-
PDF
- Split View
-
Views
-
Cite
Cite
Santosh Kesari, David Schiff, Lisa Doherty, Debra C. Gigas, Tracy T. Batchelor, Alona Muzikansky, Alison O'Neill, Jan Drappatz, Alice S. Chen-Plotkin, Naren Ramakrishna, Stephanie E. Weiss, Brenda Levy, Joanna Bradshaw, Jean Kracher, Andrea Laforme, Peter McL. Black, Judah Folkman, Mark Kieran, Patrick Y. Wen, Phase II study of metronomic chemotherapy for recurrent malignant gliomas in adults, Neuro-Oncology, Volume 9, Issue 3, July 2007, Pages 354–363, https://doi.org/10.1215/15228517-2007-006
Close - Share Icon Share
Abstract
Preclinical evidence suggests that continuous low-dose daily (metronomic) chemotherapy may inhibit tumor endothelial cell proliferation (angiogenesis) and prevent tumor growth. This phase II study evaluated the feasibility of this antiangiogenic chemotherapy regimen in adults with recurrent malignant gliomas. The regimen consisted of low-dose etoposide (35 mg/m2 [maximum, 100 mg/day] daily for 21 days), alternating every 21 days with cyclophosphamide (2 mg/kg [maximum, 100 mg/day] daily for 21 days), in combination with daily thalidomide and celecoxib, in adult patients with recurrent malignant gliomas. Serum and urine samples were collected for measurement of angiogenic peptides. Forty-eight patients were enrolled (15 female, 33 male). Twenty-eight patients had glioblastoma multiforme (GBMs), and 20 had anaplastic gliomas (AGs). Median age was 53 years (range, 33-74 years), and median KPS was 70 (range, 60-100). Therapy was reasonably well tolerated in this heavily pretreated population. Two percent of patients had partial response, 9% had a minor response, 59% had stable disease, and 30% had progressive disease. For GBM patients, median progression-free survival (PFS) was 11 weeks, six-month PFS (6M-PFS) was 9%, and median overall survival (OS) was 21 weeks. For AG patients, median PFS was 14 weeks, 6M-PFS was 26%, and median OS was 41.5 weeks. In a limited subset of patients, serum and urine angiogenic peptides did not correlate with response or survival (p > 0.05). Although there were some responders, this four-drug, oral metronomic regimen did not significantly improve OS in this heavily pretreated group of patients who were generally not eligible for conventional protocols. While metronomic chemotherapy may not be useful in patients with advanced disease, further studies using metronomic chemotherapy combined with more potent antiangiogenic agents in patients with less advanced disease may be warranted.
Malignant gliomas, the most common primary brain tumors in adults, carry a dismal prognosis. First-line chemotherapy, usually with temozolomide, has been demonstrated to improve survival when given with radiotherapy.1 However, tumors almost always recur, and at the time of recurrence, no systemic chemotherapeutic regimens have shown clear efficacy in delaying disease progression. More effective therapies based on novel mechanisms of action are needed.
Gliomas are highly angiogenic,2-6 and the higher grade tumors show evidence of greater angiogenic activity in the form of increased microvessel density and neovascularization. Moreover, tumor vessel density is an independent prognostic parameter for gliomas.7
Malignant gliomas may induce angiogenesis by secreting angiogenic factors.8, 9 Examples of these factors include acidic and basic fibroblast growth factor (bFGF), vascular endothelial growth factor (VEGF), platelet-derived growth factor (PDGF), angiogenin, interleukin-8, and tumor necrosis factor-alpha. Experimental work in animals10-15 has demonstrated promising reductions in tumor growth using approaches that inhibit angiogenesis. Several studies of novel antiangiogenic agents such as the VEGF monoclonal antibody bevacizumab (Avastin) have shown significant activity in a number of systemic cancers.16,17 Inhibition of angiogenesis holds promise as a therapeutic strategy for patients with malignant glioma.18 However, until recently, effective application of such a strategy has been constrained by the limited availability of potent inhibitors of angiogenesis and by the limited knowledge of their safety and efficacy.
An alternative and immediately accessible strategy to inhibit angiogenesis is antiangiogenic chemotherapy, also referred to as metronomic chemotherapy.19 With such an approach, conventional cytotoxic chemotherapeutic agents are given nearly continuously at a low dose, disrupting rapidly proliferating tumor endothelium and preventing tumor growth.20 In a mouse model, Browder et al.19 demonstrated that tumor cells made resistant to traditional chemotherapeutic agents, such as cyclophosphamide, could be killed in vivo when the drug was given on a more frequent schedule. They further showed that this frequent chronic dosing targeted the tumor endothelium, resulting in endothelial apoptosis followed by tumor cell death.19 The activity and mechanism of antiangiogenic chemotherapy have been confirmed in animal models using different chemotherapy agents.21,22
Clinically, select patients with breast23 and ovarian24 cancer have demonstrated responses to agents to which they were previously resistant when these agents were delivered in near-continuous dosing. In human malignant gliomas, chemotherapeutic regimens targeting angiogenesis have been well tolerated, with at least a few patients showing minor or partial responses (PR).25,26 Additionally, a recent pediatric study using a metronomic regimen that we used in this study showed that it was tolerable and prolonged disease-free status in a heavily pretreated pediatric population.27 Responses were observed in patients with ependymomas.
In this phase II study, we sought to assess the safety and activity of a four-drug metronomic regimen (etoposide, cyclophosphamide, thalidomide, celecoxib) in patients with recurrent malignant gliomas. The treatment protocol consisted of two cytotoxic agents, etoposide and cyclophosphamide, administered continuously in alternating 21-day sequence at low doses,28,29 in combination with daily administration of two inhibitors of angiogenesis, thalidomide and celecoxib.30-32 All four drugs were administered orally and have been well tolerated in this population, offering a significant quality-of-life advantage for patients.
