Abstract
With evolving diagnostic and therapeutic advances, the survival of patients with acute leukaemia has considerably improved. This has led to an increase in the variability of ocular presentations in the form of side effects of the treatment and the ways leukaemic relapses are being first identified as an ocular presentation. Leukaemia may involve many ocular tissues either by direct infiltration, haemorrhage, ischaemia, or toxicity due to various chemotherapeutic agents. Ocular involvement may also be seen in graft-versus-host reaction in patients undergoing allogeneic bone marrow transplantation, or simply as increased susceptibility to infections as a result of immunosuppression that these patients undergo. This can range from simple bacterial conjunctivitis to an endophthalmitis. Leukaemia can present as pathology in the adnexae, conjunctiva, sclera, cornea, anterior chamber, iris, lens, vitreous, retina, choroid, and optic nerve. Recognition of the varied ocular presentations is also important in assessing the course and prognosis of leukaemia. We have presented a systematic approach taking each part of the eye in turn and outlining how leukaemia has been shown to affect it.
Similar content being viewed by others
Introduction
The leukaemias are malignant neoplasms of the haematopoietic stem cells, characterized by diffuse replacement of the bone marrow by neoplastic cells. Ophthalmic involvement can be classified into two major categories: (1) primary or direct leukaemic infiltration, (2) secondary or indirect involvement. The direct leukaemic infiltration can show three patterns: anterior segment uveal infiltration, orbital infiltration, and neuro-ophthalmic signs of central nervous system leukaemia that include optic nerve infiltration, cranial nerve palsies, and papilloedema. The secondary changes are the result of haematological abnormalities of leukaemia such as anaemia, thrombocytopenia, hyperviscosity, and immunosuppression. These can manifest as retinal or vitreous haemorrhage, infections, and as vascular occlusions. In some cases the ocular involvement may be asymptomatic. In one prospective study, there was a high prevalence of asymptomatic ocular lesions in childhood acute leukaemia.1 In the era before effective antileukaemic therapy, retinopathy was believed to be of no prognostic significance in acute leukaemia.2, 3 However, recent reports have demonstrated that the presence of ocular involvement is associated with poor prognosis in acute childhood leukaemias.3 Therefore, it is important to consider an ophthalmic evaluation at the time of diagnosis of acute leukaemia in adults and children.
In the review that follows, we present a current classification of leukaemia with a brief outline of cytochemical features and immunophenotype of acute leukaemia, and describe varied leukaemic involvement of the ocular tissues.
Classification of the leukaemia
(Table 1)
Various classifications of leukaemia exist in the literature based on cell type involved, state of maturity, morphological and cytochemical characteristics, and immunophenotype of the leukaemic cells. 4, 5 The most widely used and clinically useful classification divides leukaemia into acute and chronic forms. The acute leukaemias are characterized by replacement of the bone marrow with very immature cells called blasts. Chronic leukaemias are associated, at least initially, with well-differentiated (mature) leucocytes. Two major variants of acute and chronic leukaemia are recognized: lymphocytic and myelocytic (myelogenous). This simple classification has four patterns of leukaemia: acute lymphocytic (lymphoblastic) leukaemia (ALL), chronic lymphocytic leukaemia (CLL), acute myelocytic (myeloblastic) leukaemia (AML), and chronic myelocytic leukaemia (CML). Approximately 5% of acute leukaemias are difficult to characterize as either ALL or AML, and some may be classified as acute undifferentiated leukaemia (AUL). This simple classification separates leukaemia from closely related neoplastic disorders of haematopoietic stem cells.
It is widely accepted that CML, polycythemia vera, essential thrombocytosis, and myeloid metaplasia represent clonal neoplastic proliferation of the myeloid or, possibly, pluripotent stem cells. If the erythrocyte precursors dominate, the resulting clinical disorder is classified as polycythemia vera; on the other hand, dominance of the granulocytic series is manifested as CML. Analogous to CML, it is possible to segregate chronic lymphoproliferative disorders. This group includes CLL and hairy cell leukaemia, both of which are neoplastic proliferations of lymphoid cells, most often of B-cell lineage. As proliferative disorders of lymphoid cells, these are related to Non Hodgkin's lymphomas.
Morphologic, cytochemical features, and immunophenotype in acute leukaemia
Under normal conditions, blast forms constitute fewer than 5% of the nucleated cells of the bone marrow and are seen in the peripheral blood except during periods of profound overproduction of blood cells. Blast cells are primitive precursors, lacking many of the features of differentiation. Lymphoid blasts are differentiated from myeloid blast on the basis of standard morphologic and cytochemical differences, based on these, acute leukaemias are subdivided. Within the French-American-British (FAB) classification, ALL has been subdivided into three types: L1, L2, and L3, while subdivisions of AML are called M0–M7. Some early clinical trials have suggested that L2 morphology conveys a worse prognosis than L1 morphology, but in most recent trials of ALL, FAB classification was not found to be an independent prognostic variable. This led to correlation of cytochemical reactions with both morphology and immunologic cell surface markers.5 Identification of immunophenotypic subtypes of ALL allowed a classification that was more precise and biologically oriented than the morphologic approach, although in AML expression of immunologic markers is heterogeneous. Immunophenotyping has prognostic implications in B-cell ALL, while in AML it has diagnostic value in differentiation from ALL, especially in M0. Classification of leukaemia by immunophenotype is based on the expression of a pattern of lineage-associated antigens; no single antigen is truly lineage specific.5 Most ALLs are of B-cell origin, mainly of the early B-precursor type. ALLs of B-cell lineage express CD19 and CD22, the latter antigen is first expressed in the cytoplasm and later in the membrane. T-cell lineage ALL is defined by the cytoplasmic or membrane expression of CD3. Detailed description of various immunophenotypes is beyond the scope and objective of this article.
