-
PDF
- Split View
-
Views
-
Cite
Cite
Luis A. Diaz, Ian Cheong, Catherine A. Foss, Xiaosong Zhang, Brock A. Peters, Nishant Agrawal, Chetan Bettegowda, Baktiar Karim, Guosheng Liu, Khalid Khan, Xin Huang, Manu Kohli, Long H. Dang, Paul Hwang, Ahava Vogelstein, Elizabeth Garrett-Mayer, Barry Kobrin, Martin Pomper, Shibin Zhou, Kenneth W. Kinzler, Bert Vogelstein, David L. Huso, Pharmacologic and Toxicologic Evaluation of C. novyi-NT Spores, Toxicological Sciences, Volume 88, Issue 2, December 2005, Pages 562–575, https://doi.org/10.1093/toxsci/kfi316
Close - Share Icon Share
Abstract
Clostridium novyi-NT (C. novyi-NT) spores have been shown to be potent therapeutic agents in experimental tumors of mice and rabbits. In the present study, pharmacologic and toxicologic studies were performed to better understand the factors influencing the efficacy and toxicity of this form of therapy. We found that spores were rapidly cleared from the circulation by the reticuloendothelial system. Even after large doses were administered, no clinical toxicity was observed in healthy mice or rabbits. The spores were also not toxic in mice harboring poorly vascularized non-neoplastic lesions, including myocardial infarcts. In tumor-bearing mice, toxicity appeared related to tumor size and spore dose, as expected with any bacterial infection. However, there was no laboratory or histopathologic evidence of sepsis, and the toxicity could be effectively controlled by simple hydration.
INTRODUCTION
Most solid tumors in humans contain relatively large areas of necrosis resulting from inadequate oxygenation and nutrition (Cerar et al., 2004). The low oxygen tensions within and immediately surrounding these necrotic regions make them susceptible to infection with anaerobic bacteria (Brown, 2002; Jain, 1994; Jain and Forbes, 2001). More than 50 years ago, Parker and colleagues were the first to evaluate anaerobic bacteria and their effects in mouse tumors. (Parker et al., 1947). In the 1950s and 1960s, the first clinical studies to evaluate this approach employed Clostridium butyricum, spores of which were injected into patients suffering from a variety of cancer types including glioblastoma, lung carcinoma, head and neck cancer, osteosarcoma, and malignant teratomas. Many patients had significant germination and destruction of large portions of their tumors, but difficulty managing toxicity and a lack of a complete clinical responses delayed further pursuit of such therapy (Heppner and Mose, 1978; Mose, 1963; Mose et al., 1967).
More recently, an attenuated strain of Salmonella typhimurium (VNP20009) has been used in a phase I clinical trial in the United States to treat metastatic melanoma and renal cell carcinoma (Toso et al., 2002). These studies failed to demonstrate significant clinical efficacy, presumably because of insufficient bacterial colonization in the tumors.
Our research group has been studying the therapeutic potential of spores derived from C. novyi-NT. C. novyi-NT is a clone derived from C. novyi after elimination of the major systemic toxin gene from the parental strain (Dang et al., 2001). C. novyi bacteria are exquisitely sensitive to oxygen (Topley, 1997). We have shown that intravenous injection of C. novyi-NT spores into animals bearing tumors leads to hemorrhagic necrosis exclusively in the tumor tissue (Dang et al., 2001). In immune competent mice or rabbits, the extensive destruction of tumors, coupled with an induced immune response, leads to complete eradication of tumors and cure in ∼30% of the animals (Agrawal et al., 2004). In the others, the tumor regrows from a well-vascularized rim that is resistant to C. novyi-NT infection. Cures can be achieved more often in mice by combining C. novyi-NT with chemotherapeutic agents or radiation, both of which target well-oxygenated cells more effectively than hypoxic cells (Bettegowda et al., 2003; Dang et al., 2001, 2004).
Oncolytic bacterial therapies can be considered purposeful attempts to convert tumors into infectious lesions. The resulting abscesses lead to the local and systemic toxicities that are expected to result from any serious infection. In our initial experiments with C. novyi-NT, we observed that 10–25% of animals with large tumors died after receiving therapeutic doses of C. novyi-NT spores (Dang et al., 2001). In the present study, we describe experiments to understand the basis for the toxicity and our attempts to control it.
MATERIALS AND METHODS
Cell lines and other reagents.
CT26 cells were purchased from The American Type Culture Collection and grown in McCoy's 5A Medium (Invitrogen, Carlsbad, CA) supplemented with 5–10% fetal bovine serum (FBS; HyClone, Logan City, UT) at 37°C with 5% CO2.
Animal models.
All experimental procedures were in compliance with United States laws governing animal experimentation and were approved and overseen by the Johns Hopkins University Animal Care and Use Committee. Six- to eight-week-old C57BL/6, BALB/c as well as outbred, athymic nu/nu mice were purchased from Harlan Breeders (Indianapolis, IN). Ten-week-old New Zealand White (NZW) rabbits were purchased from Myrtle's Rabbitry (Thompson Station, TN). Cage side and clinical observations of animals was performed using the “Standard Clinical Observation Criteria and Terms,” which are listed in Supplementary Data of the Supplementary Data online. Mice were euthanized by carbon dioxide inhalation. Rabbits were euthanized by an intracardiac injection of a lethal dose of phenobarbital (100 mg/kg) after intramuscular sedation with ketamine (50 mg/kg). In some experiments, Imipenem (Merck, White House Station, NJ) was injected intraperitoneally (ip), initiated with a single loading does of 60 mg/kg followed by 40 mg/kg ip injections every 12 h through day 4 (Traub, 1988). Tissues were dissected and preserved in buffered formalin (Sigma, St. Louis, MO). Selected tissues and tumors were fixed, embedded and stained using standard histologic methods. At least five mice per treatment group were used unless otherwise indicated in the Materials and Methods or Results.
Hypoxia models.
ApoE knockout (Piedrahita et al., 1992) or ApoE/LDLR double knockout mice (Ishibashi et al., 1994) in C57Bl/6J genetic backgrounds were obtained from the Jackson Laboratory (Bar Harbor, ME). In each model, at least two mice were evaluated after spore injection, and an equal number of mice were used as controls. These mice were fed normal chow (Harlan Teklab 18% Protein Diet), resulting in the development of aortic plaques by 18 weeks of age (Ishibashi et al., 1994; Zhang et al., 1994). To ensure that severe and chronic atheromatous lesions were present, mice older than 30 weeks of age were used. Myocardial infarction was induced in C57Bl/6J mice by suturing the anatomic course of the left anterior descending coronary artery one third of the length of the left ventricle from the apex, as previously described (Patten et al., 1998). Successful interruption of coronary blood flow was indicated by immediate blanching and bulging of the affected myocardium.
Sepsis models.
BALB/c mice bearing subcutaneous CT26 tumors were injected intravenously with 100 μg Escherichia coli–derived, phenol-extracted lipopolysaccharide (LPS; Sigma, St. Louis, MO) or intramuscularly with 5 × 108Staphylococcus aureus (#25923 isolate from the Johns Hopkins Hospital Microbiology Lab). To prepare S. aureus, Mueller Hinton broth with cations was inoculated with 0.01 volumes of an overnight bacterial culture and shaken vigorously at 37°C until mid-logarithmic phase was reached. The bacteria were washed five times with normal saline before injection. Lipopolysaccharide and S. aurerus were resuspended in normal saline prior to administration. Experiments were conducted with at least three mice per group.
Blood analysis.
Blood analysis was performed in the clinical pathology laboratory at Johns Hopkins Hospital. The following blood chemistries and blood cell counts were performed on all tested mice: phosphate, uric acid, sodium, chloride, blood urea nitrogen, glucose, serum creatinine, calcium, total protein, albumin, total bilirubin, alanine transaminase (ALT), aspartate aminotransferase (AST), alkaline phosphatase, CO2, white blood cell count, hematocrit, and platelet count. Potassium concentrations were not determined because the hemolysis that often occurred during blood collection made these measurements unreliable.
Tumor inoculation and spore administration.
Five million CT26 cells were injected subcutaneously into the right flank of each mouse. Tumor volume was calculated as length × width2 × 0.5. Mice were treated with C. novyi-NT spores when tumors reached ∼500 mm3 in size except when specifically indicated otherwise in the text. In general, 10–14 days were required for tumors to reach the target size. C. novyi-NT spores were prepared as described elsewhere (Cheong et al., in preparation, Dang et al., 2001). Rabbits were injected with 25–1250 × 106 spores/kg of C. novyi-NT suspended in 3 ml of normal saline (NS) via a vessel in the ear. Mice received 500–25,000 × 106 spores/kg suspended in 200 μl of phosphate buffered saline (PBS) or NS via the tail vein. Experiment-specific doses are provided in the text.
