blocked in 5% blocking buffer (Amersham) for 18 h, and incubated with a p21 polyclonal antibody (Calbiochem) for 1 h. Protein-antibody complexes are detected by the ECL/ECL-Plus method according to manufacturer's instructions (Amersham). Blots were stripped at 50°C (100 mM β-mercaptoethanol, 2% SDS, 62.5 mM Tris-HCl pH 6.7) for 30 min, blocked overnight, and reprobed with a mouse monoclonal anti-β tubulin antibody (Boehringer Mannheim) as a loading control.
Statistical analysis
All statistics were performed by analysis of variance (ANOVA) using the Stats Plus program (Human Systems Dynamics).
Abbreviations
- p53ts:
-
temperature-sensitive p53
- p53mut:
-
constitutively mutant p53
- PS:
-
phosphatidylserine
- UDS:
-
unscheduled DNA synthesis
- aph:
-
aphidicolin
- FITC:
-
fluoresceine isothyocyanate
- FACS:
-
fluorescent activated cell sorting
- HU:
-
hydroxyurea
References
Kerr JFR, Wyllie AH and Currie AR . 1972 Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br. J. Cancer 26: 239–257
Kerr JFR . 1971 Shrinkage necrosis: a distinct mode of cellular death. J. Pathol. 105: 13–20
Granville DJ, Carthy CM, Hunt DWC and McManus BM . 1998 Apoptosis: molecular aspects of cell death and disease. Lab. Invest. 78: 893–913
Garfield E and Melino G . 1997 The growth of the cell death field: an analysis from the ISI-Science citation index. Cell Death Differ. 4: 352–361
Fadok VA, Voelker DR, Campbell PA, Cohen JJ, Bratton DL and Henson PM . 1992 Exposure of phosphatidylserine on the surface of apoptotic lymphocytes triggers specific recognition and removal by macrophages. J. Immun. 148: 2207–2216
Fadok VA, Bratton DL, Frasch SC, Warner ML and Henson PM . 1998 The role of phosphatidylserine in recognition of apoptotic cells by phagocytes. Cell Death Differ. 5: 551–562
Oberhammer F, Wilson JW, Dive C, Morris ID, Hickman JA, Wakeling AE, Walker PR and Sikorska M . 1993 Apoptotic death in epithelial cells: cleavage of DNA to 300 and/or 50 kb fragments prior to or in the absence of internucleosomal fragmentation. EMBO J. 12: 3679–3684
Bicknell GR, Snowden RT and Cohen GM . 1994 Formation of high molecular mass DNA fragments is a marker of apoptosis in the human leukaemic cell line, U937. J. Cell Sci. 107: 2483–2489
Cohen GM, Sun X-M, Fearnhead H, MacFarlane M, Brown DG, Snowden RT and Dinsdale D . 1994 Formation of large molecular weight fragments of DNA is a key committed step of apoptosis in thymocytes. J. Immunol. 153: 507–516
Beere HM, Chresta CM, Alejo-Herberg A, Skladanowski A, Dive C, Larsen AK and Hickman JA . 1995 Investigation of the mechanism of higher order chromatin fragmentation observed in drug-induced apoptosis. Mol. Pharmacol. 47: 986–996
Ko LJ and Prives C . 1996 p53: puzzle and paradigm. Genes Dev. 10: 1054–1072
Agarwal ML, Taylor WR, Chernov MV, Chernova OB and Stark GR . 1998 The p53 network. J. Biol. Chem. 273: 1–4
Kastan MB, Onyekwere O, Sidransky D, Vogelstein B and Craig RW . 1991 Participation of p53 protein in the cellular response to DNA damage. Cancer Res. 51: 6304–6311
Nelson WG and Kastan MB . 1994 DNA stand breaks: the DNA template alterations that trigger p53-dependent DNA damage response pathways. Mol. Cell. Biol. 14: 1815–1823
Chen X, Ko LJ, Jayaraman L and Prives C . 1996 p53 levels, functional domains, and DNA damage determine the extent of the apoptotic response of tumor cells. Genes Dev. 10: 2438–2451
Lakin ND and Jackson SP . 1999 Regulation of p53 in response to DNA damage. Oncogene 18: 7644–7655
Linke SP, Clarkin KC, Di Leonardo A, Tsou A and Wahl GM . 1996 A reversible, p53-dependent G0/G1 cell cycle arrest induced by ribonucleotide depletion in the absence of detectable DNA damage. Genes Dev. 10: 934–947