Patients and Methods
Objectives
The primary objective of this study was to determine the efficacy of oral administration of thalidomide, celecoxib, etoposide, and cyclophosphamide in patients with recurrent malignant gliomas as measured by the six-month progression-free survival (6M-PFS). We also evaluated the safety and toxicity of this four-drug combination. In addition, we sought to correlate response with levels of serum angiogenic peptides.
Patient Eligibility
Between March 2002 and October 2004, eligible adult patients were enrolled after providing written informed consent. The institutional review boards of the Dana-Farber Cancer Institute, Massachusetts General Hospital, and University of Virginia approved this protocol before enrollment (DFCI protocol 01-278). Eligibility criteria included patients with histologically proven recurrent intracranial malignant glioma. In addition, they had to meet the following criteria: unequivocal evidence of tumor recurrence or progression, by MRI or CT scan or by tumor resection, within 14 days prior to registration; on a stable dose of steroids for at least five days prior to entry and subsequent scans; failed prior radiation therapy, with an interval of at least four weeks from the completion of radiation therapy to study entry; age ≥ 18 years; life expectancy > two months; KPS ≥ 60; adequate hematological function (hemoglobin ≥ 10, absolute neutrophil count [ANC] ≥ 1,500/mm3, white blood cells [WBC] ≥ 3,000/mm3, platelets ≥ 100,000/mm3); adequate liver function (alanine transaminase and alkaline phosphatase < 2.5 times greater than normal, bilirubin < 1.5 mg); adequate renal function (blood urea nitrogen or creatinine < 1.5 times greater than normal). All patients had measurable disease on baseline MRI. Patients were excluded if they were pregnant or nursing, had greater than two months of prior oral therapy with any of the agents used in this study, had peripheral neuropathy greater than grade 1 (G1), had serious concurrent medical illness, had history of other cancers for which they received therapy within the past three years, refused to follow birth control measures during and for four weeks after treatment with thalidomide, or were concurrently using other investigational agents. There was no limit on the number of prior treatment regimens.
Treatment Regimen
Each six-week cycle began with oral etoposide administered daily for 21 days, followed by oral cyclophosphamide administered daily for 21 days. These chemotherapeutic agents were alternated to reduce the induction of autometabolism through hepatic microsomal enzyme activation and thus more rapid clearance of cyclophosphamide. Thalidomide and celecoxib were given daily, continuously, throughout the cycle. If etoposide or cyclophosphamide was held, thalidomide and celecoxib continued to be given at the treating physician's discretion. Alternating 21-day courses of etoposide and cyclophosphamide, along with daily thalidomide and celecoxib, were continued until tumor progression or the development of intolerable side effects. Oral etoposide was initially given at a dose of 50 mg/m2 daily, adjusted to the nearest 50-mg increment, but after the development of grade 3 (G3) and grade 4 (G4) hematological toxicities (leukopenia and neutropenia) in four out of the first seven patients, the protocol was amended and subsequent patients were treated with 35 mg/m2 of etoposide daily. Oral cyclophosphamide was given at a dose of 2 mg/kg daily, with a maximum daily dose of 100 mg daily; this drug was given in the morning to provide for adequate hydration. Thalidomide was begun at a dose of 50-200 mg p.o. at bedtime at the treating physician's discretion and escalated by 50 mg every week to a maximum of 1,200 mg daily. Celecoxib was begun at a dose of 200 mg twice daily and increased to 400 mg twice daily in patients weighing more than 50 kg. Therapy was continued until evidence of progression or toxicity. Patient diaries were used to monitor compliance with the regimen.
Dose Modifications and Patient Follow-Up
Patients were closely monitored throughout therapy for drug-related toxicity, and all adverse events were recorded and graded according to the NCI Common Toxicity Criteria (CTC, version 2.0). Pregnancy testing on women was performed weekly during the first month of thalidomide therapy and monthly thereafter in those with regular menstrual cycles or every two weeks in those with irregular menstrual cycles. All patients were required to participate in the mandatory System for Thalidomide Education and Prescribing Safety (S.T.E.P.S.) Program. Physical and neurological examinations were performed every three weeks (when changing cytotoxic agents). Hematological testing was performed weekly for the first six weeks and every three weeks thereafter. Study drugs were held for one week if a patient experienced a drug-related, G3 nonhematological toxicity and thrombocytopenia or G4 anemia and neutropenia. Dose reductions were performed until all symptomatic toxicities had resolved to grade 2 (G2) or lower. A new six-week cycle could begin when there was adequate hematological recovery (ANC ≥ 1,500/mm3, WBC ≥ 3,000/mm3, hemoglobin ≥ 10, and platelet count ≥ 100,000/mm3) and any nonhematological, symptomatic toxicities were ≤ G1.
Imaging and Response Assessment
MRI of the brain was performed every six weeks. Among others, axial and coronal T1 pre- and postgadolinium images were obtained and used for this study. Responses were determined using a modified Macdonald criteria33: complete response (CR), complete disappearance of tumor; partial response (PR), at least 50% decrease in the sum of products of the two largest perpendicular diameters of all measurable lesions; minor response (MR), between 0% and 50% decrease in the sum of products of the two largest perpendicular diameters of all measurable lesions; progressive disease (PD), at least 25% increase in the products of the two largest perpendicular diameters of all measurable lesions; stable disease (SD), neither MR, PR, nor PD. These criteria were applied when patients were on stable doses of steroids and also did not experience clinical deterioration other than that attributable to progressive tumor burden (e.g., systemic or metabolic disturbances). Responses (CR, PR, MR) had to be sustained on two successive scans taken at least four weeks apart compared to the best-response scan.
Angiogenic Peptide Measurements
Antiangiogenic activity was measured with assays of angiogenic peptides, as a biomarker of tumor response to the drug regimen.27 VEGF, bFGF, endostatin, and thrombospondin-1 levels were evaluated from batched samples of serum and urine (when available and with consent) using commercially available ELISA kits (VEGF and bFGF kits were obtained from R&D Systems, Minneapolis, MN, USA; the endostatin and thrombospondin kits were obtained from Cytimmune Sciences Inc., College Park, MD, USA) in accordance with the manufacturers' recommended methodology. Patients were requested to provide blood and/or urine samples before therapy and every ninth week, although samples were accepted whenever provided.