Tissue processing.
Tissues from all necropsied animals were preserved in neutral buffered 10% formalin solution (Sigma, St. Louis, MO). The following tissues/organs were evaluated: adrenals, aorta, bone marrow, bone (femur), brain, cecum, cervix, colon, duodenum, epididymis, esophagus, eye, fallopian tube, gallbladder, gross lesions, harderian gland, heart, ileum, injection site, jejunum, kidneys, lacrimal gland, larynx, liver, lungs, lymph nodes (cervical, mandibular, mesenteric), mammary gland, nasal cavity, optic nerves, ovaries, pancreas, parathyroid, peripheral nerve, pharynx, pituitary, prostate, rectum, salivary gland, sciatic nerve, seminal vesicles, skeletal muscle, skin, spinal cord, spleen, sternum, stomach, testes, thymus, thyroid, tongue, trachea, urinary bladder, uterus, vagina, and zymbal gland. A full set of tissues from animals in the high-dose and control groups, as well as any tissues with gross lesions in the other groups, were embedded in paraffin, sectioned, stained with hematoxylin and eosin (H&E) or with Gram-staining solution, and examined microscopically. When an abnormality in a specific tissue was observed histologically in the high-dose group, microscopic examination of the affected tissue from all the remaining dose groups was performed.
Tissue analysis.
Macroscopic and microscopic analyses were performed by a board certified veterinary pathologist (D.L.H). Liver lesions were graded as (1) background, occasional small foci of mononuclear cells; (2) minimal, less than 10% portal triads or centrilobular areas infiltrated with small numbers of mononuclear cells and occasional neutrophils; (3) mild, 10–50% of portal triads or centrilobular areas infiltrated by minor populations of mononuclear cells and neutrophils; rare, randomly distributed foci in parenchyma; (4) moderate, majority (>50%) of portal triads or centrilobular areas infiltrated; limited random foci in the parenchyma comprised of a mixture of neutrophils and mononuclear infiltrates; (5) severe, extensive PMN infiltrates affecting all periportal and centrilobular areas; extensive randomly distributed foci throughout parenchyma with some coalescence of foci.
Radiolabeling studies.
C. novyi-NT spores were radiolabeled with 125I-NaI using a modification of the Iodogen method for labeling cell membrane proteins (Fraker and Speck, 1978). In brief, ∼3 × 109 spores suspended in 1 ml of PBS were placed in a 4-ml glass vial containing 100 μg of coated Iodogen (Pierce, Rockford, IL). Immediately after the spores were added, 5 mCi of 125I-NaI (MP Biomedicals, Costa Mesa, CA) was added to the suspension, and the contents were gently mixed. The reaction proceeded for 15 min at ambient temperature with occasional mixing. The suspension was then transferred to a 1.5-ml sterile microcentrifuge tube and briefly centrifuged at 1000 × g to pellet the spores. The supernatant was removed and the spores were washed three times with PBS. The radiochemical yield was typically 80%, and the radiochemical purity was ∼95%.
Fluorescent labeling of spores.
For fluorescent labeling, 50 μl of 10 mM carboxyfluorescein diacetate succinimidyl ester (Molecular Probe, Eugene, OR0 was added to 2.5 × 109C. novyi-NT spores in a total volume of 0.5 ml. After vortexing, the suspension was incubated at room temperature for 1 h on a Labquake Rotisserie Shaker (Barnstead Thermolyne, Dubuque, IA). Unreacted fluorescein was removed by successive washes, and the spores were resuspended in PBS.
Gamma scintigraphy.
Mice were anesthetized through intraperitoneal administration of ketamine (72 mg/kg) plus acepromazine (6 mg/kg). Mice were then injected intravenously (iv) via the tail vein with 44–137 μCi (1.63–5.07 MBq, 3 × 107 spores) of labeled spores in 200 μl of normal saline. Imaging was performed with a Gamma Medica X-SPECT small animal scanner (Northridge, CA) using a low-energy, high-resolution parallel-hole collimator. Ten-minute planar scans at 1, 3, 7, and 14 days post-injection were obtained. Prior to each scan, mice were weighed and their total radioactivity was measured with a dose calibrator (Capintec, Ramsey, NJ).
In vivo biodistribution studies.
Female BALB/c mice were injected with 2 μCi (74 Bq) of 125I-labeled spores supplemented with unlabeled spores such that the total number of spores injected was 15,000 × 106/kg. Mice in groups of three or four were sacrificed by CO2 narcosis at 1 h, 1 day, 3 days, 7 days, and 14 days post-injection, and their blood (0.1 ml), brain, heart, lungs, liver, spleen, kidneys, muscle, bone, small and large intestines, and tumor were removed, weighed, and counted in an automated gamma counter (1282 Compugamma CS, Pharmacia/LKB Nuclear, Inc, Gaithersburg, MD). The percentage of the injected dose per gram of tissue (%ID/g) was calculated by comparison with samples of a standard dilution of the initial dose. All measurements were corrected for radioactive decay.
Bacterial colony counts.
Tissues were removed, weighed, and homogenized in PBS, using 10 ml per gram of tissue and an IKA-ULTRA-TURRAX T 25 homogenizer set (IKA-WERKE, Staufen, Germany) at a speed of ∼24,000 rpm for 30–60 s. The resulting suspension was diluted in PBS and spread onto blood agar (Brucella agar with 5% sheep blood, PML Microbiologic, Wilsonville, OR) in duplicate 50-μl aliquots. Blood was obtained from intracardiac punctures and mixed with 9 volumes of PBS, after which 50 μl of suspension was spread on blood agar plates in duplicate. Blood agar plates were incubated for 18 h at 37°C in a sealed anaerobic chamber (GasPak Systems, Becton Dickinson, Franklin Lakes, NJ). Following incubation, plates were removed from the incubator and colonies were counted. At least three mice were used for each time point. To determine whether they were derived from C. novyi-NT, any morphologically atypical colonies were evaluated by polymerase chain reaction (PCR), using the following primers to amplify the C. novyi-NT flagellin gene: 5′-AACAAATGTACAAAAAGAAATAGC-3′ and 5′-CTAATCTATTTTGGATAGCTCC-3′. For determination of viability under varying oxygen concentrations, ∼1000 spores were spread onto blood agar plates and incubated in sealed chambers (Billups-Rothenberg, Del Mar, CA), which were purged with oxygen/nitrogen gas mixtures (Puritan Medical Products, Lithicum Heights, MD).
Statistical analysis.
The statistical significance of percent survival and toxicity was tested using the chi-squared test for trends in proportions and the Fisher's exact test. The null hypothesis was rejected if p < 0.05.
RESULTS
Clearance of C. novyi-NT Spores from the Circulation Is Rapid
Past experiments have employed several strains of mice, including C57BL/6, BALB/c, 129, and outbred nu/nu mice (Agrawal et al., 2004; Bettegowda et al., 2003; Dang et al., 2001, 2004). Toxicity was more often observed in tumor-bearing BALB/c mice than in the other strains, and these mice were chosen for detailed toxicological analysis. To determine the optimal route for systemic spore administration, 15,000 × 106 spores/kg were injected into BALB/c mice bearing subcutaneous (sc) tumors derived from CT26 cells, an isogenic murine colon cancer cell line. Germination and tumor necrosis were evident in >95% of mice after iv injection, in ∼60% of mice following intraperitoneal (ip) injection, and in no mice after sc injection of spores on the flank contralateral to that of the tumor. Germination was more rapid after iv injection, generally occurring within 18 h, than after ip injection (∼48 h); iv injection was therefore employed for all further experiments.