Ophthalmic involvement
(Table 2)
The eye is involved directly or indirectly, far more often in the acute leukaemia than in chronic cases. Various other effects occur from opportunistic infection or from therapeutic procedures, such as chemotherapy, radiotherapy, or bone marrow transplantation.
Leukaemic involvement in the various ocular tissues is described below.
Conjunctiva
Conjunctiva involvement, although not a common presentation of leukemias, occurs most often in patients with lymphocytic leukaemias.6.Cellular involvement is found at all levels of the substantia propria and can be diffuse or patchy, tending to concentrate along blood vessels.7 Comma-shaped venial abnormalities (cork screw vessels similar to those found in sickle cell disease) have also been reported. These are believed to be secondary to hyperviscosity.8 ALL has been reported to present as conjunctival lymphoma in adults.9 Lei et al described an unusual occurrence of bilateral conjunctival tumours in a 25-year-old woman. This was the first sign of relapse of ALL.10
Cornea and sclera
The cornea is an avascular structure and therefore is not commonly involved in leukaemia, especially in the form of direct invasion by leukaemic cells. Allen and Straatsma's series reported no corneal involvement beyond limbal infiltration.7
Sterile ring ulcers with iritis and pannus have been reported in leukaemias.11 Keratitis can occur secondary to immunosuppression or graft-versus-host disease (GVHD). Keratitis in a patient with GVHD can cause severe thining of cornea, threatening corneal perforation.12 Corneal involvement is also seen when corneal epithelial changes result secondary to chemotherapy.13 These changes include thinning irregularity, faulty maturation, and keratinisation. Peripheral corneal ulceration has also been reported in a patient with leukaemia and herpes zoster ophthalmicus.14
Scleral infiltration is usually an autopsy finding and occurs in acute leukaemia. These cells are most often found in the episclera in a perivascular pattern.7
Iris and anterior segment
Clinically evident infiltration of the iris by leukaemic cells is not common. It occurs with the involvement of choroid and ciliary body. Clinically, it is characterised by a change in iris colour, and a pseudohypopyon, which is grey/yellow in colour.15 On histopathological examination, the iris may show diffuse involvement, especially at the root and sphincter.7 The intra-ocular pressure (IOP) can be high enough to cause signs and symptoms of acute glaucoma with normal depth of anterior chamber.16 It is postulated that the raised IOP is probably due to infiltration of the trabecular meshwork.17 In children, spontaneous hyphaema is also a presentation of leukaemia.15 Usually clinically apparent involvement of the iris and anterior segment occurs with ALL.17, 18 It may also occur less commonly with CLL19 and myeloid leukaemias.15 Extramedullary relapse of acute leukaemias may masquerade as hypopyon uveitis.20 Primary relapse of acute leukaemia in the anterior segment is not uncommon.20, 21, 22, 23 Leukaemias have been identified as the cause of uveitis in 5% of paediatric uveitis cases.23 Ocular involvement is unusual in acute non-lymphoblastic leukaemia, however, one case was reported where an infant who had evidence of active central nervous system disease showed infiltration of the anterior chamber during therapy. Treatment was with topical corticosteroids, chemotherapy, and bilateral ocular radiotherapy.24 Any ophthalmic manifestation in children with leukaemia should be detected and treated early. Radiotherapy is warranted in infiltration of the anterior chamber. The presence of ocular or CNS involvement indicates poor prognosis in acute childhood leukaemia.
Choroid
Choroid shows leukaemic infiltration most consistently on histopathological examination, though clinically the retina is most commonly involved in leukaemia.7.The involvement of choroid by leukaemic cells tends to be perivascular, and may be patchy or diffuse.7 Choroid may be thickened to many times normal at the posterior pole. The overlying retinal pigment epithelium (RPE) may show secondary alterations, including atrophy and hypertrophy. There may be secondary photoreceptor cell loss, drusen formation or serous detachment. Clinically, choroidal involvement presents as a serous retinal detachment, which is generally shallow, and located at the posterior pole. These detachments have been reported in CLL25, ALL26, 27, CML28 and AML.29
Retina
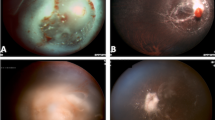
The retina is involved in leukaemia more often than any other ocular tissue. It is estimated that up to 69% of all patients with leukaemia show fundus changes at some point in the course of their disease, although at that time no effective treatments were present.30 The early manifestations (because of haematological disturbance) are venous dilatation and tortuosity.31 Haemorrhages may occur in all levels of the retina, usually in the posterior pole, and may extend into the vitreous. They may be round or flame shaped, and often has a white component. The white area consists of leukaemic cells and debris, platelet-fibrin aggregates, or septic emboli. A similar clinical picture can be seen in severe anaemia (Figure 1), thrombocytopenia, and hyperviscosity. Histopathological examination shows discrete and diffuse haemorrhage and leukaemic infiltration.7 The haemorrhages and infiltrates are found at all levels of the retina, but especially in the inner layers with focal destruction. The infiltrates and aggregates of leukaemic cells are usually but not always seen with surrounding haemorrhage.32 Large leukaemic infiltrates can cause total retinal detachment that may present as an isolated relapse.33 Smaller infiltrates tend to be perivascular. Subretinal infiltration in leukaemia has been referred as subretinal hypopyon.34 Cotton wool spots can be seen and are probably due to ischaemia from anaemia, hyperviscosity, or leukaemic infiltration.
