-
PDF
- Split View
-
Views
-
Cite
Cite
Morten F. Gjerstorff, Kirsten Kock, Ole Nielsen, Henrik J. Ditzel, MAGE-A1, GAGE and NY-ESO-1 cancer/testis antigen expression during human gonadal development, Human Reproduction, Volume 22, Issue 4, April 2007, Pages 953–960, https://doi.org/10.1093/humrep/del494
Close - Share Icon Share
Abstract
Cancer/testis antigens (CTAs) are expressed in several cancers and during normal adult male germ cell differentiation. Little is known about their role in fetal development of human germ cells.
We examined expression of the CTAs MAGE-A1, GAGE and NY-ESO-1 in fetal gonads by single and double immunohistochemical staining.
We found that GAGE was expressed in the primordial germ cells of the gonadal primordium, whereas MAGE-A1 and NY-ESO-1 were first detected in germ cells of both testis and ovary after sexual differentiation was initiated. The number of positive germ cells and the staining intensity of all three CTAs peaked during the second trimester and gradually decreased towards birth in both male and female germ cells. In oocytes, MAGE-A1 expression terminated around birth, whereas NY-ESO-1 expression persisted through the neonatal stage and GAGE expression was maintained until adulthood. The population of GAGE-expressing male and female germ cells partially overlapped the population of OCT4-positive cells, whereas MAGE-A1 and NY-ESO-1 were clearly expressed only by OCT4-negative cells.
Our results suggest that MAGE-A1 and NY-ESO-1 are associated with highly proliferating germ cells, whereas GAGE proteins have a more general function in germ cells unrelated to any specific developmental stage. The recognition of differential cellular expression of GAGE, MAGE-A1, NY-ESO-1 and OCT4 may help define biologically distinct germ cell subpopulations.
Introduction
Cancer/testis antigens (CTAs) are comprised of at least 44 distinct families that are traditionally considered to be expressed only in normal adult testis germ cells and in different types of cancer. Most CTA genes are located in clusters at chromosome X, comprising multiple genes of high identity (e.g. MAGE-A, GAGE and SSX), whereas others exist as autosomally encoded single genes (Simpson et al., 2005).
Spermatogonia are testicular stem cells that can proliferate throughout life to maintain the stem cell pool or undergo differentiation to produce spermatozoa. This process includes two meiotic divisions from tetraploid primary spermatocytes to diploid secondary spermatocytes to haploid spermatids that, in turn, develop into spermatozoa (Sadler, 1985). Many CTAs, including most of the chromosome X-encoded CTAs, such as MAGE-A, GAGE and NY-ESO-1, are found in spermatogonia and primary spermatocytes, whereas a smaller number of CTAs have been identified in the more differentiated stages of spermatogenesis (Simpson et al., 2005).
The functional biology of germ cells and tumour cells remains to be determined for most CTAs, although clues have emerged indicating that several members of the MAGE family (MAGE-A1 and -A4) interact with the nuclear proteins SKIP and histone deacetylase 1, thereby inhibiting transcriptional activation mediated by Notch-IC (Laduron et al., 2004). Moreover, MAGE-A2 was observed to down-regulate p53 transactivation by recruiting histone deacetylase 3 (Monte et al., 2006). GAGE proteins have been shown to localize to the nucleus of a subset of spermatogonia and cancer cells, indicating that GAGE may also be a regulator of gene expression (Gjerstorff et al., 2006).
The primordial germ cells (PGCs) are the progenitors of both male and female germ cells, and they migrate from the dorsal yolk sac via the dorsal mesentery to arrive at the indifferent gonad during the fifth week of gestation (Burger and de Kretser, 1981). In the male gonad, the PGCs become enclosed by Sertoli cells in the seminiferous cords, where they acquire a different morphology and become gonocytes. The gonocytes proliferate and turn into prospermatogonia, which then arrest in the G0/G1 phase until spermatogenesis is initiated at puberty (Burger and de Kretser, 1981). In the female gonad, the PGCs are the progenitors of oogonia, which increase in number during the first and second trimesters by mitotic cell divisions. This proliferation coincides with a major wave of cell degeneration by apoptosis (McGee et al., 1998; McGee, 2000). At the end of the first trimester, the epithelial precursors of human granulosa cells begin to encircle the oogonia, and at birth all female germ cells are enclosed in primordial follicles. During the second trimester, the oogonia develop into oocytes when they initiate meiosis to arrest in the prophase of the first meiotic division. These cells constitute the resting pool of adult female germ cells and will complete the meiotic division just prior to ovulation (Sadler, 1985).