Statistical Methods
Patients were also stratified into two groups for statistical analysis: (1) glioblastoma multiforme (GBM) or gliosarcoma and (2) anaplastic glioma (AG), which included anaplastic astrocytoma (AA), anaplastic oligodendroglioma (AO), and anaplastic oligoastrocytoma (AOA). The primary endpoint was 6M-PFS from the time of registration. In a retrospective review of eight consecutive negative phase II trials in recurrent malignant gliomas from the M.D. Anderson Cancer Center, the 6M-PFS was 15% for GBM and 31% for AG.34 Our trial cohort included both GBM and AG patients who were entered at an approximately 2:1 ratio. The trial was sized to be able to discriminate between a 20% and 40% rate of 6M-PFS for the entire cohort and a 15% and 35% rate for the GBM group alone. The GBM comparison was the one of primary concern. Twenty-eight patients provide an 81% power, using a one-sided, binomial hypothesis test with significance level of 0.05 for this comparison of 6M-PFS. We planned to reject the null hypothesis if eight or more patients were progression free by six months. Assuming an accrual of approximately 16 AG patients, there would be a reasonable power to discriminate between a 20% and 40% rate of 6M-PFS for the entire group. For the group as a whole, the regimen would be considered effective if at least 30% 6M-PFS was observed. This rule gave at least a 0.9 probability of detecting a 40% rate of 6M-PFS, with at least a 0.9 probability of rejecting the drug combination if the 6M-PFS were only 20%. PFS and overall survival (OS) were estimated using the Kaplan-Meier method. The difference between response groups (PR + MR + SD vs. PD) and GBM status in OS or PFS curves was assessed using the log-rank test. Angiogenic peptides analysis included the assessment of differences between the response categories, in baseline values and the change from baseline to response values (which was obtained within 14 days of response date), in its absolute form and adjusted for baseline value. We used the Wilcoxon rank-sum test to determine the significance of these differences. The Cox proportional hazard model was used to determine the effect of angiogenic peptide levels on OS and PFS. All analyses were performed using SAS statistical software (version 8.0; SAS Institute, Inc., Cary, NC, USA).
Results
Population Characteristics
Forty-eight patients were enrolled with measurable, enhancing high-grade gliomas (Table 1). One patient never started treatment after signing informed consent and was lost to follow-up. Ages ranged from 33 to 74 years, with a median age of 53 years. There were 33 men and 15 women. Of the forty-eight patients, 20 had AG (four AA, seven AOA, nine AO), and 28 had GBM. Median KPS was 70 (range, 60-100). All patients had PD on MRI. The median number of prior recurrences was two. All patients received at least one adjuvant chemotherapy regimen after radiotherapy. The median number of prior chemotherapies was two; 32% had one prior chemotherapy, 34% had two, and 33% had three or more prior chemotherapies. Thus, all patients were well beyond the postradiation period.
Characteristics of the 48 patients
| Patient Characteristic . | Data . |
|---|---|
| Age | |
| Median (years) | 53 |
| Range (years) | 33-74 |
| Sex | |
| Male | 33 (68%) |
| Female | 15 (32%) |
| Median number of prior recurrences | 2 |
| Number of prior chemotherapy regimens | |
| 1 | 32% |
| 2 | 34% |
| ≥3 | 34% |
| Pathology | |
| Glioblastoma | 28 (64%) |
| Anaplastic oligodendroglioma | 9 (20%) |
| Mixed anaplastic oligoastrocytoma | 7 (16%) |
| Anaplastic astrocytoma | 4 (9%) |
| KPS | |
| Median | 70 |
| Range | 60-100 |
| Patient Characteristic . | Data . |
|---|---|
| Age | |
| Median (years) | 53 |
| Range (years) | 33-74 |
| Sex | |
| Male | 33 (68%) |
| Female | 15 (32%) |
| Median number of prior recurrences | 2 |
| Number of prior chemotherapy regimens | |
| 1 | 32% |
| 2 | 34% |
| ≥3 | 34% |
| Pathology | |
| Glioblastoma | 28 (64%) |
| Anaplastic oligodendroglioma | 9 (20%) |
| Mixed anaplastic oligoastrocytoma | 7 (16%) |
| Anaplastic astrocytoma | 4 (9%) |
| KPS | |
| Median | 70 |
| Range | 60-100 |
Characteristics of the 48 patients
| Patient Characteristic . | Data . |
|---|---|
| Age | |
| Median (years) | 53 |
| Range (years) | 33-74 |
| Sex | |
| Male | 33 (68%) |
| Female | 15 (32%) |
| Median number of prior recurrences | 2 |
| Number of prior chemotherapy regimens | |
| 1 | 32% |
| 2 | 34% |
| ≥3 | 34% |
| Pathology | |
| Glioblastoma | 28 (64%) |
| Anaplastic oligodendroglioma | 9 (20%) |
| Mixed anaplastic oligoastrocytoma | 7 (16%) |
| Anaplastic astrocytoma | 4 (9%) |
| KPS | |
| Median | 70 |
| Range | 60-100 |
| Patient Characteristic . | Data . |
|---|---|
| Age | |
| Median (years) | 53 |
| Range (years) | 33-74 |
| Sex | |
| Male | 33 (68%) |
| Female | 15 (32%) |
| Median number of prior recurrences | 2 |
| Number of prior chemotherapy regimens | |
| 1 | 32% |
| 2 | 34% |
| ≥3 | 34% |
| Pathology | |
| Glioblastoma | 28 (64%) |
| Anaplastic oligodendroglioma | 9 (20%) |
| Mixed anaplastic oligoastrocytoma | 7 (16%) |
| Anaplastic astrocytoma | 4 (9%) |
| KPS | |
| Median | 70 |
| Range | 60-100 |
Toxicity of Regimen
Therapy was reasonably well tolerated in this heavily pretreated patient cohort. The median number of completed cycles was one (range, 0-15). The median dose of thalidomide was 400 mg. Toxicity was monitored and graded (G1 to G4) based on the NCI CTC throughout the trial. There were no treatment-related deaths. Constipation and fatigue were common. In most patients these were usually mild, but three patients (6%) experienced G3 constipation, two (4%) experienced G4 constipation, and one patient (2%) experienced G3 fatigue. Oral etoposide was initially given at a dose of 50 mg/m2 daily, adjusted to the nearest 50-mg increment, but after the development of G3 and G4 hematological toxicities (leukopenia and neutropenia) in four of the first seven patients, the protocol was amended and subsequent patients were treated with 35 mg/m2 of etoposide daily. Six patients discontinued therapy secondary to toxicities possibly or probably related to the treatment protocol. Of these six patients, four had G4 leukopenia or neutropenia, one had a G3 tremor probably related to treatment, and one had a G2 rash and leukopenia. Five venous thromboembolic events were noted in this study. The G3 and G4 toxicities associated with treatment are listed in Table 2.