Prior to the era of modern chemotherapy, massive leukaemic infiltrates were often seen accompanying haemorrhages, partially or completely destroying retinal architecture.35 Less common manifestations include microaneurysms that tend to be peripheral. The presence of microaneurysms is probably related to increased viscosity from elevated white blood cell (WBC) count, and does not correlate with the haemoglobin level or platelet count.36 Peripheral retinal neovascularisation, similar to that seen in sickle cell anaemia, has been recorded in CML.37 Various studies have reported no correlation between retinal involvement and WBC or platelet level.36 It may be that the blood profile varies during the course and emergence of retinal findings, and may correlate better with the blood cell counts in the preceding weeks or months.
The internal limiting membrane generally acts as an effective barrier to leukaemic cell infiltration.32 However, leukaemic cells occasionally do invade the vitreous possibly emerging from the optic nerve head. Reese and Guy38 were the first to mention leukaemic cells in the vitreous cavity in the absence of haemorrhage. Swartz and Schumann39 reported such a case, in which their patient (in clinical remission from ALL) had lymphoblasts in the vitreous, diagnosed by examination of specimens obtained by pars plana vitrectomy. There is also a case study of a 5-year-old child with ALL who had a bilateral dense cellular infiltration resulting in significant bilateral visual loss (counting fingers at 20 cm distance). In this case, because the vitreous was not cleared by chemotherapy, a bilateral vitrectomy was performed with vision improving to 6/10 in both eyes that remained stable at 3-year follow up.40
Optic nerve
As a result of increased survival, involvement of the central nervous system became more frequent particularly in acute leukemia. Shaw et al41 and co-workers described the clinical entity of CNS leukaemia, which may appear even when bone marrow is in remission. As the blood brain barrier restricts free passage of certain chemotherapeautic agents, prophylactic therapy to the CNS and posterior pole of the eye is usually advocated.42 CNS leukaemia occurs in both children and adults (less common)43 and more often in ALL compared to AML.44
Symptoms of CNS leukaemia include nausea, vomiting, lethargy, and seizures.42 Ocular symptoms include blurred vision and diplopia due to the involvement of cranial nerves. CNS leukaemia may also present as an asymptomatic papilloedema.45 Optic nerve involvement may be an extension of CNS leukaemia: by direct infiltration of the nerve head in which case the intracranial pressure may be normal; or by passive swelling because of retrolaminar leukaemic invasion; or by passive swelling secondary to increased intracranial pressure. Massive direct infiltration of the optic nerve head by leukaemic cells can give rise to a clinical picture identical to that of papilloedema (Figure 2). Clinically, papilloedema and direct optic nerve head infiltration may sometimes be differentiated by the typical retinal perivascular infiltration seen in the latter.35 Vision may remain good, despite direct leukaemic invasion of the optic nerve head, although vision is more likely to be impaired if the retrolaminar portion of optic nerve is involved.46 Examination of the spinal fluid for leukaemic cells will enable the clinician to determine the presence of CNS disease, but this does not necessarily imply the presence of direct optic nerve invasion.46 Ellis and Little47 reported a patient with CML, treated with intrathecal methotrexate.47 The leukaemic infiltrates were present distal to the termination of the arachnoid sheaths (2–3 mm posterior to the disc). This indicates that the intraocular portion of the optic nerve is beyond the reach of intrathecal chemotherapy and should be locally irradiated when involved.
Orbit
Orbital infiltration in leukaemia presents with exophthalmos (Figure 3), lid oedema, chemosis and pain.48, 49.All types of leukaemia may involve the orbit; however, orbital involvement is more common in acute leukaemias compared to chronic leukaemias, and it occurs more commonly in lymphoid leukaemias compared to myeloid ones.50 Orbital involvement could also present as orbital abscess due to infections of periocular tissues secondary to neoplastic infiltration or immunosuppression.51 The orbital mass of cells formed in myeloid leukaemia has been called a chloroma or granulocytic sarcoma. The orbital chloroma has a high chance of eroding into the cranial cavity. The histopathological evaluation of orbital chloroma shows infiltration by typical leukaemic cells. There has also been a report of a retro-orbital mass in patients with relapse of ALL.52, 53, 54 Whenever relapse of acute leukaemia is documented in extramedullary tissues, it is important to perform a full haematological work-up of the patient (including bone marrow examination and blood counts), because this is often followed by bone marrow relapse within a few weeks or months.
Leukaemic cells may also infiltrate other orbital structures such as lacrimal glands. Leukaemic cells may rarely infiltrate extraocular muscles, but this has not been evident clinically as isolated muscle palsy. Leukaemic infiltrate may also invade the adjoining sinuses simulating sinusitis.