Little is known about the complex processes of human sex determination and gonadal differentiation, although it has been shown that human fetal germ cells are heterogeneous in terms of morphology and marker expression (Wartenberg et al., 1998; Gaskell et al., 2004). To enhance our understanding of the maturation process, the identification of further markers that can distinguish between germ cells with distinct biological properties is needed. Observations of MAGE-A4 and NY-ESO-1 expression in fetal gonocytes (Satie et al., 2002; Rajpert-De Meyts et al., 2003; Gaskell et al., 2004; Pauls et al., 2006; Rajpert-De Meyts, 2006) have suggested that at least some CTAs play a role in the development and differentiation of fetal germ cells, but a systematic evaluation of CTAs in fetal gonads has never been conducted.
In this study, we characterized the protein expression patterns of three different types of CTAs, e.g. MAGE-A1, GAGE and NY-ESO-1, in gonads of first and second trimester fetuses. Using immunohistochemical double staining, the cellular expression of these proteins was further correlated to the expression of the pluripotency marker OCT4 and to the gonadal parenchymal cell markers p450scc and steroidogenic factor 1 (SF-1).
Materials and methods
Specimens
Tissues were obtained from abortion specimens collected for diagnostic purposes and deposited in the tissue bank of Odense University Hospital. The tissues included were indifferent human embryonic gonads (weeks 6 and 8), fetal testis [weeks 9 (n = 1), 12 (n = 1), 13 (n = 1), 14 (n = 3), 15 (n = 1), 16 (n = 2), 17 (n = 2), 18 (n = 2), 19 (n = 2), 20 (n = 2), 21 (n = 1), 22 (n = 2), 23 (n = 2) and 27 (n = 1)] and fetal and neonatal ovaries [weeks 13 (n = 1), 17 (n = 1), 18 (n = 1), 19 (n = 3), 22 (n = 2), 28 (n = 1), 29 (n = 1), 34 (n = 1), 38 (n = 1), 39 (n = 1) and 41 (n = 1)]. The study was approved by the ethical committee of Funen and Vejle County (VF20050069).
True fetal age was deduced from the gestational age (e.g. the time after the first day of the last period), supplemented with physiological data (e.g. fetal weight, fetal length, organ weight, etc.).
Immunohistochemistry
Tissue biopsies were fixed in 4% formaldehyde (pH 7.4) for 24 h and paraffin-embedded. Sections of tissues were cut, deparaffinized, treated with 1.5% H2O2 in Tris-buffered saline (TBS; pH 7.5) for 10 min to block endogenous peroxidase activity, rinsed in distilled H2O, demasked, processed for antigen retrieval and washed in TNT buffer (0.1 M Tris, 0.15 M NaCl, 0.05% Tween-20, pH 7.5). A panel of antigen retrieval protocols was initially evaluated, including microwave boiling for 15 min in (i) T-EG buffer (10 mM Tris, 0.5 mM EGTA, pH 9.0), (ii) 10 mM citrate buffer, pH 6.0 or (iii) Dako Target retrieval solution (Dako S1699), or proteolytic treatment using (iv) 0.05% protease type XIV (pronase E, Sigma; cat. no. P5147) in TBS, pH 7.4 for 15 min at 37°C or (v) 0.4% pepsin (Sigma; cat. no. P7012) in 0.01 M HCl for 20 min at 37°C. Microwave boiling in T-EG buffer for 15 min proved to be the optimal antigen retrieval method for anti-GAGE, anti-MAGE-A1 and anti-NY-ESO-1 mAbs and was used in the successive experiments. Sections were subsequently incubated with anti-GAGE mAb M3 [1/100; produced in-house (Gjerstorff et al., 2006)], anti-GAGE-7 mAb (1/2000; Clone 26, BD Biosciences), anti-MAGE-A1 mAb (1/200; Clone MA454, Lab Vision Corporation, Newmarket