Grade 3 and 4 toxicities per patient (n = 48)
| . | Number (%) of Incidents . | . | |
|---|---|---|---|
| Toxicity . | Grade 3 . | Grade 4 . | |
| Anemia | 1 (2) | 0 | |
| Ataxia | 1 (2) | 0 | |
| Colitis | 0 | 2 (4) | |
| Constipation | 3 (6) | 2 (4) | |
| Dizziness | 1 (2) | 0 | |
| Dysphagia | 1 (2) | 0 | |
| Fatigue | 1 (2) | 0 | |
| Hepatotoxicity | 2 (4) | 0 | |
| Hyperglycemia | 2 (4) | 0 | |
| Hypoxia | 1 (2) | 0 | |
| Infection | 4 (8) | 0 | |
| Leukopenia | 7 (14.6) | 6 (12.5) | |
| Lymphopenia | 9 (18.8) | 0 | |
| Nausea/vomiting | 4 (8) | 0 | |
| Neutropenia | 2 (4) | 8 (16.7) | |
| Rash | 1 (2) | 0 | |
| Somnolence | 1 (2) | 0 | |
| Thrombocytopenia | 1 (2) | 0 | |
| Thrombosis | 1 (2) | 4 (8) | |
| Tremor | 1 (2) | 0 | |
| . | Number (%) of Incidents . | . | |
|---|---|---|---|
| Toxicity . | Grade 3 . | Grade 4 . | |
| Anemia | 1 (2) | 0 | |
| Ataxia | 1 (2) | 0 | |
| Colitis | 0 | 2 (4) | |
| Constipation | 3 (6) | 2 (4) | |
| Dizziness | 1 (2) | 0 | |
| Dysphagia | 1 (2) | 0 | |
| Fatigue | 1 (2) | 0 | |
| Hepatotoxicity | 2 (4) | 0 | |
| Hyperglycemia | 2 (4) | 0 | |
| Hypoxia | 1 (2) | 0 | |
| Infection | 4 (8) | 0 | |
| Leukopenia | 7 (14.6) | 6 (12.5) | |
| Lymphopenia | 9 (18.8) | 0 | |
| Nausea/vomiting | 4 (8) | 0 | |
| Neutropenia | 2 (4) | 8 (16.7) | |
| Rash | 1 (2) | 0 | |
| Somnolence | 1 (2) | 0 | |
| Thrombocytopenia | 1 (2) | 0 | |
| Thrombosis | 1 (2) | 4 (8) | |
| Tremor | 1 (2) | 0 | |
Grade 3 and 4 toxicities per patient (n = 48)
| . | Number (%) of Incidents . | . | |
|---|---|---|---|
| Toxicity . | Grade 3 . | Grade 4 . | |
| Anemia | 1 (2) | 0 | |
| Ataxia | 1 (2) | 0 | |
| Colitis | 0 | 2 (4) | |
| Constipation | 3 (6) | 2 (4) | |
| Dizziness | 1 (2) | 0 | |
| Dysphagia | 1 (2) | 0 | |
| Fatigue | 1 (2) | 0 | |
| Hepatotoxicity | 2 (4) | 0 | |
| Hyperglycemia | 2 (4) | 0 | |
| Hypoxia | 1 (2) | 0 | |
| Infection | 4 (8) | 0 | |
| Leukopenia | 7 (14.6) | 6 (12.5) | |
| Lymphopenia | 9 (18.8) | 0 | |
| Nausea/vomiting | 4 (8) | 0 | |
| Neutropenia | 2 (4) | 8 (16.7) | |
| Rash | 1 (2) | 0 | |
| Somnolence | 1 (2) | 0 | |
| Thrombocytopenia | 1 (2) | 0 | |
| Thrombosis | 1 (2) | 4 (8) | |
| Tremor | 1 (2) | 0 | |
| . | Number (%) of Incidents . | . | |
|---|---|---|---|
| Toxicity . | Grade 3 . | Grade 4 . | |
| Anemia | 1 (2) | 0 | |
| Ataxia | 1 (2) | 0 | |
| Colitis | 0 | 2 (4) | |
| Constipation | 3 (6) | 2 (4) | |
| Dizziness | 1 (2) | 0 | |
| Dysphagia | 1 (2) | 0 | |
| Fatigue | 1 (2) | 0 | |
| Hepatotoxicity | 2 (4) | 0 | |
| Hyperglycemia | 2 (4) | 0 | |
| Hypoxia | 1 (2) | 0 | |
| Infection | 4 (8) | 0 | |
| Leukopenia | 7 (14.6) | 6 (12.5) | |
| Lymphopenia | 9 (18.8) | 0 | |
| Nausea/vomiting | 4 (8) | 0 | |
| Neutropenia | 2 (4) | 8 (16.7) | |
| Rash | 1 (2) | 0 | |
| Somnolence | 1 (2) | 0 | |
| Thrombocytopenia | 1 (2) | 0 | |
| Thrombosis | 1 (2) | 4 (8) | |
| Tremor | 1 (2) | 0 | |
Response and Survival
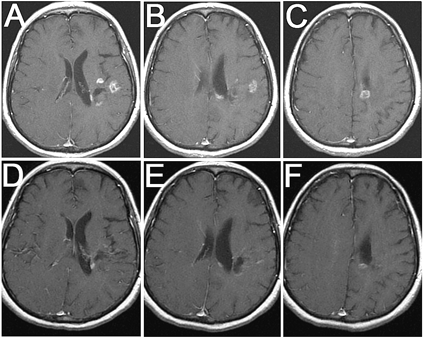
Two percent (n = 1) of patients showed PR (Fig. 1), 9% (n = 4) showed MR, 59% (n = 26) had stable disease (SD), and 30% (n = 13) had PD at their first scan. For the vast majority of these patients, this metronomic chemotherapy represented their final treatment. When comparing responders (SD + MR + PR, n = 31) to nonresponders (PD, n = 13) for the whole cohort, there was a significant difference in OS (p = 0.049), with median point estimates for responders and nonresponders of 33 and 20 weeks, respectively; PFS was also significantly different (p < 0.0001), with 6M-PFS estimates of 21% versus 0% in the responder and the nonresponder groups, respectively. The median duration of response was 24 weeks for PR/MR and six weeks for SD.