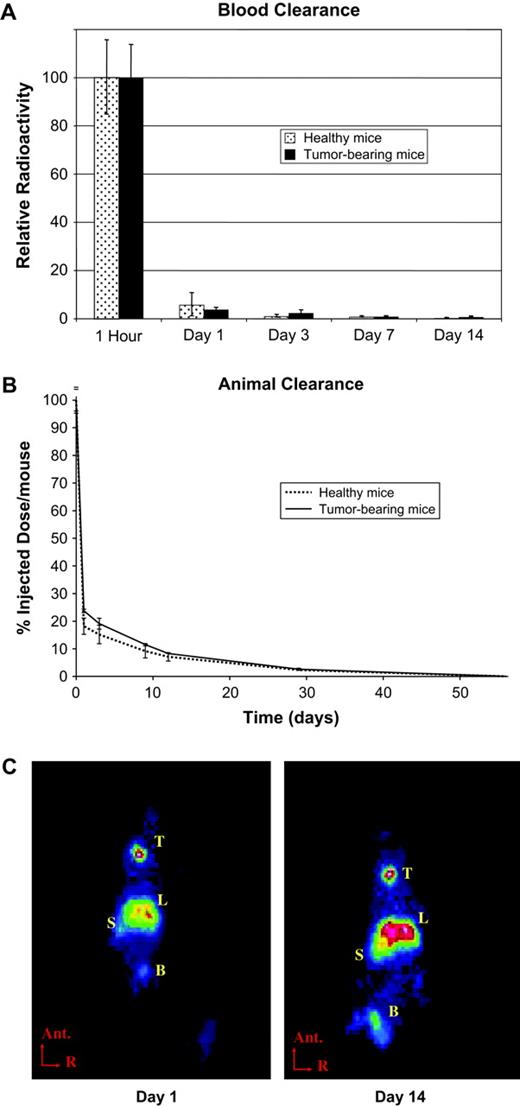
To quantitatively assess the clearance of C. novyi-NT spores from the circulation, 125I- labeled spores were intravenously injected into healthy and tumor-bearing BALB/c mice. Radioactivity in the blood was measured at various times after injection using four mice per time point. Greater than 95% of the radioactivity was cleared from the circulation within 1 day, and activity fell to barely detectable levels over the next 2 weeks (Fig. 1A). Measurements of whole-body radioactivity showed that >70% of the injected dose was eliminated within 24 h of administration and >90% was eliminated within 14 days (Fig. 1B). No residual radioactivity was detectable 2 months after administration of spores, and there were no differences in spore clearance between healthy animals and those bearing tumors (Fig. 1A and 1B).
Spore clearance. 125I-labeled C. novyi-NT spores (15,000 × 106 spores/kg) were injected into healthy or CT26 tumor-bearing BALB/c mice. (A) Radioactivity in 1 ml of blood was measured in a gamma counter and normalized to the 1-h value. (B) Whole-animal radioactivity was measured in a dose calibrator. (C) Planar gamma scintigraphy images. Ant. = anterior, R = right, T = thyroid, L = liver, S = spleen, B = bladder. (D, E) Radioactivity in various organs was measured with a gamma counter in healthy and tumor-bearing mice. Values in A, B, D, and E represent the mean activity (with standard deviation) of at least three mice per time point.
Biodistribution of C. novyi-NT Spores—Labeling Studies
To define the distribution of C. novyi-NT spores after intravenous injection, healthy and tumor-bearing BALB/c mice were injected with 125I-labeled spores as described above and imaged using gamma scintigraphy. Concentration of activity was observed within the liver and spleen 1 day after injection, and this pattern persisted for the entire 14-day experimental imaging period (Fig. 1C). Radioactivity was also observed in the thyroid and bladder, presumably emanating from free 125I released from the spores (Fig. 1C).
To quantify this distribution, individual tissues were assessed. A significant fraction of the radioactivity was present in several tissues (particularly the lung), in addition to liver and spleen, at 1 h post-injection, but the radioactivity in these tissues disappeared quickly and was barely detectable 24 h later (Fig. 1D and 1E). Consistent with the imaging studies, the liver and spleen were the major repositories of radioactivity, and signals persisted, but gradually decreased, until the end of the experiment (day 14). The tumor-bearing mice retained radioactivity in their livers and spleens slightly longer than the healthy mice (Fig. 1D and 1E). No other differences in biodistribution patterns were observed between tumor-bearing and healthy mice in the radiolabeling studies, assessed either by imaging or by direct radioactivity measurements in excised tissues.
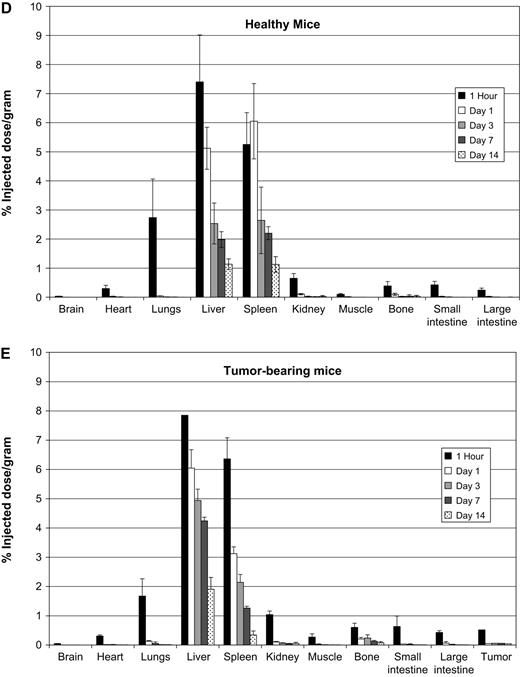
To define the histological distribution of C. novyi-NT spores, fluorescein-labeled spores were injected into BALB/c mice bearing CT26 tumors. After 24 h, mice were humanely euthanized, and frozen sections were prepared from brain, liver, spleen, and tumor tissues. Fluorescence microscopy showed only rare fluorescent spores in the brains or tumors of these mice, consistent with their expected presence within the vasculature. In contrast, there was a diffuse, uniform distribution of spores throughout the liver (Fig. 2, left panels). In the spleen (Fig. 2, right panels), the majority of spores were trapped within the marginal zone, corresponding to the region enriched in macrophages and other antigen-presenting cells (Aichele et al., 2003).
Spore imaging. Sections of liver and spleen were imaged using fluorescence microscopy 24 h after injection with 15,000 × 106 fluorescein-labeled spores/kg. Adjacent sections were stained with H&E (scale bar represents 50 μm) WP = white pulp, MZ = marginal zone, RP = Red pulp.
Biodistribution of C. novyi-NT Spores—Microbiologic Studies
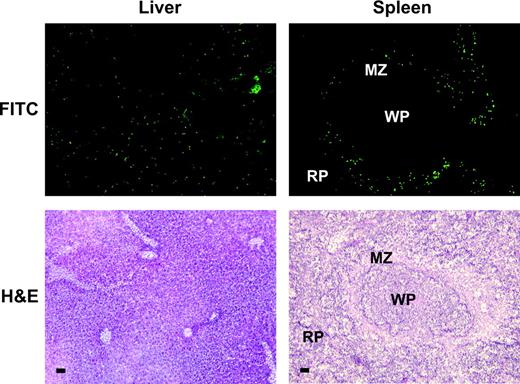
To independently confirm the clearance and biodistribution data derived from radioactively labeled spores, we used microbiologic methods to assess spore viability. At various times after iv injection of unlabeled spores, mice were euthanized, and blood and various tissues were removed, homogenized, and then spread onto blood agar plates. Approximately 50,000 viable spores/ml of blood could be detected in the circulation 1 h after injection, but the number of viable spores was reduced by ∼95% 1 week later, and they were undetectable 1 month later. The majority of viable spores were present in the lungs, liver, and spleen at 1 h (Fig. 3), consistent with the results of the studies with radiolabeled spores. Viable spores quickly disappeared from the lung and all other organs except the liver and spleen (Fig. 3). Qualitatively, similar biodistribution patterns were observed in healthy and tumor-bearing mice. Quantitatively, tumor-bearing mice initially retained a larger proportion of viable spores in the liver than healthy mice, although this difference was no longer apparent by day 28. No viable spores were detected in blood or in any other organ tested 1 year after spore injection.
Spore distribution. Unlabeled 15,000 × 106C. novyi-NT spores/kg were injected into healthy Balb/c (A) or tumor-bearing Balb/c mice (B). Tissues were homogenized, and viable spores were assessed by plating on blood-agar media under anaerobic conditions. At least three mice were evaluated per time point.
Toxicological Studies in Healthy Mice and Rabbits—Clinical Observations
Detailed studies were performed to examine acute toxicity in adult mice and rabbits. The mouse study involved 50 female and 50 male 6–8 week-old BALB/c mice that were randomly assigned to receive different doses of C. novyi-NT spores. All groups were divided equally by sex, and each mouse received a single intravenous injection of spores. Group 1, the control cohort, consisted of 30 mice that received only the vehicle (0.9% NaCl). Groups 2–4 each consisted of at least 20 mice that received C. novyi-NT doses of 500 × 106, 5000 × 106, or 25,000 × 106 spores/kg, respectively, as detailed in Table 1. All mice underwent daily observation and weighing on days 0–14. Groups 1 and 4 contained 10 extra mice that were used as recovery groups and followed for 28 days. No signs of clinical toxicity were observed in any mouse during the acute toxicology or recovery phase studies. All mice gained weight during both phases of the study, and there were no significant differences in the weights of the mice in any of the groups (data not shown).