Miscellaneous manifestations
Other less common ocular manifestations of leukaemia include anterior segment necrosis, dacryocystitis, and skin infiltration.55 Anterior segment necrosis in leukaemia occurs because of hyperviscosity or anaemia. It may present as ocular pain, corneal oedema, chemosis, decreased vision, anterior uveitis, increased IOP, and cataract. Both acute and chronic leukaemias have been reported to cause dacryocystitis.56 Lid involvement in leukaemia can be secondary to cranial nerve palsy or orbital involvement. A recent report presented a case of granulocytic sarcoma involving the eyelids and caruncles as the first sign of AML relapse after bone marrow transplantation, although primary involvement of the lid skin is very rare.57 Leukaemia uncommonly infiltrates the dermis.58
Opportunistic infections
Patients with leukaemia are prone to develop unusual and potentially life-threatening infections during periods of neutropenia, which results both from the underlying disease as well as chemotherapy. These patients are susceptible to a wide variety of infections by viral, fungal, protozoal, and bacterial organisms.59
One of the common viruses infecting immunocompromised host is cytomegalovirus (CMV).56 In the immunocompromised host, there may be considerable involvement of neuroectodermal tissue by CMV. The virus invades the retina, causing necrosis, vascular sheathing, haemorrhage, and combined exudative and rhegmatogenous retinal detachment.60 Other viruses (herpes simplex, varicella zoster, and mumps) may also cause necrotising retinitis in immunocompromised hosts.59 Herpes zoster can also cause peripheral corneal ulcer, keratitis, and scleritis.61 Mumps virus has been reported to be a cause of granulomatous uveitis.62
Fungi are common causes of ocular infection in leukaemias. Candida infection causes uveitis and retinitis with characteristic cotton balls in the vitreous.59 Aspergillus is also a common fungal infection in leukaemic patients.59 Nerikelun reported a case of acute blindness caused by fungal infection in CML.63 Autopsy showed mucormycosis of the brain and right optic nerve sheath. In one report, a patient with AML developed a cavernous sinus syndrome during the aplastic phase after induction chemotherapy. The clinical, serological, and radiological findings suggested an invasive sphenoid sinus aspergillosis. Endoscopic ethmoidosphenoidectomy allowed definitive diagnosis of this infection. After surgery, fungal eradication and reversal of neuro-ophthalmological damage paralleled complete haematological remission.64
This report showed that early recognition, prompt intervention, and immunological reconstitution are essential for successful outcome of paranasal mycoses in immunosuppressed patients.
Pseudomonas is a common bacterial infection in immunosuppressed leukaemic patients. This infection may occasionally start as a blepharoconjunctivitis and spread to cause orbital cellulitis.65 It is important to mention here Sweet's syndrome.66 This is characterised by abrupt onset of fever, neutrophilic leucocytosis, and erythematous, tender pseudovesiculated plaques or nodules that respond readily to corticosteroid therapy. It is usually distinguished by the presence of mature neutrophils on histopathologic examination. In its initial presentation, this syndrome can be confused with orbital cellulitis.66 Endophthalmitis is also a well-recognised eye manifestation of acute leukaemia with its potentially lethal effects upon visual potential.67
Toxicity to antileukaemic drugs
The past few years have seen the introduction of many new anticancer drugs, both in routine clinical practice and as a part of research protocols. For many of these, the full range of side effects is still not known. We will mention a few important drugs known to have ocular side effects. Busulphan is reported to cause posterior subcapsular cataract.68
Vincristine and vinblastine are toxic to the CNS and have been clinically shown to affect motor nerves of the eye (cranial nerves III, IV, VI, and VII). They can also cause corneal hypoaesthesia.69 Vincristine has also been shown to cause optic atrophy.70 Cytosine-arabinoside has been shown to be toxic to corneal epithelium when given topically or systemically.71 Stentoft highlighted that corneal toxicity is frequent in high-dose cytarabine therapy (as is used in the therapy of AML), but is often reversible and usually preventable with prophylactic steroid eye drops.72
There have been reports of a condition called posterior leukoencephalopathy syndrome.73 The clinical presentation usually includes seizures, headache, altered mental status, and blindness associated with immunosuppressants like cyclosporin used for prophylaxis of GVHD. The condition improves with drug discontinuation. This syndrome can also present with oculogyric crisis. Recognising this association is important because patients on cyclosporin often receive other medications that can cause dystonic eye movements. This can lead to delay in diagnosis and treatment, which can result in irreversible neurological deficit.