MRI response to antiangiogenic regimen: 64-year-old woman with recurrent anaplastic astrocytoma treated previously with surgery, radiation therapy, temozolomide, PCV (procarbazine, chloroethyl cyclohexylnitrosourea, and vincristine), CCI-779 (temsirolimus), and fenretinide before (A, B, C) and six months after (D, E, F) treatment with antiangiogenic chemotherapy. All images are axial T1-weighted postgadolinium images.
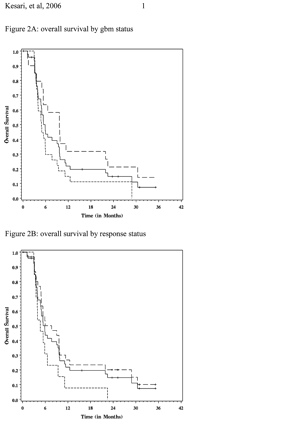
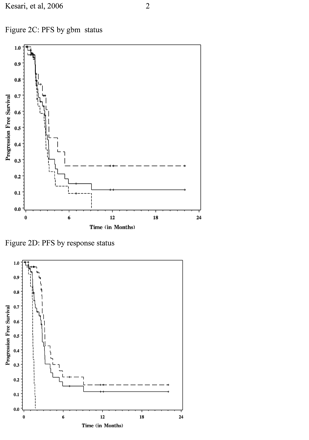
For GBM patients, median PFS was 11 weeks, 6M-PFS was 9%, and median OS was 21 weeks (Fig. 2). There was no difference (p = 0.48) in the median point estimates for OS (21 vs. 20 weeks) for responders (n = 16; 15 died, 1 censored) and nonresponders (n = 9; 9 died) in this subgroup. 6M-PFS was significantly different (p < 0.0001), with estimates of 14% versus 0% in the responder and the nonresponder groups, respectively.
For AG patients, median PFS was 14 weeks, 6M-PFS was 26%, and median OS was 42 weeks. There was no difference (n too small to report p-value) in the median point estimates for OS (42 vs. 22 weeks) for responders (n = 15; 11 died, 4 censored) and nonresponders (n = 4; 4 died) in this subgroup. 6M-PFS was significantly different (p = 0.0008), with estimates of 31% versus 0% in the responder and the nonresponder groups, respectively.
In this cohort, 24 patients were on enzyme-inducing antiepileptic drugs (EIAEDs), 16 patients were on non-EIAEDs, and 8 were not on any seizure medications at baseline. When comparing patients on EIAEDs to the rest, the response categorization by antiepileptic drug status was not affected (p = 0.74), and the 6M-PFS (21% vs. 6%; p = 0.74) and OS (median 32 vs. 23 weeks; p = 0.45) were not statistically different in these cohorts.
Angiogenic Peptide Measurements and Correlation with Response and Survival
We sought in vitro correlates predictive of tumor response to the drug regimen by measuring serum and urine angiogenic peptides during the course of treatment. Serum and urine levels of VEGF, bFGF, endostatin, and thrombospondin-1 were evaluated in patients who consented to the biological analysis. Of the 48 patients, a subset of samples was available for angiogenic peptide testing from serum (n = 25) and urine (n = 31). We did not find any statistically significant differences between responders (PR + MR + SD) and nonresponders (PD) in changes in serum or urine levels of bFGF or VEGF from baseline to best response (absolute and adjusted for baseline values; all p > 0.05). For the comparison of baseline levels, only urine VEGF was significantly different (p = 0.02) between responders (n = 23) and nonresponders (n = 9). Baseline or change in urine and serum bFGF and VEGF levels did not appear to affect OS or PFS. However, concerns over power make this result inconclusive. For thrombospondin-1 and endostatin, we were unable to perform a statistically meaningful analysis because data were available for only two patients in one of the response categories (PD). Because we were comparing angiogenic peptide values only at our first and last observation points, the possibility remains that there may be more temporally limited correlates of disease activity and drug response that were not detected with our method.
Discussion
Malignant gliomas are poorly responsive and highly resistant to standard chemotherapies. Alternative ways of increasing the efficacy of these chemotherapeutic agents would be of tremendous benefit for patients with malignant gliomas where options are limited. Antiangiogenic chemotherapeutic (metronomic) regimens offer a novel approach to these resistant tumors by inhibiting the tumor vasculature with conventional chemotherapy, preventing tumor growth. In addition, metronomic chemotherapy may prevent angiogenesis by increasing plasma levels of the angiogenesis inhibitor thrombospondin-121 and decreasing the numbers and viability of circulating endothelial progenitor (CEP) cells, which may play an important role in tumor angiogenesis.35 Many standard chemotherapeutic agents used in conventional schedules do not normally cross an intact blood-brain barrier. However, metronomic regimens that target and disrupt the tumor vasculature theoretically may also give these same drugs improved access to the tumor.