Suffolk, UK), anti-NY-ESO-1 mAb (1/25; Clone E978, Zymed Laboratories Inc., San Francisco, CA, USA), anti-p450scc pAb (1/8.000; Corgen Inc., Taipei, Taiwan), anti-SF-1 pAb (1/500; a gift from Professor Ken-Ichirou Morohashi, National Institute of Natural Sciences, Aichi, Japan) or anti-OCT3/4 mAb (1/400; Clone C10, Santa Cruz Biotechnology, Heidelberg, Germany) diluted in antibody diluent (S2022, DAKO, Glostrup, Denmark) for 1 h at room temperature. Sections were washed with TNT buffer and incubated with horse-radish peroxidase (HRP)-conjugated ‘Ready-to-use’ EnVision™ + polymer K4001 (mouse mAb) or polymer K4003 (rabbit pAb) (DAKO) for 30 min, followed by another wash with TNT buffer. The final reaction product was visualized by incubating with 3,3′-diaminobenzidine (DAB) + substrate–chromogen (DAKO) for 10 min, followed by washing with H2O and counterstaining of sections with Mayers hematoxylin before mounting in AquaTex (Merck Inc., Whitehouse Station, NJ, USA). A significant portion of the specimens were examined twice with similar results. For each experiment, samples of either an isotype-matched antibody or no primary antibody were included as controls. For the immunohistochemical double staining, antigen retrieval and primary antibody incubations were carried out as above. The first layer primary antibody incubation was followed by incubations with alkaline phosphatase conjugated PowerVision polymer (ImmunoVision Technologies, Brisbane, CA, USA) for 30 min and Vector Blue substrate-chromogen (Vector Laboratories, Burlingame, CA, USA) for 20 min, whereas the second layer primary antibody was followed by incubations with HRP-conjugated EnVision polymerTM ± K4001 (mouse mAb) or K4003 (rabbit pAb) (DAKO) for 30 min and DAB ± substrate-chromogen (DAKO) for 10 min. A 15 min microwave boiling in T-EG buffer was carried out between the two antibody-staining sequences to block antibody cross-reactivity. Stained sections were mounted in AquaTex (Merck Inc.).
Morphometric quantification
The frequencies of GAGE-, MAGE-A1- or NY-ESO-1-positive female germ cells in third trimester and neonatal specimens were calculated as the mean of the frequencies of positive cells (number of positive cells/number of germ cells) in three to five randomly chosen microscope frames using the × 20 microscope lens.
Results
Protein expression of MAGE-A1, NY-ESO-1 and GAGE was evaluated by immunohistochemistry on indifferent human embryonic gonads (6–8 weeks), fetal testis (9–27 weeks) and fetal and neonatal ovaries (13–41 weeks).
GAGE expression in PGCs of the indifferent gonad
In the gonadal primordium of a 6-week-old embryo, PGCs derived from the dorsal yolk sac exhibited GAGE expression (Figure 1a), whereas no MAGE-A1 and NY-ESO-1 expression was observed. At 8 weeks, the PGCs, now associated with the primitive sex cords, remained GAGE-positive (Figure 1b) and MAGE-A1- and NY-ESO-1-negative (Figure 1c and d). No MAGE-A1, GAGE and NY-ESO-1 staining was observed in the gonadal mesenchymal cells. At 6 and 8 weeks, only a subset of the PGCs was GAGE-positive and varied in staining intensity.
Expression of GAGE in primordial germ cells (PGCs). GAGE was expressed in the PGCs of the gonadal primordium of 6-(a) and 8-week-old (b) embryos. MAGE-A1 (c) and NY-ESO-1 (d) were not detected in the embryonal gonad. No staining was observed using the negative control antibody (Magnification: × 40).