Bone marrow transplant (BMT)/GVHD
Ocular symptoms and signs can develop following bone marrow transplantation either due to GVHD or radiation treatment given along with bone marrow transplant. Recent improvements in the systemic management of these patients have led to more frequent recognition of the ocular problems. Ocular manifestations of GVHD include keratoconjunctivitis sicca, cicatricial lagophthalmos, sterile and pseudomembranous conjunctivitis, corneal epithelial defects, corneal ulceration and melting, uveitis, and ectropion of the lid.74, 75 In severe cases, the cornea may undergo keratinisation. Cataracts are a common complication after autologous bone marrow transplantation.76
The manifestations are more frequent in patients with chronic GVHD than in patients with acute GVHD. The more severe ocular complications are associated with severe chronic GVHD and poorer survival.74 Chronic GVHD often presents as Sjogren or scleroderma like illness.77 Since the era of bone marrow transplantation, the picture of acute and/or chronic transplant reaction of the host cells against grafted bone marrow has become more frequent. The so-called ‘adaptive immune therapy’ is based on the fact that patients who present with a low grade of GVHD less often suffer from a relapse of the malignant leukaemic disease. Therefore, new therapeutic regimens are now performed, which aim to keep the patient on a low level of GVHD to prevent a recurrence of leukaemia. Kasmann and Ruprecht75 showed that in acute or chronic GVHD, conjunctival disease is the most important form of ocular involvement. There is a lymphocytic infiltration in the conjunctiva and lacrimal glands, which can lead to a severe sicca syndrome. Acute GVHD of the conjunctiva can be classified into four stages: injection/exudation and chemosis/formation of pseudomembranes/defects of the corneal epithelium. These stages correlate directly to the prognosis of the survival time of the patient.75 A pathognomonic sign for the chronic GVHD of the conjunctiva are the fibrotic Arlt lines of the tarsal conjunctiva. Even asymptomatic patients should be screened for ocular sicca and started on artificial tear replacement if indicated. A close cooperation of oncologists and ophthalmologists is important for the optimal management of these patients.
A common cause of impaired visual acuity following BMT is cataract formation. It is probably related to steroid intake and the use of total body irradiation (TBI) for pretransplant conditioning. Ocular manifestations of BMT in children are not uncommon.78 The most common anterior segment problem is tear dysfunction. Posterior segment conditions although less common do occur; unilateral epiretinal membrane and or bilateral multiple discrete chorioretinal hypopigmented lesions in the middle to peripheral part of the retina are described. A high incidence of cataract formation was also reported, this probably being the most important long-term ‘amblyogenic’ problem in these immature eyes. Ischaemic retinopathy is a well-characterized complication of BMT. Although the aetiology is unclear, it is most probably multifactorial, and may be related to treatments such as radiation and cyclosporin. Webster et al79 reported two patients who developed such a retinopathy and the ocular histology from one of these cases showed evidence of retinal capillary endothelial loss. The retinal endotheliopathy in this case was similar to that described in radiation retinopathy. Again, awareness and management of these problems with routine eye examination and early intervention are recommended.78
Treatment of leukaemic eye infiltrates
The eye is a pharmacological sanctuary, which may not be treated adequately by drugs given systemically. 80 Ellis and Little47 demonstrated that the eye is beyond the reach of chemotherapeutic agents injected intrathecally. Ridgway et al42 advocated prophylactic irradiation to the eye as well as brain, but were unable to suggest doses. Radiation has been given for leukaemic infiltration of the eyes (both anterior and posterior) and for the orbit. Local irradiation along with systemic chemotherapy for underlying disease can help resolve iris infiltration, pseudohypopyon, and the accompanying elevated intraocular pressure.81, 82 Masera and co workers used 390 rads during a 22-day period to induce clinical resolution. Fonken and Ellis18 used 250 rads over 5 days to treat iris infiltration, glaucoma, and leukaemic retinopathy. Clinically they were successful, but eyes that obtained post-mortem 2 months later showed leukaemic infiltrates.
Decreased vision with leukaemic infiltration of the optic nerve is an ophthalmic emergency.83 Various authors have used doses ranging from 700 to 2000 rads with success.35 They suggested that invasion of the retrobulbar optic nerve is much more devastating for the vision than leukaemic invasion of the disc, although both may coexist. Orbital involvement causes a poor prognosis despite radiation and chemotherapy. Conjunctival leukaemic infiltration responds well to systemic chemotherapy.84 A prompt recognition of the ocular manifestation and their importance as a sign of possible extramedullary disease is crucial if appropriate therapy is to be initiated.
Conclusion
In this review, we have given a systematic account of the ocular manifestations of leukaemia. We have discussed the pathogenesis and pathophysiology of different types of leukaemic ocular involvement including the side effects of its treatment. It is sometimes difficult for the physician to fully appreciate how frequently leukaemia involves the eye, probably because many patients remain asymptomatic in the earlier stages of ocular involvement.
Numerous intra-ocular and extra-ocular changes may be rare, but can still be of prognostic significance. All of the main manifestations of leukaemic involvement can be explained on the basis of anaemia, hypoxia, blood viscosity, compression by masses of cells, or direct tumour infiltration. Various other effects occur from opportunistic infection or from therapeutic procedures, such as chemotherapy, radiotherapy, or bone marrow transplantation.
Although the ophthalmologist has a secondary role in the treatment of leukaemias, a prompt recognition of the ocular manifestations and their importance as a sign of possible extramedullary disease is crucial if appropriate therapy is to be initiated. We suggest that full collaboration among physicians, oncologists, and ophthalmologists is needed, with prompt ophthalmic assessment of patients suspected to have eye manifestations.
References
Reddy SC, Menon BS . A prospective study of ocular manifestations in childhood acute leukaemia. Acta Ophthalmol Scand 1998; 76: 700–703.
Curto Mlo, Zingone A, Aquaviva A, Bagnulo S, Calculli L, Cristiani L et al. Leukaemic infiltration of the eye: results of therapy in a retrospective multicentric study. Med Pediatr Oncol 1989; 17: 134–139.
Ohkoshi K, Tsiaras WG . Prognostic importance of ophthalmic manifestations in childhood leukaemia. Br J Ophthalmol 1992; 76: 651–655.