Estimates of Kaplan-Meier overall survival (A, B) and progression-free survival (C, D) for entire study population and subsets. (A) Overall survival for all patients (solid line, n = 48) and subsets of glioblastoma (small dashed line, n = 28) and anaplastic glioma (large dashed line, n = 20) patients. (B) Overall survival for all patients (solid line) and subsets of responders (large dashed line, n = 31) and nonresponders (small dashed line, n = 13). (C) Progression-free survival for all patients (solid line) and subsets of glioblastoma (small dashed line, n = 28) and anaplastic glioma (large dashed line, n = 20) patients. (D) Progression-free survival for all patients (solid line) and subsets of responders (large dashed line, n = 31) and nonresponders (small dashed line, n = 13).
We used the conventional chemotherapeutic agents etoposide and cyclophosphamide, given orally in a metronomic fashion (daily, alternating each drug every 21 days). Oral etoposide has been studied in patients with recurrent malignant glioma and shown to be well tolerated, with an overall response rate (including SD) of 42%.28 Oral cyclophosphamide has been used as palliative chemotherapy in systemic malignancies29 and in non-malignant disease,36 where it has been well tolerated.
Thalidomide was initially developed as a sedative in the 1950s but subsequently achieved notoriety for its teratogenic effects. In the mid-1990s, the observation that thalidomide has potent antiangiogenic properties sparked renewed interest in the drug.37 Several phase II trials of thalidomide for recurrent high-grade astrocytomas showed that the drug had modest activity in malignant gliomas and was reasonably well tolerated.38,39 In one study of 36 evaluable patients treated with up to 1,200 mg of thalidomide daily, four patients had objective radiographic regressions on MRI scans, and another 12 patients had stabilization of disease for at least two months.38 In this study, a statistically significant relationship between changes in serum bFGF levels (arguably a marker for angiogenic activity) and radiographic response and survival was observed. Other studies using lower doses of thalidomide have shown similar evidence of modest benefit. Marx et al.39 treated patients with recurrent malignant gliomas with thalidomide doses up to 500 mg/day and obtained 5% PR and 42% SD, while Short et al.,40 using only 100 mg/day of thalidomide, found a 6% PR.
Celecoxib is a cyclooxygenase-2 (COX-2) inhibitor that has recently been shown to have antiangiogenic activity, in addition to its better known antiinflammatory and analgesic actions. The COX enzymes, which catalyze the synthesis of prostaglandin-E from arachidonic acid, differ in their patterns of expression. While COX-1 is constitutively expressed in most tissues, COX-2 is induced in inflammatory cells by cytokines. Furthermore, COX-2 expression is up-regulated in human tumors,41 and COX-2 inhibition can suppress tumor formation and growth.42 Malignant gliomas strongly express COX-243; furthermore, COX-2 inhibitors can reduce proliferation of glioma cells in vitro.44 That at least some of this antitumor activity is due to inhibition of angiogenesis is suggested by studies showing that COX-2 inhibitors can down-regulate VEGF expression and activity,42,45 impair integrin signaling pathways,46 and inhibit bFGF-induced neovascularization.47 Taken together, these data suggest that COX-2 inhibitors such as celecoxib may have a role in the treatment of malignant gliomas.
The four-drug combination was generally well tolerated in this group of heavily pretreated patients with recurrent malignant gliomas (Table 2). Five patients developed venous thromboembolic disease (VTD). Thalidomide has been associated with an increased incidence of VTD in other malignancies such as multiple myeloma.48 However, the number of patients with VTD observed in this study does not appear to be high for a group of malignant glioma patients with advanced disease who are known to be at greater risk of VTD.49
The regimen showed only minimal antitumor activity. One patient (2%) showed PR, and four (8%) showed MR. Since all patients had at least one adjuvant chemotherapy regimen, they were well beyond the postradiation period, so these objective responses cannot be attributable to the resolution of postradiation changes. Six patients completed at least four cycles of treatment. The sample size was too small to determine the clinical features of any subgroup of patients who would most benefit from this regimen. Because the tumor growth inhibitory effects of antiangiogenesis inhibitors may be delayed and take several weeks or months to be fully appreciated, it is possible that the strict use of the Macdonald criteria may have underestimated the benefit of the metronomic chemotherapy. Some studies of angiogenesis inhibitors have allowed patients to remain on study if they have up to 50% increase in tumor size (as measured by the sum of products of the two largest perpendicular diameters of all measurable lesions) provided that the patients remain clinically stable. The benefit of this regimen may have been limited by the fact that most patients in this study had extensive disease and were heavily pretreated. Metronomic chemotherapy may potentially be more effective when used in patients with small tumor burden or in the adjuvant setting. In addition, while antiangiogenic approaches may prevent enlargement of the tumor mass, they may still allow—and possibly promote—the diffuse infiltration of tumor cells to other areas of brain tissue.11,50
There are several studies using metronomic chemotherapy for a variety of cancers, including brain tumors.51-55 An interim analysis of a phase II study of 16 adults with recurrent AGs using thalidomide and daily low-dose cyclophosphamide appeared promising, with three responses (one CR, two PR) reported.56 The four-drug regimen used in this study was reported by Kieran et al.27 to be well tolerated in pediatric patients with recurrent malignancies. PRs were observed in three patients (two ependymomas, one glioma), and all three finished the six months of treatment. Additionally, Kieran et al. found that one of the antiangiogenic factors (thrombospondin-1) appeared to correlate with prolonged response.27 In contrast, a regimen of continuous low-dose cyclophosphamide (100 mg) and twice-weekly methotrexate (5 mg) in 10 recurrent GBM patients showed no responders and a disappointing 6M-PFS of 0%, resulting in early closure of the trial.25