Graeber TG, Osmanian C, Jacks T, Housman DE, Koch CJ, Lowe SW and Giaccia AJ . 1996 Hypoxia-mediated selection of cells with diminished apoptotic potential in solid tumours. Nature 379: 88–91
Ohnishi T, Wang X, Ohnishi K, Matsumoto H and Takahashi A . 1996 p53-dependent induction of WAF1 by heat treatment in human glioblastoma cells. J. Biol. Chem. 271: 14510–14513
Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M and Krammer PH . 1998 p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J. Exp. Med. 188: 2033–2045
Sheard MA, Vojtesek B, Janakova L, Kovarik J and Zaloudik J . 1997 Up-regulation of Fas (CD95) in human p53 wild-type cancer cells treated with ionizing radiation. Int. J. Cancer 73: 757–762
Miyashita T, Krajewski S, Krajewska M, Wang HG, Lin HK, Liebermann DA, Hoffman B and Reed JC . 1994 Tumor suppressor p53 is a regulator of bcl-2 and bax gene expression in vitro and in vivo. Oncogene 9: 1799–1805
Zhan Q, Fan S, Bae I, Guillouf C, Liebermann DA, O'Connor PM and Fornace AJ Jr. . 1994 Induction of bax by genotoxic stress in human cells correlates with normal p53 status and apoptosis. Oncogene 9: 3743–3751
Wu GS, Burns TF, McDonald ERI, Jiang W, Meng R, Krantz ID, Kao G, Gan D-D, Zhou J-Y, Muschel R, Hamilton SR, Spinner NB, Markowitz S, Wu G and El-Deiry WS . 1997 KILLER/DR5 is a DNA damage-inducible p53-regulated death receptor gene. Nat. Genet. 17: 141–143
Polyak K, Xia Y, Zweier JL, Kinzler KW and Vogelstein B . 1997 A model for p53-induced apoptosis. Nature 389: 300–305
Gu Z, Flemington C, Chittenden T and Zambetti GP . 2000 ei24, a p53 response gene involved in growth suppression and apoptosis. Mol. Cell. Biol. 20: 233–241
Kastan MB, Zhan Q, El-Deiry WS, Carrier F, Jacks T, Walsh WV, Plunkett BS, Vogelstein B and Fornace AJ Jr . 1992 A mammalian cell cycle checkpoint pathway utilizing p53 and GADD45 is defective in Ataxia-Telangiectasia. Cell 71: 587–597
Wang XW, Yeh H, Schaeffer L, Roy R, Moncollin V, Egly J-M, Wang Z, Friedberg EC, Evans MK, Taffe BG, Bohr VA, Weeda G, Hoeijmakers JHJ, Forrester K and Harris CC . 1995 p53 modulation of TFIIH-associated nucleotide excision repair activity. Nat. Genet. 10: 188–195
Leveillard T, Andera L, Bissonnette N, Schaeffer L, Bracco L, Egly J-M and Wasylyk B . 1996 Functional interactions between p53 and the TFIIH complex are affected by tumour-associated mutations. EMBO J. 15: 1615–1624
Ford JM and Hanawalt PC . 1995 Li-Fraumeni syndrome fibroblasts homozygous for p53 mutations are deficient in global DNA repair but exhibit normal transcription-coupled repair and enhanced UV resistance. Proc. Natl. Acad. Sci. 92: 8876–8880
Mirzayans R, Enns L, Dietrich K, Barley RDC and Paterson MC . 1996 Faulty DNA polymerase δ/ε-mediated excision repair in response to gamma radiation or ultraviolet light in p53-deficient fibroblast strains from affected members of a cancer-prone family with Li-Fraumeni syndrome. Carcinogenesis 17: 691–698
Geske FJ, Nelson AC, Lieberman R, Strange R, Sun T and Gerschenson LE . 2000 DNA repair is activated in early stages of p53-induced apoptosis. Cell Death Differ. 7: 393–401
Cleaver JE and Thomas GH. . 1981 Measurement of unscheduled synthesis by autoradiography. In DNA repair: a lab manual. 277–281
Althaus FR, Lawrence SD, Sattler GL, Longfellow DG and Pitot HC . 1982 Chemical quantification of unscheduled DNA synthesis in cultured hepatocytes as an assay for the rapid screening of potential chemical carcinogens. Cancer Res. 42: 3010–3015
Ahnstrom G . 1989 Inhibition of DNA strand break rejoining in ultraviolet-irradiated human cells: comparison of aphidicolin and cytosine arabinoside. Bioch. Biophys. Acta 1007: 357–358
Suter W and Romagna F . 1990 DNA repair induced by various mutagens in rat hepatocyte primary cultures measured in the presence of hydroxyurea, guanazole or aphidicolin. Mut. Res. 231: 251–264