Macintyre E, Flandrin G . Biological classification of acute leukemias: federalization or centralization? Leukemia 1995; 9: 2152–2154.
Willman CL . Acute leukemias: a paradigm for the integration of new technologies in diagnosis and classification. Mod Pathol 1999; 12: 218–228.
Duke-Elder S . System of ophthalmology. Cornea and conjunctiva, Vol V111. CV Mosby: St Louis, 1965; 1190–1195.
Allen RA, Straatsma BR . Ocular involvement in leukaemia and allied disorders. Arch Ophthalmol 1961; 66: 490–508.
Swartz M, Jampol LM . Comma-shaped venular segments of conjunctiva in chronic granulocytic leukaemia. Can J Ophthalmol 1975; 10: 458–461.
Cook BE J, Bartley GB . Acute lymphoblastic leukaemia manifesting in an adult as a conjunctival mass. Am J Ophthalmol 1997; 124: 104–105.
Lei KI, Liew CT, Lam DS, Chan AT, Wickham NW . Acute monoblastic leukaemia with conjunctival tumours. Clint Once (R Cull Radial) 1995; 7: 405–406.
Bhadresa GN . Changes in the anterior segment as a presenting feature in leukaemia. Br J Ophthalmol 1971; 55: 133–135.
Spraul CW, Lang GE, Lang GK . Corneal ulcer in chronic graft-versus-host disease: treatment with collagen shields. Klin Monatsbl Augenheilkd 1994; 205: 161–166.
Jabs DA, Hirst LW, Green WR, Tutschka PJ, Santos GW, Beschorner WE . The eye in bone marrow transplantation. II. Histopathology. Arch Ophthalmol 1983; 101: 585–590.
Mondino BJ, Brown SI, Mondzelewski JP . Peripheral corneal ulcers with herpes zoster ophthalmicus. Am J Ophthalmol 1978; 86: 611–614.
Perry HD, Mallen FJ . Iris involvement in granulocytic sarcoma. Am J Ophthalmol 1979; 87: 530–532.
Wolintz AH, Goldstein JH, Seligman BR, Rosner F, Wesely AC, Lee SL . Secondary glaucoma in leukaemia. Ann Ophthalmol 1971; 3: 1211–1213.
Rowan PJ, Sloan JB . Iris and Anterior chamber involvement in leukaemia. Ann Ophthalmol 1976; 8: 1081–1085.
Fonken HA, Ellis PP . Leukaemic infiltrates in the iris. Arch Ophthalmol 1966; 76: 32–36.
Martin B . Infiltration of the iris in chronic lymphatic leukaemia. Br J Ophthalmol 1968; 52: 781–785.
Ayliffe W, Foster CS, Marcoux P, Upton M, Finkelstein M, Legmann A . Relapsing acute myeloid leukaemia manifesting as hypopyon uveitis. Am J Ophthalmol 1995; 119: 361–364.
Jankovic M, Conter V, Pretto G, Placa F, D’Incalci M, Masera G . Isolated bilateral anterior chamber eye relapse in a child with acute lymphoblastic leukaemia. Med Pediatr Oncol 1995; 25: 109–112.
MacLean H, Clarke MP, Strong NP, Kernahan J . Primary relapse of acute leukaemia in anterior segment of the eye. Lancet 1995; 346: 500.
Soylu M, Ozdemir G, Anli A . Pediatric uveitis in southern Turkey. Ocul Immunol Inflamm 1997; 5: 197–202.
Garrido Colino C, Mateos Gonzalez M, Torres Valdivieso M, Lopez Perez J, Melero Moreno C, Vivanco Martinez J . Ocular infiltration in the anterior chamber in a female infant with acute non-lymphoblastic leukaemia. Anales Espanoles de Pediatria 2001; 55: 69–72.
Newman NM, Smith ME, Gay AJ . An unusual case of leukaemia involving the eye: a clinico-pathological study. Surv Ophthalmol 1972; 16: 316–321.
Burns CA, Blodi FC, Williamson BK . Acute lymphocytic leukaemia and central serous retinopathy. Trans Am Acad Ophthalmol Otolaryngol 1965; 69: 307–309.
Zimmerman LE, Thoreson HT . Sudden loss of vision in acute leukaemia. A clinico-pathological report of two unusual cases. Surv Ophthalmol 1964; 9: 467–473.
Hine JE, KIngham JD . Myelogenous leukaemia and bilateral exudative retinal detachment. Ann Ophthalmol 1979; 11: 1867–1872.
Gass JDM . Differential Diagnosis of Intraocular Tumours: A Stereoscopic Presentation. CV Mosby: St Louis, 1974, pp 160–176.
Alemayehu W, Shamebo M, Bedri A, Mengistu Z . Ocular manifestations of leukaemia in Ethiopians. Ethiop Med J 1996; 34: 217–224.
Ballantyne AJ, Michaelson IC . Textbook of the Fundus of the Eye. Williams and Wilkins: Baltimore, 1970; 290–292.
Kuwabara T, Aiello L . Leukaemic military nodule in retina. Arch Ophthalmol 1964; 72: 494–497.
Primack JD, Smith ME, Tychsen L et al. Retinal detachment in a child as the first sign of leukemic relapse. J Pediatr Ophthalmol Strabismus 1995; 32: 253–256.