Several potential strategies could improve the effectiveness of metronomic chemotherapy. In this study, we used thalidomide and celecoxib, which were the only antiangiogenic agents available to us at the time the study was conceived. It is likely that the combination of metronomic chemotherapy with more potent inhibitors of angiogenesis will lead to greater antitumor activity. Potential agents include bevacizumab (humanized VEGF monoclonal antibody), lenalidomide (newer thalidomide analogue), tyrosine kinase inhibitors such sorafenib (multikinase inhibitor of VEGF, Raf, and PDGF receptor), sunitinib (multikinase inhibitor of PDGF receptor, VEGF receptor, and c-Kit), AZD2171 (VEGF receptor inhibitor), enzastaurin (protein kinase C beta inhibitor), and combination of imatinib and hydrea.57 Some novel chemotherapeutic agents such as gimatecan (topoisomerase inhibitor)58 also appear to have antiangiogenic activity when administered continuously. A second approach involves combining metronomic chemotherapy with drugs that prevent invasion. One possible agent is cilengitide, an αvβ3 and αvβ5 inhibitor, which has both antiangiogenic and antiinvasive activity and is under evaluation in clinical trials. It is likely that in the near future, other more effective agents inhibiting invasion will become available. Third, metronomic chemotherapy may be more effective in patients with a smaller tumor burden such as in the adjuvant setting or in patients with recurrent gliomas following surgical debulking. Fourth, using more active agents for gliomas, such as temozolomide, in a metronomic fashion may be more efficacious.26,59,60
Recently, the mechanism by which metronomic chemotherapy mediates some of the antiangiogenic effect has been reported to be via increasing thrombospondin-1 levels in animals.21 We sought to identify such biomarkers (serum and urine) of antiangiogenic activity, but we did not find any of the four tested markers (VEGF, bFGF, endostatin, and thrombospondin-1) to be of prognostic significance. This may be related in part to the low response rate and the small sample size. We did not measure CEP cells,35 which were reported to be decreased by metronomic chemotherapy in preclinical studies. Evaluation of CEP cells and circulating endothelial cells may be of interest in future studies using this approach.
In summary, this four-drug metronomic chemotherapy regimen in adults with recurrent malignant gliomas was fairly well tolerated but produced only minimal benefit. Nonetheless, this approach warrants further evaluation. Future studies, combining metronomic chemotherapy with more potent antiangiogenic agents and antiinvasive agents may result in greater antitumor activity.
This work was supported in part by grant from Celgene Corporation. We gratefully acknowledge the support of the Neil Harrington Brain Tumor Research Fund.
References
Stupp R, Mason WP, van den Bent MJ, et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma.
Brem S, Tsanaclis AM, Gately S, Gross JL, Herblin WF. Immunolocalization of basic fibroblast growth factor to the microvasculature of human brain tumors.
Kesari S, Ramakrishna N, Sauvageot C, Stiles CD, Wen PY. Targeted molecular therapy of malignant gliomas.
Plate KH, Breier G, Weich HA, Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo.
Kargiotis O, Rao JS, Kyritsis AP. Mechanisms of angiogenesis in gliomas.
Leon SP, Folkerth RD, Black PM. Microvessel density is a prognostic indicator for patients with astroglial brain tumors.
Pluda JM. Tumor-associated angiogenesis: mechanisms, clinical implications, and therapeutic strategies.
Segal DH, Germano IM, Bederson JB. Effects of basic fibroblast growth factor on in vivo cerebral tumorigenesis in rats.
Kunkel P, Ulbricht U, Bohlen P, et al. Inhibition of glioma angiogenesis and growth in vivo by systemic treatment with a monoclonal antibody against vascular endothelial growth factor receptor-2.
Laird AD, Christensen JG, Li G, et al. SU6668 inhibits Flk-1/KDR and PDGFRbeta in vivo, resulting in rapid apoptosis of tumor vasculature and tumor regression in mice.
Laird AD, Vajkoczy P, Shawver LK, et al. SU6668 is a potent antiangiogenic and antitumor agent that induces regression of established tumors.
Man S, Bocci G, Francia G, et al. Antitumor effects in mice of low-dose (metronomic) cyclophosphamide administered continuously through the drinking water.
Ohlfest JR, Demorest ZL, Motooka Y, et al. Combinatorial antiangiogenic gene therapy by nonviral gene transfer using the sleeping beauty transposon causes tumor regression and improves survival in mice bearing intracranial human glioblastoma.
Hurwitz H, Fehrenbacher L, Novotny, W, et al. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer.
Kabbinavar F, Hurwitz HI, Fehrenbacher L, et al. Phase II, randomized trial comparing bevacizumab plus fluorouracil (FU)/leucovorin (LV) with FU/LV alone in patients with metastatic colorectal cancer.
Bogler O, Mikkelsen T. Angiogenesis and apoptosis in glioma: two arenas for promising new therapies.
Browder T, Butterfield CE, Kraling BM, et al. Antiangiogenic scheduling of chemotherapy improves efficacy against experimental drug-resistant cancer.
Miller KD, Sweeney CJ, Sledge GW Jr. Redefining the target: chemotherapeutics as antiangiogenics.
Bocci G, Francia G, Man S, Lawler J, Kerbel RS. Thrombospondin 1, a mediator of the antiangiogenic effects of low-dose metronomic chemotherapy.
Klement G, Baruchel S, Rak J, et al. Continuous low-dose therapy with vinblastine and VEGF receptor-2 antibody induces sustained tumor regression without overt toxicity.
Seidman AD, Hochhauser D, Gollub M, et al. Ninety-six-hour paclitaxel infusion after progression during short taxane exposure: a phase II pharmacokinetic and pharmacodynamic study in metastatic breast cancer.