Wood RD and Shivji MKK . 1997 Which DNA polymerases are used for DNA-repair in eukaryotes? Carcinogenesis 18: 605–610
Lane DP . 1992 p53, guardian of the genome. Nature 358: 15–16
Waring P, Lambert D, Sjaarda A, Hurne A and Beaver J . 1999 Increased cell surface exposure of phosphatidylserine on propidium iodide negative thymocytes undergoing death by necrosis. Cell Death Differ. 6: 624–637
Hammill AK, Uhr JW and Scheuermann RH . 1999 Annexin V staining due to loss of membrane asymmetry can be reversible and precede commitment to apoptotic death. Exp. Cell Res. 251: 16–21
Lane DP and Hall PA . 1997 MDM2 – arbiter of p53's destruction. Trends Biochem. Sci. 22: 372–374
Grossman SR, Perez M, Kung AL, Joseph M, Mansur C, Xiao Z-X, Kumar S, Howley PM and Livingston DM . 1998 p300/MDM2 complexes participate in MDM2-mediated p53 degradation. Mol. Cell 2: 405–415
Thomas A and White E . 1998 Suppression of the p300-dependent mdm2 negative-feedback loop induces the p53 apoptotic function. Genes Dev. 12: 1975–1998
Stroh C and Schulze-Osthoff K . 1998 Death by a thousand cuts: an ever increasing list of caspase substrates. Cell Death Differ. 5: 997–1000
Enari M, Sakahira H, Yokoyama H, Okawa K, Iwamatsu A and Nagata S . 1998 A caspase-activated DNase that degrades DNA during apoptosis, and its inhibitor ICAD. Nature 391: 43–50
Sakahira H, Enari M and Nagata S . 1998 Cleavage of CAD inhibitor in CAD activation and DNA degradation during apoptosis. Nature 391: 96–99
Tang D and Kidd VJ . 1998 Cleavage of DFF-45/ICAD by multiple caspases is essential for its function during apoptosis. J. Biol. Chem. 273: 28549–28552
Friedberg EC, Walker GC and Siede W. . Nucleotide excision repair: mammalian genes and proteins. In DNA repair and mutagenesis. Washington DC: ASM Press pp. 317–365
Medina D, Oborn CJ, Kittrell FS and Ullrich RL . 1986 Properties of mouse mammary epithelial cell lines characterized by in vivo transplantation and in vitro immunocytochemical methods. J. Nat. Cancer Inst. 76: 1143–1151
Kaeck M, Lu J, Strange R, Ip C, Ganther HE and Thompson HJ . 1997 Differential induction of growth arrest inducible genes by selenium compounds. Biochem. Pharm. 53: 921–926
Michalovitz D, Halevy O and Oren M . 1990 Conditional inhibition of transformation and of cell proliferation by a temperature-sensitive mutant of p53. Cell 62: 671–680.
Acknowledgements
We thank Dr. Moshe Oren for the p53 plasmids and Dr. Valerie Fadok for invaluable assistance with the Annexin-V assays. This work was supported by the National Institutes of Health and the R Herbert and Alma Manweiler Research Endowment, University of Colorado Health Sciences Center.
Author information
Authors and Affiliations
Corresponding author
Additional information
Edited by JYJ Wang
Rights and permissions
About this article
Cite this article
Geske, F., Lieberman, R., Strange, R. et al. Early stages of p53-induced apoptosis are reversible. Cell Death Differ 8, 182–191 (2001). https://doi.org/10.1038/sj.cdd.4400786
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.cdd.4400786
Keywords
This article is cited by
-
Stressed neuronal cells can recover from profound membrane blebbing, nuclear condensation and mitochondrial fragmentation, but not from cytochrome c release
Scientific Reports (2023)
-
Drug toxicity assessment: cell proliferation versus cell death
Cell Death Discovery (2022)
-
Mechanical Regulation of Apoptosis in the Cardiovascular System
Annals of Biomedical Engineering (2021)
-
Quantitative assessment of the impact of cryopreservation on human bone marrow-derived mesenchymal stem cells: up to 24 h post-thaw and beyond
Stem Cell Research & Therapy (2020)
-
Integration of EMT and cellular survival instincts in reprogramming of programmed cell death to anastasis
Cancer and Metastasis Reviews (2020)