Schworm HD, Nasemann JE, Schriever S . Subretinal hypopyon in prolymphocytic leukaemia. Br J Ophthalmol 1995; 79: 863–864.
Kincaid MC, Green WR . Ocular and orbital involvement in Leukaemia. Surv Ophthalmol 1983; 27: 211–233.
Jampol LM, Goldberg MF, Busse B . Peripheral retinal micro aneurysms in chronic leukaemia. Am J Ophthalmol 1975; 80: 242–248.
Frank RN, Ryan Jr SJ . Peripheral retinal neovascularistation with chronic myelogenous leukaemia. Arch Ophthalmol 1972; 87: 585–589.
Reese AB, Guy L . Exophthalmos in leukaemia. Am J Ophthalmol 1933; 16: 718–720.
Swartz M, Schumann GB . Acute leukaemic infiltration of the vitreous diagnosed by pars plana aspiration. Am J Ophthalmol 1980; 90: 326–330.
Zhioua R, Boussen I, Malek I, Ouertani A . Acute lymphoblastic leukaemia and vitreous infiltration. A case study. J Francais Ophtalmol 2001; 24: 180–182.
Shaw RK, Moore EW, Freireich EJ, Thomas LB . Meningeal leukaemia. Neurology 1960; 10: 823–833.
Ridgway EW, Jaffe N, Walton DS . Leukemic ophthalmopathy in children. Cancer 1976; 38: 1744–1746.
Dawson DM, Rosenthal DS, Moloney WC . Neurologic complications of acute leukaemia in adults: changing rates. Ann Int Med 1973; 79: 541–544.
Hyman CB, Bogle JM, Brubaker CA, Williams K, Hammond D . Central nervous system involvement by leukaemia in children. Blood 1965; 25: 1–12.
Brown GC, Shields JA, Augsburger JJ, Serota FT, Koch P . Leukemic optic neuropathy. Int Ophthalmol 1981; 3: 111–116.
Rosenthal AR, Egbert PR, Wilbur JR, Probert JC . Leukemic involvement of optic nerve. J Ped Ophthalmol 1975; 12: 84–93.
Ellis W, Little HL . Leukaemic infiltration of the optic nerve head. Am J Ophthalmol 1973; 75: 867–871.
Colombini A, Barzaghi A, Castagneto M, Bovo G, Rossi MR, Rovelli A et al. Retro-orbital late relapse in a child with leukaemia after allogeneic bone marrow transplantation. Acta Haematol 1995; 94: 44–47.
Cavdar AO, Gozdasoglu S, Arcasoy A, Demirag B . Chloroma-like ocular manifestations in Turkish children with acute myelo-monocytic leukaemia. Lancet 1971; 1: 680–682.
Jakobiec FA, Jones IS . Lymphomatous plasmacytic, histiocytic, haematopoietic tumours. In: Jones IS, Jakobiec FA (eds). Diseases of the Orbit. Hagerstown Md, Harper and Row, 1979, pp 309 (see pp 345-348).
Esmaeli B, Medeiros LJ, Myers J, Champlin R, Singh S, Ginsberg L . Orbital mass secondary to precursor T-cell acute lymphoblastic leukemia: a rare presentation. Arch Ophthalmol 2001; 119: 443–446.
Kajimoto H, Naya M, Hojo M, Hibi S, Imashuku S . Relapse of acute lymphoblastic leukaemia as a retro-orbital mass (review). Jpn J Clin Hematol 1999; 40: 1193–1197.
Hsu LY, Jou JR, Tseng HS, Shiue C, Hou PK, Lin KH . Relapse of acute leukemia in childhood presenting as proptosis due to an orbital mass. J Formos Med Assoc 1997; 96: 835–838.
Lakhkar BN, Banavali S, Philip P . Orbital granulocytic sarcoma in acute myelogenous leukaemia. Indian J Pediatr 2000; 67: 234–235.
Cullis CM, Hines DR, Bullock JD . Anterior segment ischaemia: classification and description in chronic myeloid leukaemia. Ann Ophthalmol 1979; 11: 1739–1744.
Shibata K, Shimamoto Y, Nishimura T, Okinami S, Yamada H, Miyahara M . Ocular manifestation in adult T cell leukaemia/lymphoma. Ann Hematol 1997; 74: 163–168.
Yaghouti F, Nouri M, Mannor GE . Ocular adnexal granulocytic sarcoma as the first sign of acute myelogenous leukaemia relapse. Am J Ophthalmol 1999; 127: 361–363.
Lever WF . Schaumburg. Histopathology of skin, 5th ed. JB, Lippincott: Philadelphia, 1975, pp 705–707.
Cogan DG . Immunosuppression and eye disease. Am J Ophthalmol 1977; 83: 777–788.
Meredith TA, Aaberg TM, Reeser FH . Rhegmatogenous retinal detachment complicating CMV retinitis. Am J Ophthalmol 1979; 87: 793–796.
Walton RC, Reed KL . Herpes zoster ophthalmicus following bone marrow transplantation in children. Bone Marrow Transplant 1999; 23: 1317–1320.
Al-Rashid RA, Cress C . Mumps uveitis complicating the course of leukaemia. J Pediatr Ophthalmol. 1977; 14: 100–102.