Ontogenic expression of MAGE-A1, GAGE and NY-ESO-1 in human testis
Germ cell expression of MAGE-A1, GAGE and NY-ESO-1 was further examined in first, second and third trimester fetal testis (Figure 2). At week 9, germ cells were strongly positive for all three CTAs (Figure 2a–c), but while GAGE expression was continuously strong throughout the fetal period, MAGE-A1 and NY-ESO-1 expression was absent (weeks 12, 14 and 15) or weak (weeks 13 and 14) in the beginning of the second trimester (Figure 2d–f). In the middle and end of the second trimester, a peak in staining intensity for MAGE-A1 and NY-ESO-1 (weeks 16, 17, 18 and 20) was followed by a reduction in the number of positive cells and in the intensity of cellular staining (weeks 21, 22 and 23) (Figure 2g–l). A third trimester specimen (27 weeks) exhibited GAGE and MAGE-A1 but not NY-ESO-1 expression. The subcellular localization of MAGE-A1 and NY-ESO-1 staining in fetal male germ cells was primarily cytoplasmic, whereas GAGE was localized to both the cytoplasm and the nucleus.
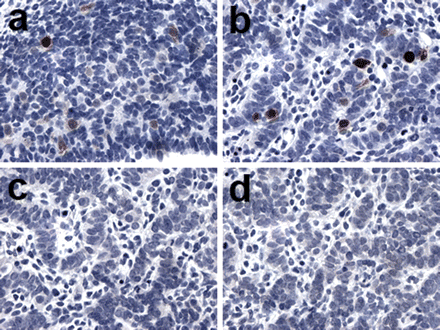
Expression of GAGE, MAGE-A1 and NY-ESO-1 in fetal testis. GAGE (a, d, g and j), MAGE-A1 (b, e, h and k) and NY-ESO-1 (c, f, i and l) were detected in a subset of testicular germ cells of 9–22-week-old fetuses. A peak in expression was observed at weeks 16–20, whereas MAGE-A1 and NY-ESO-1 expression was significantly reduced by week 22. Double immunohistochemical staining combining GAGE (DAB/brown stain) and p450scc (Vector Blue/blue stain) (m) or Steroidogenic factor 1 (Vector Blue/blue stain) (n) showed that GAGE was also expressed in some Sertoli and Leydig cells during weeks 16–20. No staining was observed using the negative control antibody [Magnification: × 40 (a–c, m and n), × 10 (d–l)].
Interestingly, we found that most Sertoli cells and a subset of the interstitial Leydig cells, in addition to the prospermatogonia, were GAGE-positive at weeks 14–20 (Figure 2g), whereas these cells were GAGE-negative at all other ages. The GAGE staining intensity in Sertoli and Leydig cells was less pronounced than that of the germ cells and was confined to the cytoplasm. No expression of MAGE-A1 and NY-ESO-1 was ever observed in Sertoli and Leydig cells, and the cells of the connective tissue were negative for all three CTAs in all sections. The identity of the GAGE-positive Leydig and Sertoli cells was confirmed by immunohistochemical double staining, showing that the GAGE-expressing cells also expressed p450scc (Figure 2m) and SF-1 (Figure 2n).
Ontogenic expression of MAGE-A1, GAGE and NY-ESO-1 in human ovary
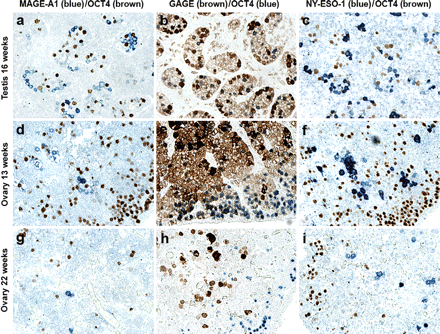
Since male and female germ cells are derived from the same progenitor cells and undergo similar prenatal proliferation and differentiation processes, we examined whether CTAs were also expressed in early female germ cells. Second trimester ovary sections (13, 17, 18, 19 and 22 weeks) were found to contain MAGE-A1-, GAGE- and NY-ESO-1-positive germ cells (Figure 3). Strong expression of all CTAs was observed in most cells at week 13 (Figure 3a–c), whereas both the number of cells positive for MAGE-A1 and NY-ESO-1 and the staining intensity of the positive germ cells were clearly reduced at weeks 17, 18, 19 and 22 compared with week 13 (Figure 3e, f, h and i). In contrast, GAGE expression remained unchanged in the number of positive cells and the staining intensity (Figure 3d and g). Similar to male germ cells, GAGE staining of female germ cells was localized to the cytoplasm and the nucleus, whereas MAGE-A1 and NY-ESO-1 staining was detected only in the cytoplasm (Figure 3). Female germ cells, exhibiting strong expression of CTAs, were often located in clusters (Figure 3a–c).