Fennelly D, Aghajanian C, Shapiro F, et al. Phase I and pharmacologic study of paclitaxel administered weekly in patients with relapsed ovarian cancer.
Herrlinger U, Rieger J, Steinbach JP, et al. UKT-04 trial of continuous metronomic low-dose chemotherapy with methotrexate and cyclophosphamide for recurrent glioblastoma.
Tuettenberg J, Grobholz R, Korn T, et al. Continuous low-dose chemotherapy plus inhibition of cyclooxygenase-2 as an antiangiogenic therapy of glioblastoma multiforme.
Kieran MW, Turner CD, Rubin JB, et al. A feasibility trial of antiangiogenic (metronomic) chemotherapy in pediatric patients with recurrent or progressive cancer.
Fulton D, Urtasun R, Forsyth P, et al. Phase II study of prolonged oral therapy with etoposide (VP16) for patients with recurrent malignant glioma.
Asou N, Suzushima H, Nishimura S, et al. Long-term remission in an elderly patient with mantle cell leukemia treated with low-dose cyclophosphamide.
Fine HA, Wen PY, Maher EA, et al. Phase II trial of thalidomide and carmustine for patients with recurrent high-grade gliomas.
Gately S, Li WW. Multiple roles of COX-2 in tumor angiogenesis: a target for antiangiogenic therapy.
Wei D, Wang L, He Y, et al. Celecoxib inhibits vascular endothelial growth factor expression in and reduces angiogenesis and metastasis of human pancreatic cancer via suppression of Sp1 transcription factor activity.
Macdonald DR, Cascino TL, Schold SC Jr, Cairncross JC. Response criteria for phase II studies of supratentorial malignant glioma [see comment].
Wong ET, Hess KR, Gleason MJ, et al. Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials [see comment].
Bertolini F, Paul S, Mancuso P, et al. Maximum tolerable dose and low-dose metronomic chemotherapy have opposite effects on the mobilization and viability of circulating endothelial progenitor cells.
Lien YH, Scott K. Long-term cyclophosphamide treatment for recurrent type I membranoproliferative glomerulonephritis after transplantation.
D'Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis.
Fine HA, Figg WD, Jaeckle K, et al. Phase II trial of the antiangiogenic agent thalidomide in patients with recurrent high-grade gliomas.
Marx GM, Pavlakis N, McCowatt S, et al. Phase II study of thalidomide in the treatment of recurrent glioblastoma multiforme.
Short SC, Traish D, Dowe A, et al. Thalidomide as an anti-angiogenic agent in relapsed gliomas.
Fujiwaki R, Iida K, Kanasaki H, et al. Cyclooxygenase-2 expression in endometrial cancer: correlation with microvessel count and expression of vascular endothelial growth factor and thymidine phosphorylase.
Tortora G, Caputo R, Damiano V, et al. Combination of a selective cyclooxygenase-2 inhibitor with epidermal growth factor receptor tyrosine kinase inhibitor ZD1839 and protein kinase A antisense causes cooperative antitumor and antiangiogenic effect.
Deininger MH, Weller M, Streffer J, Mittelbronn M, Meyermann R. Patterns of cyclooxygenase-1 and -2 expression in human gliomas in vivo.
Joki T, Heese O, Nikas DC, et al. Expression of cyclooxygenase 2 (COX-2) in human glioma and in vitro inhibition by a specific COX-2 inhibitor, NS-398.
Hernandez GL, Volpert OV, Iniguez MA, et al. Selective inhibition of vascular endothelial growth factor-mediated angiogenesis by cyclosporin A: roles of the nuclear factor of activated T cells and cyclooxygenase 2.
Dormond O, Foletti A, Paroz C, Reugg C. NSAIDs inhibit alpha V beta 3 integrin-mediated and Cdc42/Rac-dependent endothelial-cell spreading, migration and angiogenesis.
Daniel TO, Liu H, Morrow JD, Crews BC, Marnett LJ. Thromboxane A2 is a mediator of cyclooxygenase-2-dependent endothelial migration and angiogenesis.
Osman K, Comenzo R, Rajkumar SV. Deep venous thrombosis and thalidomide therapy for multiple myeloma.
Wen PY, Schiff DS, Kesari S, et al. Medical management of patients with brain tumors.
Lamszus K, Kunkel P, Westphal M. Invasion as limitation to antiangiogenic glioma therapy.
Correale P, Cerretani D, Remondo C, et al. A novel metronomic chemotherapy regimen of weekly platinum and daily oral etoposide in high-risk non-small cell lung cancer patients.
Kerbel RS, Kamen BA. The anti-angiogenic basis of metronomic chemotherapy.
Nicolini A, Mancini P, Ferrari P, et al. Oral low-dose cyclophosphamide in metastatic hormone refractory prostate cancer (MHRPC).
Desjardins A, Quinn J, Reardon D, et al. Phase II study of thalidomide and daily low-dose cyclophosphamide for adults with anaplastic gliomas [Abstract TA-16]. Paper presented at:
Kvasnicka HM, Thiele J, Staib P, et al. Reversal of bone marrow angiogenesis in chronic myeloid leukemia following imatinib mesylate (STI571) therapy.
Petrangolini G, Pratesi G, De Cesare M, et al. Antiangiogenic effects of the novel camptothecin ST1481 (gimatecan) in human tumor xenografts.
Kim JT, Kim JS, Kow KW, et al. Metronomic treatment of temozolomide inhibits tumor cell growth through reduction of angiogenesis and augmentation of apoptosis in orthotopic models of gliomas.
- angiogenesis
- endothelial cells
- chemotherapy regimen
- cyclophosphamide
- etoposide
- glioblastoma
- adult
- angiogenesis inhibitors
- phase 2 clinical trials
- glioma
- peptides
- thalidomide
- neoplasms
- urine
- celecoxib
- tumor growth
- glioma, malignant
- chemotherapy, metronomic
- partial response
- progressive neoplastic disease
- progression-free survival
- urine specimens