Nerkelun S, Kellermann S, Nenning H . Acute blindness caused by fungal infection in chronic myeloid leukaemia. Klin Manatslbl Augenhertkd 1997; 211: 272–274.
Carta A, Cesana C . Ocular presentation and successful outcome of invasive sphenoid sinus aspergillosis in acute myelogenous leukaemia. Haematologica 1998; 83: 1116–1119.
Giagounidis AA, Meckenstock G, Flacke S, Burk M, Wehmeier A, Aul C et al. Pseudamonas aeruginosa blepharoconjunctivitis during cytoreductive chemotherapy in a woman with acute lymphocytic leukaemia. Ann Hematol 1997; 75: 121–123.
Morgan KW, Callen JP . Sweet's syndrome in acute myelogenous leukaemia presenting as periorbital cellulitis with an infiltrate of leukemic cells. J Am Acad Dermatol 2001; 45: 590–595.
Yoken J, Forbes B, Maguire AM, Prenner JL, Carpentieri D . Microsporidial endophthalmitis in a patient with acute myelogenous leukaemia. Retina 2002; 22: 123–125.
Ravindranathan MP, Paul VJ, Kuriakose ET . Cataract after busulphan treatment. Br Med J 1972; 1: 218–219.
Albert DM, Wong VG, Henderson ES . Ocular complications of vincristine therapy. Arch Ophthalmol 1967; 78: 709–713.
Shurin SB, Rekate HL, Annable W . Optic atrophy induced by vincristine. Pediatrics 1982; 70: 288–291.
Hopen G, Mondino BJ, Johnson BL, Chervenick PA . Corneal toxicity with systemic cytarabine. Am J Ophthalmol 1981; 91: 500–504.
Stentoft J . The toxicity of cytarabine. Drug Saf 1990; 5: 7–27.
Antunes NL, Small TN, George D, Boulad F, Lis E . Posterior leukoencephalopathy syndrome may not be reversible. Pediatr Neurol 1999; 20: 241–243.
Claes K, Kestelyn P . Ocular manifestations of graft versus host disease following bone marrow transplantation. Bull Soc Belge Ophtalmol 2000; 77: 21–26.
Kasmann B, Ruprecht KW . Ophthalmologic findings in graft versus host disease (GvHD). Klin Monatsbl Augenheilkd 1993; 202: 491–499.
Frisk P, Hagberg H, Mandahl A, Soderberg P, Lonnerholme G . Cataract in children after autologous bone marrow transplantation. A common but curable complication. Lakartidningen 2002; 99: 1444–1447.
Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE et al. Chronic graft vs host disease in 52 patients: adverse natural course and successful treatment with combination immunosuppression. Blood 1981; 57: 267–276.
Ng JS, Lam DS, Li CK, Chik KW, Cheng GP, Yuen PM et al. Ocular complications of pediatric bone marrow transplantation. Ophthalmology 1999; 106: 160–164.
Webster AR, Anderson JR, Richard EM, Moore AT . Ischaemic retinopathy occurring in patients receiving bone marrow allografts and campath-1G: a clinicopathological study. Br J Ophthalmol 1995; 79: 687–691.
O’Rourke JF, O’Connor GR . The eye as a sanctuary in acute lymphoblastic leukaemia. Lancet 1980; 1: 452–453.
Ninane J, Taylor D, Day S . Leukaemic hypopyon in acute lymphoblastic leukaemia after interruption of treatment. Arch Dis Child 1979; 54: 73–74.
Masera G, Carnelli V, Uderzo C, Toselli C, Lasagni F, Lambertenghi E . Ocular involvement in leukaemia: Report of three cases. Lancet 1977; 1: 829–831.
Murray KH, Paolino F, Goldman JM, Galton DA, Grindle CF . Optic nerve head infiltration in acute leukemia in children: an indication for emergency optic nerve radiation therapy. Med Pediatr Oncol 1996; 26: 101–104.
O’Rourke JF, O’Connor GR . Unusual ocular involvement in acute leukaemia. Arch Ophthalmol 1957; 57: 585–589.
Acknowledgements
We thank Mr TTQ Reuser and Miss Marie Tsaloumas for their help with the clinical photographs, used in the article.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sharma, T., Grewal, J., Gupta, S. et al. Ophthalmic manifestations of acute leukaemias: the ophthalmologist's role. Eye 18, 663–672 (2004). https://doi.org/10.1038/sj.eye.6701308
Published:
Issue Date:
DOI: https://doi.org/10.1038/sj.eye.6701308
Keywords
This article is cited by
-
Detection of Retinal Microvascular Changes with Optical Coherence Tomography Angiography in Patients with Acute Leukemia Without Retinopathy
Ophthalmology and Therapy (2024)
-
Optic neuropathy associated with GVHD after bone marrow allogeneic hematopoietic stem cell transplantation for B-Acute lymphoblastic leukemia: case report
BMC Ophthalmology (2022)
-
Leukemic retinopathy presenting as concurrent bilateral subhyaloid hemorrhage and subarachnoid hemorrhage in a patient with acute monocytic leukemia: a case report
Journal of Medical Case Reports (2022)
-
The recognition of oral manifestations of haematological disease saves lives: a case report
Bulletin of the National Research Centre (2022)
-
Relapsed acute myeloid leukemia presenting as conjunctival myeloid sarcoma: a case report
BMC Ophthalmology (2022)