Dosing Chart for Mouse Toxicity Study
. | . | . | . | Number of animals sacrificed . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Animal no. . | . | . | Day 14 . | . | Day 28 . | . | |||
| Group . | Male . | Female . | Dose (× 106 spores/kg) . | Male . | Female . | Male . | Female . | |||
| 1 | 15 | 15 | 0 | 10 | 10 | 5 | 5 | |||
| 2 | 10 | 10 | 500 | 10 | 10 | 0 | 0 | |||
| 3 | 10 | 10 | 5,000 | 10 | 10 | 0 | 0 | |||
| 4 | 15 | 15 | 25,000 | 10 | 10 | 5 | 5 | |||
. | . | . | . | Number of animals sacrificed . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Animal no. . | . | . | Day 14 . | . | Day 28 . | . | |||
| Group . | Male . | Female . | Dose (× 106 spores/kg) . | Male . | Female . | Male . | Female . | |||
| 1 | 15 | 15 | 0 | 10 | 10 | 5 | 5 | |||
| 2 | 10 | 10 | 500 | 10 | 10 | 0 | 0 | |||
| 3 | 10 | 10 | 5,000 | 10 | 10 | 0 | 0 | |||
| 4 | 15 | 15 | 25,000 | 10 | 10 | 5 | 5 | |||
Dosing Chart for Mouse Toxicity Study
. | . | . | . | Number of animals sacrificed . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Animal no. . | . | . | Day 14 . | . | Day 28 . | . | |||
| Group . | Male . | Female . | Dose (× 106 spores/kg) . | Male . | Female . | Male . | Female . | |||
| 1 | 15 | 15 | 0 | 10 | 10 | 5 | 5 | |||
| 2 | 10 | 10 | 500 | 10 | 10 | 0 | 0 | |||
| 3 | 10 | 10 | 5,000 | 10 | 10 | 0 | 0 | |||
| 4 | 15 | 15 | 25,000 | 10 | 10 | 5 | 5 | |||
. | . | . | . | Number of animals sacrificed . | . | . | . | |||
|---|---|---|---|---|---|---|---|---|---|---|
| . | Animal no. . | . | . | Day 14 . | . | Day 28 . | . | |||
| Group . | Male . | Female . | Dose (× 106 spores/kg) . | Male . | Female . | Male . | Female . | |||
| 1 | 15 | 15 | 0 | 10 | 10 | 5 | 5 | |||
| 2 | 10 | 10 | 500 | 10 | 10 | 0 | 0 | |||
| 3 | 10 | 10 | 5,000 | 10 | 10 | 0 | 0 | |||
| 4 | 15 | 15 | 25,000 | 10 | 10 | 5 | 5 | |||
Eighteen 10-week-old female New Zealand white rabbits were used for the second toxicity study, which is described in Table 2. Rabbits received intravascular injections of various numbers of spores suspended in 3 ml of vehicle (0.9% NaCl). As in the mouse study, four dosing groups were selected (vehicle alone, 25 × 106, 250 × 106, and 1250 × 106 spores/kg, respectively). The basic design of the study was similar to that of the mice, with six female rabbits in the control and high-dose groups and three rabbits in the low- and intermediate-dose groups. Animals were euthanized on day 14, except for three rabbits in the control and high-dose groups that were observed through day 28 and euthanized on that day. All rabbits in all groups gained weight, and none showed any clinical signs of toxicity during either the acute or the recovery phase of the study (data not shown).
Dosing Chart for Rabbit Toxicity Study
. | . | . | Number of animals sacrificed . | . | |
|---|---|---|---|---|---|
| Group . | Number of rabbits . | Dose (× 106 spores/kg) . | Day 14 . | Day 28 . | |
| 1 | 6 | 0 | 3 | 3 | |
| 2 | 3 | 25 | 3 | 0 | |
| 3 | 3 | 250 | 3 | 0 | |
| 4 | 6 | 1,250 | 3 | 3 | |
. | . | . | Number of animals sacrificed . | . | |
|---|---|---|---|---|---|
| Group . | Number of rabbits . | Dose (× 106 spores/kg) . | Day 14 . | Day 28 . | |
| 1 | 6 | 0 | 3 | 3 | |
| 2 | 3 | 25 | 3 | 0 | |
| 3 | 3 | 250 | 3 | 0 | |
| 4 | 6 | 1,250 | 3 | 3 | |
Dosing Chart for Rabbit Toxicity Study
. | . | . | Number of animals sacrificed . | . | |
|---|---|---|---|---|---|
| Group . | Number of rabbits . | Dose (× 106 spores/kg) . | Day 14 . | Day 28 . | |
| 1 | 6 | 0 | 3 | 3 | |
| 2 | 3 | 25 | 3 | 0 | |
| 3 | 3 | 250 | 3 | 0 | |
| 4 | 6 | 1,250 | 3 | 3 | |
. | . | . | Number of animals sacrificed . | . | |
|---|---|---|---|---|---|
| Group . | Number of rabbits . | Dose (× 106 spores/kg) . | Day 14 . | Day 28 . | |
| 1 | 6 | 0 | 3 | 3 | |
| 2 | 3 | 25 | 3 | 0 | |
| 3 | 3 | 250 | 3 | 0 | |
| 4 | 6 | 1,250 | 3 | 3 | |
Toxicological Studies in Healthy Mice and Rabbits—Histopathologic Observations
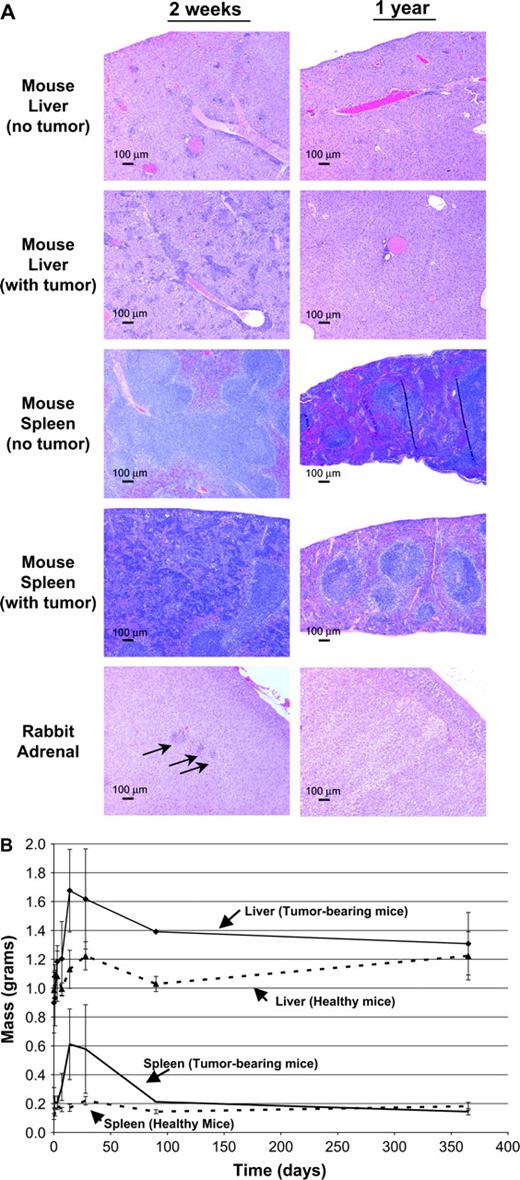
No gross pathologic abnormalities, other than mild hepatosplenomegaly in the high-dose groups, were noted in the mice that received C. novyi-NT. However, in treated-female mice, mild hepatic congestion (p = 0.007) was noted. Upon microscopic analysis of 58 organs (see Materials and Methods), abnormal findings in mice were confined to the spleen and liver. At day 14 all mice showed moderate multifocal subacute hepatitis at the highest dose (25,000 × 106 spores/kg) and mild subacute multifocal hepatitis at the intermediate dose (5000 × 106 spores/kg). In the lowest-dose group (500 × 106 spores/kg), 6 of 10 female mice developed minimal multifocal subacute hepatitis. At day 28, the liver lesions in the high-dose group were regressing and were characterized as mild in all 10 mice examined. Fifty percent of mice in the high-dose group developed reactive splenic hyperplasia at 14 days, and 90% had developed this abnormality by day 28. No splenic hyperplasia was observed in the mice in the intermediate- or low-dose groups. In a longer-term study, all splenic abnormalities had resolved and only rare regions of mild hepatic scarring were noted 1 year after treatment with C. novyi-NT spores (Fig. 4A). All dose-dependent trends in toxicity were statically significant, with p ≤ 0.01.
Toxicity studies. (A) Necropsy of mice and rabbits in the acute toxicity studies revealed inflammatory changes in the spleen and liver of mice and in the adrenal glands of rabbits. Representative slides made at 14 days and 1 year after spore treatment from indicated animal organs are shown. Scale bars: 100 μm. (B) As a measure of long-term toxicity, various tissues were weighed at the indicated times after spore injections into healthy and tumor-bearing mice. The only significant differences among organ weights between tumor-bearing and healthy mice were observed in the spleen and liver.