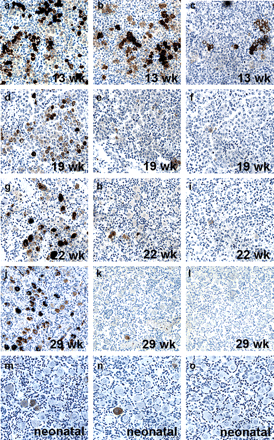
Expression of GAGE, MAGE-A1 and NY-ESO-1 in the developing fetal ovary. GAGE (a, d, g and j), NY-ESO-1 (b, e, h and k) and MAGE-A1 (c, f, i and l) were detected in a subset of germ cells in the ovary of 13–29-week-old fetuses. Expression of all three CTAs was strongest at 13 weeks, and while GAGE expression was detected in most germ cells up to week 29, MAGE-A1 and NY-ESO-1 expression was significantly reduced by the middle of second trimester. GAGE (m) and NY-ESO-1 (n) expression persisted in ∼11% and ∼3% of oocytes of neonatal primordial follicles, respectively, whereas no or very few MAGE-A1-positive oocytes (<1%) were detected either at this stage (o) or in the third trimester specimens (l). No staining was observed using the negative control antibody (Magnification: × 10).
In the third trimester specimens (weeks 28 and 29), most germ cells (<95%) remained GAGE-positive, whereas ∼3% were NY-ESO-1-positive. A few MAGE-A1-positive oocytes (<1%) were detected at week 28, whereas the 29-week-old specimen was negative. Moreover, among the resting primordial follicles of neonatal ovaries (weeks 34, 38, 39 and 41), the number of GAGE-positive cells was reduced to ∼11%, while still ∼3% of oocytes were NY-ESO-1-positive (Figure 3j–o). In neonatal ovaries, MAGE-A1 was detected at 34 and 41 weeks (<1%), but not at 38 and 39 weeks.
Co-expression studies of OCT4 and MAGE-A1, NY-ESO-1 or GAGE in the normal fetal gonad
The pluripotency marker OCT4 has been shown to be expressed in migrating PGCs, and a large number of germ cells in both the testis and ovary remain OCT4-positive until the beginning of the second trimester, where the number of positive cells and the staining intensity decline (Rajpert-De Meyts et al., 2004). We compared the OCT4 immunolocalization to that of MAGE-A1, NY-ESO-1 and GAGE to identify germ cell subpopulations (Figure 4). As expected, a subset of germ cells of both second trimester male and female gonads was OCT4-positive (weeks 13–22), and the frequency of positive cells declined gradually with age. Double immunohistochemical staining revealed that a subset of the OCT4-positive germ cells in both male and female gonads also expressed GAGE (Figure 4b, e and h). Interestingly, not all GAGE-positive cells were OCT4-positive, demonstrating the existence of three different populations of cells: OCT4pos/GAGEpos, OCT4neg/GAGEpos and OCT4pos/GAGEneg. Double-staining combining OCT4 and MAGE-A1 or NY-ESO-1 staining clearly showed that these proteins were never expressed in the same germ cells (Figure 4a, c, d, f, g and i).
Identification of subpopulations of fetal germ cells by double immunostaining of OCT4 and MAGE-A1, GAGE or NY-ESO-1. The GAGE-positive cell population partially overlapped the population of OCT4-positive cells, allowing the identification of three subpopulations of germ cells: OCT4pos/GAGEpos, OCT4neg/GAGEpos and OCT4pos/GAGEneg (b, e and h). In contrast, dual staining combining OCT4- and MAGE-A1- or NY-ESO-1-reactivity clearly showed that OCT4 and MAGE-A1 or NY-ESO-1 were not co-expressed (a, c, d, f, g and i). No staining was observed using the negative control antibody (Magnification: × 10).
In ovaries, a clear difference in the macroscopic tissue distribution of OCT4-positive germ cells compared with germ cells positive for any of the three CTAs was observed. The OCT4-positive germ cells tended to be located in the peripheral part of the ovary, whereas the CTA-positive germ cells were located primarily in the central part of the ovary (Figure 4d–i).