Systematic pathologic examinations of healthy rabbits were performed after 14 days of observation in all groups and at 28 days in the control and high-dose groups. Rabbits in the low- and intermediate-dose groups demonstrated no specific gross or microscopic abnormalities associated with spore administration. All rabbits in the high-dose group showed minimal multifocal hepatitis (1250 × 106 spores/kg) that was no longer present at day 28. This dose-dependent trend was statistically significant with a p value < 0.05. In the high-dose group, two of three rabbits at day 14 were observed to have multifocal nonsuppurative adrenalitis (Fig. 4A, arrows), whereas at day 28 only one of three rabbits demonstrated this adrenal abnormality. This trend was not statistically significant (p > 0.99). Tumor-bearing rabbits from another study that received 600 × 106 spores/kg were euthanized after 1 year of observation; no evidence of adrenal abnormalities was found at that time (Fig. 4A).
Toxicological Studies in Tumor-Bearing Mice
To evaluate toxicity in tumor-bearing mice, female BALB/c mice bearing CT26 tumors were injected with 15,000 × 106C. novyi-NT spores/kg and clinically evaluated for at least 28 days. A subset of mice that were cured of their tumors was observed for longer periods. Gross evaluation was performed at 1 h and on days 1, 3, 7, 14, 28, 90, and 365. Only hepatomegaly and splenomegaly were noted, which were maximal between days 14–28 and had disappeared by 1 year (Fig. 4B). Complete histopathologic analysis of 58 organs (see Materials and Methods) at day 14 revealed only the same abnormalities observed in healthy mice that had received C. novyi-NT (25,000 × 106 spores/kg), i.e., moderate multifocal hepatitis and reactive splenic hyperplasia. All splenic abnormalities had resolved, and only background levels of hepatic inflammation remained at 365 days (Fig. 4B).
C. novyi-NT Does Not Germinate in Hypoxic Tissues That Are Not Neoplastic
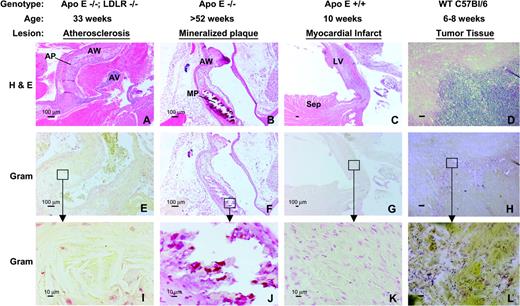
The potential ability of C. novyi-NT to colonize hypoxic tissues that were not neoplastic was assessed in three different models. In the first model, 33- to 35-week-old ApoE−/−; LDLR−/− mice were used. These mice had extensive atheromatous plaque formation in the walls of their aortas. To determine whether C. novyi-NT would germinate within these poorly vascularized plaques, mice were treated with 15,000 × 106C. novyi-NT spores/kg and observed for 4 days, a time sufficient for extensive germination within tumor tissues (Dang et al., 2001). No signs of clinical toxicity were evident in these mice. Histopathologic evaluation of atheromatous plaques from mice necropsied on day 5 (Fig. 5) showed no evidence of necrosis or acute inflammation of the type that would be expected from bacterial colonization and that was routinely observed within tumors after treatment with C. novyi-NT (Agrawal et al., 2004). Furthermore, Gram stains did not reveal any bacteria within or surrounding the cardiovascular lesions (Fig. 5).
C. novyi-NT spores fail to colonize atheromatous plaques and myocardial infarcts. (A,E,I) Atheromatous plaques in the ascending aorta and aortic arch from an ApoE−/−; LDL−/− mouse 4 days after intravenous administration of 15,000 × 106C. novyi-NT spores/kg were stained with H&E or Gram stain. (B, F, J) Mineralized atheromatous plaques from an aged, ApoE−/− mouse 4 days after the administration of 15,000 × 106 spores/kg, stained as indicated. (C, G, K) Myocardial necrosis, leukocytic infiltrate, fibrosis, and thinning of the left ventricular free wall were observed after ligation of the left anterior descending coronary artery. A typical lesion found 4 days after the administration of 15,000 × 106 spores/kg is shown. (D, H, L) Necrotic tissue juxtaposed with viable tumor tissue after treatment with 15,000 × 106C. novyi-NT spores/kg. Numerous rod-like Gram-positive organisms can be observed within and adjacent to the necrotic regions. Scale bars in A–H: 100 μm; scale bars in I–L: 10 μm. AP = atheromatous plaque, AV = aortic valve, AW = aortic wall, MP = mineralized plaque, Sep = interventricular septum, LV = left ventricular wall.
The second model involved 52- to 56-week-old ApoE−/− mice with well-developed atheromatous plaques that had undergone mineralization. At day 4 after injection of 15,000 × 106C. novyi-NT spores/kg, there were no clinical symptoms or histopathological evidence of bacterial colonization of the mineralized plaques (Fig. 5). No gross or histopathologic abnormalities were noted upon examination of any other tissues obtained from necropsies of the ApoE−/− or ApoE−/−; LDLR−/− mice.
In the third model, myocardial infarcts were created in normal, 10-week-old mice by ligation of the left coronary artery. Five days later, the mice were injected iv with 15,000 × 106C. novyi-NT spores/kg. These mice appeared clinically normal after injection. Histopathological examination of the heart 4days after injection of spores demonstrated that while the mice had the expected large myocardial infarcts with extensive areas of necrosis and fibrosis, there was no histopathological evidence of bacterial colonization within or surrounding the lesions (Fig. 5).
The Relationship Between Toxicity, Spores Dose and Tumor Size
Through the treatment of thousands of mice with C. novyi-NT spores, often in combination with other agents, it was obvious to us that toxicity, when observed, was related to the germination of the bacteria within tumors. Thus, clinical toxicity was not observed in animals without tumors, even when treated with very high doses of spores (Dang et al., 2004). Furthermore, toxicity was not observed in animals with tumors in experiments wherein bacterial germination did not occur. The toxicity most commonly manifested itself as lethargy and poor grooming, and when sufficiently severe, death within 5 days. We also noticed that toxicity appeared to be greater when larger tumors or higher doses of spores were employed.
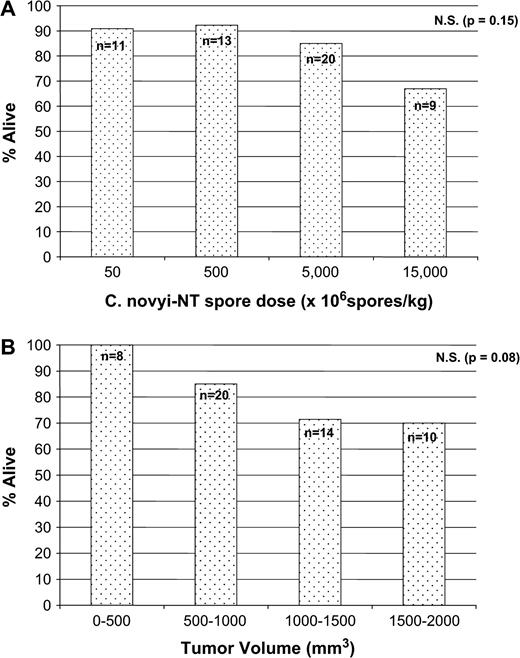
To more quantitatively evaluate the relationship between tumor size, spore dose, and toxicity, BALB/c mice bearing subcutaneous CT26 tumors measuring between 500 and 1000 mm3 were injected with C. novyi-NT spores at 50, 500, 5000, or 15,000 × 106 spores/kg (Fig. 6A). Mortality ranged from 7.6% to 33.0% and appeared to have a dose-dependent trend that was not statistically significant (p = 0.15). In a second set of experiments, mice were segregated into groups of various tumor sizes at the time of treatment, with the smallest tumors measuring 250–500 mm3 and the largest tumors measuring 1500–2000 mm3. Mice were injected with a fixed spore dose of 5000 × 106 spores/kg and monitored daily for toxicity (Fig. 6B). The mortality range was 0–30% and was greater in mice with larger tumors. This apparent trend did not achieve statistical significance (p = 0.08).
Mortality in tumor-bearing mice. (A) Balb/c mice bearing CT26 tumors with a fixed tumor volume (500–1000 mm3) were treated with the indicated doses of spores (p = 0.15). (B) Balb/c mice bearing CT26 tumors of the indicated sizes were treated with 5000 × 106C. novyi-NT spores/kg) (p = 0.08). The total number (n) of mice in each cohort is indicated above each column.
Toxicity from C. novyi-NT Therapy Is Not Characteristic of That Caused by Bacterial Sepsis
Clinical observations demonstrated that the mice treated with C. novyi-NT often appeared ill once their tumors began to undergo necrosis. As noted above, the major symptoms were lethargy and ruffled fur, and these symptoms were more severe in mice that eventually died. To determine whether the symptoms reflected a typical septic process, we compared the laboratory and pathologic responses of mice to C. novyi-NT spores, LPS, and S. aureus. In each case, mice bearing tumors of ∼500 mm3 in size were treated with doses of the three agents that were similarly toxic, i.e., that resulted in death in 10–30% of the animals. The laboratory tests performed after injection of these agents included standard chemistries, hematological analyses, and serum-based tests of liver and renal functions (details in Materials and Methods).