Discussion
To enhance our understanding of the function of MAGE-A1, NY-ESO-1 and GAGE, members of three different subfamilies of CTAs, we evaluated their expression in human embryonic and fetal gonads.
In the gonadal primordium of the first trimester embryos (6 and 8 weeks), a subset of PGCs was strongly positive for GAGE, but not MAGE-A1 or NY-ESO-1. The PGCs migrate from the dorsal yolk sac and arrive at the indifferent gonad at the fifth week of gestation, where they reside in the sex cords (Sadler, 1985). Although not conclusive, the finding of GAGE-negative PGCs in the gonadal primordium together with the lack of GAGE-positive migrating PGCs suggests that PGCs do not express GAGE until embedded in the gonadal mesenchyme.
High GAGE expression was seen in a subset of both male and female germ cells throughout the second trimester. GAGE proteins were localized to both cytoplasm and nucleus, similar to the subcellular localization in spermatogonia of adult testis (Gjerstorff et al., 2006). GAGE expression was found to persist in ∼11% of primordial follicles of the neonatal ovary, and we previously observed GAGE expression in ∼30% of adult oocytes (Gjerstorff et al., 2006). The prolonged expression of GAGE in germ cells, e.g. PGCs to neonatal germ cells, indicates that this protein has a general function in germ cells and is not associated with a specific developmental step in germ cell differentiation.
In addition to germ cells, GAGE was also detected in both Leydig and Sertoli cells in 14–20-week-old testis. This was surprising, since the ontogeny of these cells differs from that of PGCs, although the exact origin of these three cell types in humans is controversial. The PGCs are known to be derived from cells of the yolk sac (Burger and de Kretser, 1981), whereas the coelomic epithelium or the mesonephos have been suggested as the source of Leydig cells (O'Shaughnessy et al., 2006). The coelomic epithelium also seems to give rise to the Sertoli cells (Karl and Capel, 1998), and the coordinated expression of GAGE in fetal Leydig and Sertoli cells may indicate a common origin. It is also possible that local factors of the gonadal parenchyma contribute to the coordinated expression of GAGE in germ, Leydig and Sertoli cells.
In contrast to GAGE, MAGE-A1 and NY-ESO-1 were first detected in testis at 9 weeks and in ovary at 13 weeks. However, in the fetal ovary, the lack of fetuses of age 8–13 weeks prohibited us from establishing the initial induction of MAGE-A1 and NY-ESO-1 expression. The number and staining intensity of MAGE-A1- and NY-ESO-1-positive cells was significantly reduced at the end of second trimester in both testis and ovary. The strong expression of these two proteins in the beginning of the second trimester correlates with the period of high proliferation of oogonia and gonocytes and the beginning of differentiation of these cells, and the subsequent reduction in expression indicates that MAGE-A1 and NY-ESO-1 expression is reduced as the oogonia and gonocytes differentiate and enter cell cycle arrest. Strongly stained female germ cells were often located in clusters, indicating that high expression of MAGE-A1, GAGE or NY-ESO-1 is a synchronized process in interconnected oogonia.
Analysis of third trimester and neonatal ovaries demonstrated a strong expression of GAGE in ∼95% and 11% of primary follicle oocytes, respectively. During this period, NY-ESO-1 was consistently expressed in ∼3% of primary follicle oocytes, while MAGE-A1 was detected in not more than a few cells per specimen (<1%) and not at all in some specimens. These data are in accordance with previous results showing that the adult ovary also contained oocytes positive for GAGE, whereas no adult oocytes were MAGE-A1- or NY-ESO-1-positive (Gjerstorff et al., 2006). Our results indicate that, at least in oocytes, MAGE-A1 is the first of these three CTAs to be turned off, while NY-ESO-1 expression persists through the neonatal stage and GAGE expression remains through adulthood (Figure 5). GAGE and NY-ESO-1 expression in oocytes of primordial follicles did not seem to correlate with any morphologically distinct subtype of follicles, and the characteristics of the GAGE/NY-ESO-1-positive oocytes remains to be elucidated.
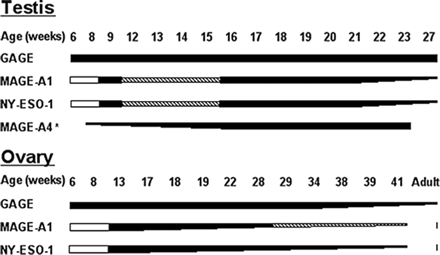
Sequential expression of CTAs during fetal development of human germ cells. Expression of GAGE, MAGE-A1, NY-ESO-1 and MAGE-A4 is initiated and terminated at different time points during development of fetal human germ cells. Black lines indicate CTA expression, a reduction in the thickness of the line indicates a reduction in CTA expression, dashed lines indicate infrequent detection of the antigen and white lines indicate no expression. *Data reported in Aubry et al. (2001).
Double immunohistochemical staining revealed that the GAGE-expressing population of germ cells in the second trimester gonads partially overlapped the population of OCT4-positive germ cells, showing that GAGE is expressed in both pluripotent germ cells and more differentiated germ cells. This observation is consistent with our finding that GAGE proteins were expressed in the PGCs of indifferent gonads, which are known to express high levels of OCT4 (Rajpert-De Meyts et al., 2004). MAGE-A1 and NY-ESO-1 expression was initiated later than GAGE expression and was clearly distinct from OCT4 expression, demonstrating that these two CTAs are only expressed in more differentiated stages of fetal germ cells. Similarly, an earlier report observed that MAGE-A4 was also expressed in fetal testis by a population of cells distinct from OCT4-positive cells. MAGE-A4 was identified in a small number of cells as early as at weeks 7–9, and the number of positive cells markedly increased from week 17 (Aubry et al., 2001; Gaskell et al., 2004). Thus, GAGE, MAGE-A1, NY-ESO-1 and MAGE-A4 seem to be sequentially expressed in fetal germ cells (Figure 5). In the adult testis, they have all been localized to the spermagonial cells, but whether they are also differentially expressed in the different known stages of spermatogonia remains to be determined.
In conclusion, we present for the first time a detailed analysis of MAGE-A1, GAGE and NY-ESO-1 expression in fetal testis, and we demonstrate that CTAs per se are also expressed in fetal ovary germ cells. Based on the time course of their expression, we found indications of the function of these proteins and provided markers that may help differentiate germ cell populations.
Acknowledgements
We would like to thank Lisbet Mortensen for her excellent technical assistance with the immunohistochemical staining, and M.K. Occhipinti-Bender for editorial assistance.



![Expression of GAGE, MAGE-A1 and NY-ESO-1 in fetal testis. GAGE (a, d, g and j), MAGE-A1 (b, e, h and k) and NY-ESO-1 (c, f, i and l) were detected in a subset of testicular germ cells of 9–22-week-old fetuses. A peak in expression was observed at weeks 16–20, whereas MAGE-A1 and NY-ESO-1 expression was significantly reduced by week 22. Double immunohistochemical staining combining GAGE (DAB/brown stain) and p450scc (Vector Blue/blue stain) (m) or Steroidogenic factor 1 (Vector Blue/blue stain) (n) showed that GAGE was also expressed in some Sertoli and Leydig cells during weeks 16–20. No staining was observed using the negative control antibody [Magnification: × 40 (a–c, m and n), × 10 (d–l)].](https://oup.silverchair-cdn.com/oup/backfile/Content_public/Journal/humrep/22/4/10.1093/humrep/del494/2/m_del49402.gif?Expires=1716501509&Signature=ahbSgmCMRf8mhAAIRWM3EhImWJvSGnK48qzCzmLvsFZl08PYzd0UovyNbGg5SJpnFB~mrg9n4RxLKT5aj2Z0OB0bLOh2MFILSfvQLRMpNOm8HtPLSvxiyS-CFPP5SPoMET8t7d3qR8QAuPrGl0rrG3uKtyhMUUfDev~erYeKNNcAxdhbhGIHG2hN0TN5PFkfhmPCO9qMB6AY9jsS1XwsZ7a~WvK995YQjQhxmuwIi57xQdhitC6Qp~yH2LYnZzHwl0B3fXE-jYZQO2635WsB54esaM79e35yLFXWUNeyB3G~VCMn0Ws3pSRZwpMBm3YptgAoVn61xTEqFEDf5ns9Mw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)