Within 24–48 h of injection, all animals that were injected with LPS or S. aureus appeared lethargic and poorly groomed, as evidenced by their ruffled fur. This was also the case, although to a lesser degree, in mice treated with C. novyi-NT (15,000 × 106 spores/kg), whereas the tumor-bearing control mice appeared healthy. All laboratory tests in the C. novyi-NT spore–treated mice, as well as in the control tumor-bearing mice, were normal. In contrast, the mice that had been injected with sublethal doses of LPS or S. aureus showed a variety of laboratory abnormalities (see Supplementary Data in the Supplementary Data online). Liver function tests in LPS-injected mice were elevated, with total bilirubin 25-fold higher than normal and aminotransferase (ALT) too high to be measured. In addition, mice administered LPS had low blood CO2 (<10 mEq/l), a large anion gap, a twofold increase in serum creatinine, and increases in BUN and phosphate. Mice challenged with S. aureus showed elevated ALT (4–9 times normal) and alkaline phosphatase levels (3–7 times normal).
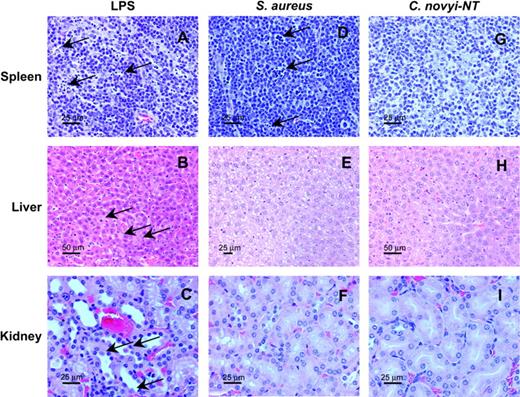
Gross and histopathologic examinations of mice necropsied 12–48 h after the injections were consistent with the laboratory abnormalities. Mice from the control, tumor-bearing group were normal. C. novyi-NT spore–treated mice had hepatosplenomegaly (as described above under Toxicological Studies in Tumor-Bearing Mice), without evidence of tissue damage, and their tissues were otherwise normal at both the gross and microscopic levels. Mice injected with LPS had hepatocellular necrosis, lymphoid necrosis in the spleen, and renal acute tubular necrosis (Fig. 7). Mice injected with S. aureus had lymphoid necrosis in the spleen but no liver or renal abnormalities. These studies indicated that the pathologic process following C. novyi-NT therapy was not that expected from typical bacterial sepsis caused by Gram-negative or Gram-positive bacteria.
Absence of histopathologic findings characteristic of sepsis in spore-treated mice. BALB/c mice bearing CT26 tumors were injected with sublethal doses of lipopolysaccharide (LPS), live Staphylococcus aureus, or C. novyi-NT spores. All animals appeared ill within 24 h, exhibiting ruffled fur and lethargy. Necropsies in LPS-injected mice revealed lymphoid necrosis in the spleen (A, arrows), scattered hepatocellular necrosis in the liver (B, arrows), and acute tubular necrosis in the kidney (C, arrows). Lymphoid necrosis in the spleen (D, arrows) was observed in mice inoculated with S. aureus. No necrotic changes typical of sepsis were observed in the mice treated with C. novyi-NT (G, H, I). Scale bars: A, C, D, E, F, G, I: 25 μm; B and H: 50 μm.
The Potential of Antibiotics to Limit Toxicity
One of the potential advantages of bacteriolytic therapies compared to conventional anti-cancer therapies is that the toxicity of the former can be controlled, in principle, through the administration of antibiotics. To test this approach for reducing toxicity, we first determined that C. novyi-NT was highly sensitive to many commonly used antibiotics, particularly to clindamycin and various formulations of penicillins, with minimum inhibitory concentrations (MICs) of < 25 μg/ml. We chose to use a penicillin derivative, imipenem, largely because of its favorable pharmacokinetics and its clinical efficacy against abscesses (Traub, 1988).
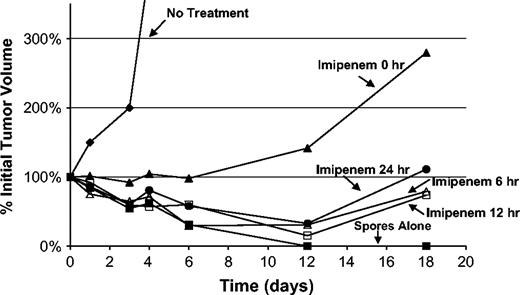
Balb/c mice bearing relatively large tumors (mean size 850 mm3) were treated with C. novyi-NT spores, and imipenem was administered 0, 6, 12, or 24 h later (Table 3). Except for the control group, mice were treated twice daily with imipenem for 4 days thereafter. The tumors in all groups of mice responded to the bacteriolytic therapy, exhibiting hemorrhagic necrosis. However, the degree of bacterial germination, as assessed by the extent of necrosis, was reduced in the animals treated with imipenem. These differential responses were reflected in tumor growth curves (Fig. 8). The tumors in animals receiving imipenem immediately after C. novyi-NT exhibited a 1-week period of delayed growth compared to animals without any spore treatment. Animals treated with antibiotics 6, 12, or 24 h after C. novyi-NT administration all had more substantial tumor responses, with tumors shrinking by an average of 60–80%. Mice treated with spores alone (no antibiotics) had complete (100%) tumor regressions in all cases (Fig. 8). No toxicity was evident in the mice treated with C. novyi-NT plus antibiotics, and all mice survived. However, the tumors regrew in 19 of these 20 mice, so that only one mouse (treated with antibiotics 24 h after C. novyi-NT administration) was cured at 1 year. Control experiments showed that 20% of mice, when treated with C. novyi-NT in the absence of antibiotics, experienced severe toxicity, and 20% were cured (no tumor recurrences at 1 year). We conclude that antibiotics can indeed protect animals from the toxicity associated with C. novyi-NT infection, even when administered once robust germination has occurred, but at the expense of efficacy.
Antibiotic rescue experiments. BALB/c mice bearing CT26 tumors were treated with imipenem beginning at the indicated times after C. novyi-NT spore injection. Imipenem was injected ip at a dose of 60 mg/kg on day 1 and at 40 mg/kg every 12 h through day 4. All time points studied consisted of five mice per group. Details of the study design and clinical outcomes are provided in Table 3.
Antibiotic Rescue of C. novyi-NT Toxicity
. | . | Mortality . | . | 28-day cure . | . | ||
|---|---|---|---|---|---|---|---|
| Time of antibiotic addition . | Number of mice tested . | n . | % . | n . | % . | ||
| 0 h | 5 | 0 | 0 | 0 | 0 | ||
| 6 h | 5 | 0 | 0 | 0 | 0 | ||
| 12 h | 5 | 0 | 0 | 0 | 0 | ||
| 24 h | 5 | 0 | 0 | 1 | 20 | ||
| Total | 20 | 0 | 0 | 1 | 5 | ||
. | . | Mortality . | . | 28-day cure . | . | ||
|---|---|---|---|---|---|---|---|
| Time of antibiotic addition . | Number of mice tested . | n . | % . | n . | % . | ||
| 0 h | 5 | 0 | 0 | 0 | 0 | ||
| 6 h | 5 | 0 | 0 | 0 | 0 | ||
| 12 h | 5 | 0 | 0 | 0 | 0 | ||
| 24 h | 5 | 0 | 0 | 1 | 20 | ||
| Total | 20 | 0 | 0 | 1 | 5 | ||
Antibiotic Rescue of C. novyi-NT Toxicity
. | . | Mortality . | . | 28-day cure . | . | ||
|---|---|---|---|---|---|---|---|
| Time of antibiotic addition . | Number of mice tested . | n . | % . | n . | % . | ||
| 0 h | 5 | 0 | 0 | 0 | 0 | ||
| 6 h | 5 | 0 | 0 | 0 | 0 | ||
| 12 h | 5 | 0 | 0 | 0 | 0 | ||
| 24 h | 5 | 0 | 0 | 1 | 20 | ||
| Total | 20 | 0 | 0 | 1 | 5 | ||
. | . | Mortality . | . | 28-day cure . | . | ||
|---|---|---|---|---|---|---|---|
| Time of antibiotic addition . | Number of mice tested . | n . | % . | n . | % . | ||
| 0 h | 5 | 0 | 0 | 0 | 0 | ||
| 6 h | 5 | 0 | 0 | 0 | 0 | ||
| 12 h | 5 | 0 | 0 | 0 | 0 | ||
| 24 h | 5 | 0 | 0 | 1 | 20 | ||
| Total | 20 | 0 | 0 | 1 | 5 | ||
Systemic Hydration Can Rescue Toxicity from C. novyi-NT
In addition to antibiotic therapy, supportive care is often necessary in the treatment of bacterial infections in humans. Fever, anorexia, and an increased metabolic demand stress an organism during severe infection and can result in fluid loss; in such cases, maintenance of circulatory volume and blood pressure is essential for survival. We attempted to provide minimal supportive care to mice through fluid administration. To this end, we treated BALB/c mice bearing large CT26 tumors (mean size 1600 mm3) with C. novyi-NT spores, and we hydrated half of them via subcutaneous injection of 2–3 ml of normal saline every 8 h. After 4 days, 3 mice of 10 had died in the control group, whereas no mice (n = 9) in the fluid-replacement group had died. On day 4, hydration was discontinued, and no further deaths occurred in any of the mice. Similar results were found in a study of similar size using smaller tumors (400–500 mm3): none of 10 animals died in the hydration group, whereas one in 10 in the control group died. We conclude that systemic hydration is a simple way to limit the toxicity from C. novyi-NT-based therapy while preserving its therapeutic efficacy.
DISCUSSION
The results described above illuminate the pharmacokinetics of C. novyi-NT spores, their toxicities in healthy and tumor-bearing animals, the nature of the toxicity and methods to control it. Each of these observations has important implications for further experimental studies of C. novyi-NT spores as a cancer therapeutic agent.
Pharmacokinetics and Distribution of Spores
The pharmacokinetic profiles we observed are consistent with those predicted from previous studies of non-infectious particles delivered intravenously into animals (Proffitt et al., 1983a, 1983b) C. novyi-NT spores are 1.3 μm long and 0.8 μm wide, in the size range that is particularly well suited for phagocytosis by reticuloendothelial cells (Proffitt et al., 1983a). Accordingly, more than 99% of spores were cleared from the systemic circulation within 1 h. At 1 h the majority of injected spores were found in the lungs, liver and spleen—the major components of the reticuloendothelial system (RES). As expected from previous studies of particulates and other clostridial spores, clearance from the lungs was completed within a few days, and the liver and spleen remained the predominant sites of spore residence thereafter. (Lambin et al., 1998). In tumor-bearing mice, as compared to healthy mice (Figs. 3A versus 3B), there was an increased number of viable spores cultured from the liver. The presence of a tumor may activate the RES such that it more aggressively clears and retains circulating microparticulate matter. Despite this difference in initial viable spore retention, spores in liver and spleen gradually diminished, so that none were detected 1 year later (Fig. 3).
The distribution studies also revealed that only a small percentage of spores (<1 % of the injected dose) were initially distributed within the tumor (Fig. 1E). The way in which spores, which are non-motile, migrate from the capillaries into the hypoxic regions of tumors is not clear. One hypothesis, supported by physiologic studies of tumor angiogenesis, is that the tumor endothelium is inherently leaky (Dvorak, 1990; Dvorak et al., 1988; Hashizume et al., 2000). Another hypothesis is that spores are ingested by phagocytic cells, which then migrate through the endothelium, carrying spores with them (O'Brien and Melville, 2000). In either case, it is obvious that only a small number of spores are required to cause a profound tumor necrosis. In experiments wherein the dose of spores was varied (unpublished data), we estimated that as few as 10 spores within a tumor, resulting from a systemic injection of 1000 spores, could result in extensive necrosis, although the responses were not as robust, rapid, or reproducible as with the higher doses used in the present study.
Within tumors, spore counts increased 30-fold by day 1 and remained elevated as long as the tumors persisted (Fig. 3B). Note that our microbiologic assays measured only spores, not live bacteria. Because C. novyi-NT is exquisitely sensitive to oxygen (see Supplementary Data in the Supplementary Data online), we found it difficult to homogenize tissues in a fashion that preserved the hypoxic environment of tumors and would provide reliable recovery of live bacteria. The increase in spore number within tumors reflected the fact that germination within tumors is followed by sporulation, as confirmed by microscopic analysis of tumors. The release of live bacteria and spores from the tumors is the likely basis, in part, for the increased number of viable spores in the liver (Fig. 3) and the significant hepatosplenomegaly observed in tumor-bearing animals after injection of spores (Fig. 4B). An important finding of the present study was that spores were eventually cleared from all organs, even in tumor-bearing animals.
Minimal Toxicity in Animals Without Tumors
Our first step in investigating the potential toxicity of C. novyi-NT spores involved traditional acute toxicology studies in two animal species. Despite the high doses of spores used in these experiments, no clinical evidence of toxicity was observed in any animal. Gross pathologic examination was also unremarkable, except for mild hepatosplenomagaly in the high-dose groups. Microscopic examination showed hepatic and adrenal inflammatory changes at day 14 that were already resolving by day 28 and had disappeared by 1 year.
The second step in toxicological investigation involved the analysis of mice with hypoxic lesions that were unrelated to neoplasia. Three different mouse models were tested, two incorporating atheromatous lesions and one incorporating myocardial infarction. Except for the expected atheromas and infarctions, no clinical or pathological abnormalities were detected in mice inoculated with C. novyi-NT spores in any of the animals. Moreover, no microscopic signs of bacterial germination or growth were observed within or surrounding these lesions. It is of interest in this regard that parental C. novyi spores are able to infect penetrating wounds induced by gunshots or contaminated drug injections (Boyd et al., 1972a, 1972b; Majumdar et al., 2004; McGuigan and Roworth, 2002; McGuigan et al., 2002; Mulleague et al., 2001). Tumors have been likened to “wounds that will not heal” (Brown et al., 1999; Metheny-Barlow and Li, 2003), and it is possible that the microenvironment within tumors is more like that observed in fresh wounds than that in fresh infarcts or in poorly vascularized tissues like atheromas. The mechanism(s) underlying the ability of C. novyi-NT spores to germinate within the hypoxic regions of tumors (or wounds) but not within other hypoxic regions is not known, but several can be imagined. First, it is possible that the oxygen levels, though lower than normal, are higher within the atheromatous/infarcted tissues than in tumors; as noted above, C. novyi-NT is exquisitely sensitive to oxygen (see Supplementary Data of the Supplementary Data online). Second, it is possible that C. novyi-NT spores cannot enter into the hypoxic regions within atheromatous or infarcted tissues because the vasculature is not leaky, as it is in tumors. And third, it is possible that some other feature of tumors or wounds, unrelated to oxygen levels or circulation per se, allows C. novyi-NT germination. In experiments in vitro, we have shown that C. novyi-NT can germinate when co-cultured with cancer cell lines in ambient air, but not when co-cultured with normal cell lines (unpublished data). The factors responsible for this tumor cell–specific germination are not understood.
Toxicity in Animals with Tumors
In contrast to healthy mice or those with atherosclerosis or myocardial infarction, mice bearing tumors did mount a pathologic response to treatment with C. novyi-NT. All available evidence suggests that the response was mediated by germinated C. novyi-NT bacteria, not the spores. Clinically, lethargy and poor grooming were commonly seen, and hepatosplenomegaly was observed pathologically. The inflammatory changes observed in the liver and spleen were those expected from infection with a relatively indolent pathogen and were fully reversible with time in surviving animals (Figs. 4A and 4B).
It is not surprising that large numbers of germinated C. novyi-NT bacteria within tumors cause toxicity in animals. Such toxicity has been established in many examples of infectious diseases (Lolis and Bucala, 2003; Ward, 2004), and its pathogenesis focuses on the cytokine storm induced in the host. The intensity of the inflammatory response leads to the release of a variety of vasoactive and immunomodulatory factors that cause sepsis or systemic inflammatory response syndrome (SIRS) (Mitaka, 2005; Rice and Bernard, 2005). Previous studies have demonstrated the release of inflammatory cytokines during therapy with C. novyi-NT (Agrawal et al., 2004). Based on studies of various infections, it would be expected that larger amounts of bacteria would result in higher levels of cytokines, and this is consistent with our observation of a relationship between the level of toxicity and both tumor size and the dose of the spore inoculum (Fig. 6). It is notable in this regard that the mouse tumors used in our experiments were much larger relative to body weight than would occur in larger animals. Perhaps this explains why treatment-related mortality from C. novyi-NT spores was not detected in rabbits (Agrawal et al., 2004). In addition, even in mice with extremely large tumors (greater than 10% of body weight), mortality rates plateaued at 30%. This may represent the self-limiting nature of an infection that destroys the anaerobic environment on which the bacteria depend for survival. The lack of the pathogenic α-toxin also limits the absolute toxicity of C. novyi-NT (Dang et al., 2001). Viewed in the context of other infectious diseases, it is therefore remarkable that the very large abscesses caused by C. novyi-NT in tumor-bearing mice were tolerated by most of the animals.
Minimizing Toxicity in Tumor-Bearing Mice
To begin to understand the basis for the toxicity observed after treatment with C. novyi-NT, we compared clinical, pathologic, and laboratory data obtained in animals treated with C. novyi-NT to the data obtained in mice administered agents known to cause typical SIRS. All mice injected with either LPS or S. aureus appeared ill and displayed poor grooming and lethargy. To a lesser and more variable extent, the mice treated with C. novyi-NT also displayed these symptoms. As expected, mice injected with LPS or S. aureus showed pathologic hepatic and renal abnormalities consistent with SIRS and laboratory findings suggestive of hepatic and renal injury. Surprisingly, clinically ill tumor-bearing mice treated with C. novyi-NT showed no such findings.
Toxicity from bacterial infections can generally be reduced by treating the infections with antibiotics. The toxicity from C. novyi-NT–induced toxicity was no exception. Antibiotics did indeed minimize toxicity, and no deaths were observed in animals treated with antibiotics, even when antibiotics were not initiated until after a large number of bacteria were present within the tumor (24 h after C. novyi-NT administration; Fig. 8). But antibiotics also reduced the efficacy of the treatment by limiting the number of tumor cells killed by the bacteria. All tumors in the antibiotic-treated mice showed evidence of central germination and necrosis, but a larger rim of viable tumor remained. We propose that antibiotics cannot penetrate the hypoxic avascular core of the tumor but do penetrate the vascular tumor rim and suppress bacterial growth, limit inflammation, and reduce complete tumor debulking.
Fluid resuscitation is another cornerstone of the therapy of severe infections (Dellinger et al., 2004; Remick et al., 2005; The SAFE Study Investigators, 2004). Fever, anorexia, and an increased metabolic demand place stress on animals during infection and can result in fluid loss. The cytokine storm (Agrawal et al., 2004; Remick et al., 2005; Schrier and Wang, 2004), together with this fluid loss, makes maintaining circulatory volume and blood pressure essential for survival. Based on the absence of distinct laboratory and histopathologic abnormalities in mice treated with C. novyi-NT spores, we suspected that hypovolemia with hypotension from fluid loss and cytokine-mediated vasodilatation was largely responsible for the deaths that were observed. This idea was consistent with clinical observations: lethargy and general malaise could have resulted in the animals' drinking insufficient quantities of water, and ruffled fur can be a sign of dehydration. This hypothesis was also consistent with the fact that the toxicity was less evident in rabbits, which are larger animals with lower metabolic rates and are thereby more tolerant to dehydration.
In the present study, we showed that fluid resuscitation alone could effectively rescue animals from death. As the infection within the tumors is self-limiting, it was only necessary to administer fluids for a few days. Our finding that the toxicity in tumor-bearing mice is reversible will be useful for future studies employing C. novyi-NT in combination with other therapeutic agents. It is likely that this simple measure would also reduce the toxicity of other forms of therapy based on relatively nontoxic bacteria or components derived from them. Most importantly, the lessons learned in mice should be applicable to humans if and when Clostridia-based therapies re-emerge in the clinical setting.
The authors thank Lattice Watson for excellent technical assistance and Harriet Chapman and the staff of the Cancer Research Building (CRB) animal facilities of the Sidney Kimmel Cancer Center for assistance with animal experiments. We thank Satoaki Matoba and Willmar Patino for providing atherosclerosis model mice and San Hong for cardiac infarction surgery. This work was supported by the Miracle Foundation, the Clayton Fund, and National Institutes of Health grants RR00171, CA062924, CA43460, CA92871, and CA103175.
References
Agrawal, N., Bettegowda, C., Cheong, I., Geschwind, J. F., Drake, C. G., Hipkiss, E. L., Tatsumi, M., Dang, L. H., Diaz, L. A., Jr., Pomper, M., et al. (
Aichele, P., Zinke, J., Grode, L., Schwendener, R. A., Kaufmann, S. H., and Seiler, P. (
Bettegowda, C., Dang, L. H., Abrams, R., Huso, D. L., Dillehay, L., Cheong, I., Agrawal, N., Borzillary, S., McCaffery, J. M., Watson, E. L., et al. (
Boyd, N. A., Thomson, R. O., and Walker, P. D. (
Boyd, N. A., Walker, P. D., and Thomson, R. O. (
Brown, J. M. (
Brown, L. F., Guidi, A. J., Schnitt, S. J., Van De Water, L., Iruela-Arispe, M. L., Yeo, T. K., Tognazzi, K., and Dvorak, H. F. (
Cerar, A., Zidar, N., and Vodopivec, B. (
Dang, L. H., Bettegowda, C., Agrawal, N., Cheong, I., Huso, D., Frost, P., Loganzo, F., Greenberger, L., Barkoczy, J., Pettit, G. R., et al. (
Dang, L. H., Bettegowda, C., Huso, D. L., Kinzler, K. W., and Vogelstein, B. (
Dellinger, R. P., Carlet, J. M., Masur, H., Gerlach, H., Calandra, T., Cohen, J., Gea-Banacloche, J., Keh, D., Marshall, J. C., Parker, M. M., et al. (
Dvorak, H. F. (
Dvorak, H. F., Nagy, J. A., Dvorak, J. T., and Dvorak, A. M. (
Fraker, P. J., and Speck, J. C., Jr. (
Hashizume, H., Baluk, P., Morikawa, S., McLean, J. W., Thurston, G., Roberge, S., Jain, R. K., and McDonald, D. M. (
Heppner, F., and Mose, J. R. (
Ishibashi, S., Herz, J., Maeda, N., Goldstein, J. L., and Brown, M. S. (
Jain, R. K., and Forbes, N. S. (
Lambin, P., Theys, J., Landuyt, W., Rijken, P., van der Kogel, A., van der Schueren, E., Hodgkiss, R., Fowler, J., Nuyts, S., and de Bruijn, E. (
Lolis, E., and Bucala, R. (
Majumdar, S., Woodcock, S., and Cheesbrough, J. (
McGuigan, C., and Roworth, M. (
McGuigan, C. C., Penrice, G. M., Gruer, L., Ahmed, S., Goldberg, D., Black, M., Salmon, J. E., and Hood, J. (
Metheny-Barlow, L. J., and Li, L. Y. (
Mitaka, C. (
Mose, J. R. (
Mose, J. R., Mose, G., Propst, A., and Heppner, F. (
Mulleague, L., Bonner, S. M., Samuel, A., Nichols, P., Khan, M., Shaw, S., and Gruning, T. (
O'Brien, D. K., and Melville, S. B. (
Parker, R. C., Plummer, H. C., Siebenmann, C. O., and Chapman, M. G. (
Patten, R. D., Aronovitz, M. J., Deras-Mejia, L., Pandian, N. G., Hanak, G. G., Smith, J. J., Mendelsohn, M. E., and Konstam, M. A. (
Piedrahita, J. A., Zhang, S. H., Hagaman, J. R., Oliver, P. M., and Maeda, N. (
Proffitt, R. T., Williams, L. E., Presant, C. A., Tin, G. W., Uliana, J. A., Gamble, R. C., and Baldeschwieler, J. D. (
Proffitt, R. T., Williams, L. E., Presant, C. A., Tin, G. W., Uliana, J. A., Gamble, R. C., and Baldeschwieler, J. D. (
Remick, D. G., Bolgos, G., Copeland, S., and Siddiqui, J. (
Rice, T. W., and Bernard, G. R. (
The SAFE Study Investigators (
Toso, J. F., Gill, V. J., Hwu, P., Marincola, F. M., Restifo, N. P., Schwartzentruber, D. J., Sherry, R. M., Topalian, S. L., Yang, J. C., Stock, F., et al. (
Traub, W. H. (
Topley, W. W. C., Balows, L. C. A., Wilson, G. S. (
Author notes
*Howard Hughes Medical Institute, The Johns Hopkins School of Medicine, Baltimore, Maryland 21231; †The Sidney Kimmel Comprehensive Cancer Center at Johns Hopkins, Baltimore, Maryland 21231; ‡Department of Otolaryngology-Head and Neck Surgery, The Johns Hopkins School of Medicine, Baltimore, Maryland 21231; §Department of Radiology and Radiological Sciences, The Johns Hopkins School of Medicine, Baltimore, Maryland 21231; ¶Department of Comparative Medicine, The Johns Hopkins School of Medicine, Baltimore, Maryland 21231; ||Department of Oncology, University of Michigan, Ann Arbor, Michigan, 48104; |||Cardiovascular Branch, National Heart Lung and Blood Institute, National Institutes of Health, Bethesda, Maryland 20892




Comments