Immunotherapy in colorectal cancer: for the select few or all?
Introduction
Chemotherapy and novel biologics targeting the epidermal growth factor receptor (EGFR) and vascular endothelial growth factor (VEGF) pathways have been the mainstay treatments for advanced or metastatic colorectal cancer (mCRC) (1). However, in heavily treated, advanced refractory CRC patients, these agents only modestly improve survival (2). The median survival of patients with mCRC following failure of 5-florouracil (5-FU)-based chemotherapy and anti-VEGF and/or anti-EGFR therapy is approximately 6 months (3). Primary or acquired resistance to these therapeutics likely accounts for dismal outcomes. Thus, the efficacy of novel agents targeting alternate pathways need to be investigated in CRC. Immunotherapy has changed the course of many cancers that had dismal prognoses in the past, such as advanced lung, melanoma, bladder and head and neck cancers (4-9). Over the past few years, we have had more promise for the role of immunotherapy in colorectal cancer (CRC), specifically in subsets of CRC with microsatellite instability (MSI) for which newer agents, such as programmed death-1 (PD-1) inhibitors, are efficacious. While other immunotherapeutic agents are more immature in development, these may be possible immunotherapeutics for all CRC patients. Pre-clinical and early phase studies show activity, which does not always translate to efficacy for all patients. We will review the later phase studies that demonstrate the role of immunotherapy in CRC and provide hope for changing treatment algorithm for CRC in the future.
The role of immune system in CRC
The immune system plays a complex role in cancer carcinogenesis and treatment (10). CRC evades the immune system via several methods. Tumors avoid destruction by circulating T lymphocytes and natural killer (NK) cells by releasing immunosuppressive factors such as TGF-beta from cancer cells. Tumors recruit immunosuppressive cells like regulatory T cells (Treg) and myeloid-derived suppressor cells (MDSCs) to evade lymphocyte-induced death (11). CRC has also increased pro-tumor inflammatory responses, such as high levels of IL-6 that activates STAT-3, leading to increased tumor activity (12). Also, CRC has high levels of macrophage-derived MMP-9, which degrades type IV collagen in the basement membrane, allowing for metastasis (13). Additionally, chronic inflammatory states, such as inflammatory bowel disease, increase the risk of CRC. One cause may be related to changes in the microbiome, resulting in an increase in inflammatory and pro-tumorigenic cytokines (14).
Exploiting the immune system to control CRC
Immunotherapy harnesses the immune system to eliminate cancer by aiming to augment the anti-tumor immune responses through multiple strategies, such as vaccines and checkpoint inhibitors, which target the immunosuppressive pathways and enable an anti-tumor immune response. The following groups of agents have been studied in later phase clinical trials, and we will review the efficacy of these agents in patients with CRC.
Immune-modulating agents
Immunotherapy in CRC has been investigated in agents with immune-modulating properties, such as levamisole. Levamisole is thought to induce antibodies against tumor antigens but also may help prevent cancer growth by cell-mediated immunity (15). However, several randomized controlled trials, including a large adjuvant studies in CRC, have not demonstrated efficacy (16,17). Other agents have been investigated but have not proceeded on to later phase studies.
Checkpoint inhibitors
While various immunomodulating agents have been investigated showing minimal efficacy, checkpoint inhibition has recently shown the most promise as a treatment for CRC, but efficacy may be reserved for a specific subset of patients. Cancers exploit major histocompatibility complex-T-cell receptor (MHC-TCR) signaling pathways by upregulating co-inhibitory molecules to suppress the immune system, and thus, result in T cell apoptosis or dysfunction. Co-inhibitory molecules include PD-1, PD-L 1, PD-L2, cytotoxic T-lymphocyte-associated protein 4 (CTLA-4), T cell immunoglobulin mucin-3 (TIM-3), B and T lymphocyte attenuator (BTLA), indoleamine 2,3-dioxygenase (IDO) and lymphocyte-activation gene 3 (LAG-3). Checkpoint inhibitors are monoclonal antibodies that selectively re-activate immune checkpoints to kill tumor cells.
Mismatch repair-deficient (MMR-d) CRC, which is as high as 15% of all CRC (18,19), is highly immunogenic and responsive to immune checkpoint blockade versus mismatch repair-proficient (MMR-p) CRC (20). CTLA-4, PD-1, and LAG-3 are expressed at considerably higher levels in MSI compared with microsatellite stable (MSS) tumors (P<0.05 in all compartments for CTLA4, in tumor infiltrating lymphocytes (TILs) and the invasive front for LAG3; P>0.05 in all compartments for PD-1) (21).
CTLA-4
CTLA-4 is present on the surface of CD4 and CD8 T cells and binds to B7 ligands on APCs, thus preventing B7 binding to CD28 receptors on T cells, leading to inhibition of immune stimulation. To date, as monotherapy, CTLA-4 agents have been effective in melanoma; however, they have yet to show efficacy in CRC. Tremelimumab, a fully human anti-CTLA-4 monoclonal antibody, was given to patients with refractory CRC with good tolerability (22). One partial response was observed, but most patients had progressive disease after one dose (22). Thus, as a single agent, blockage of CTLA4 was not clinically efficacious; however, it is possible that with combination with another agent, such as chemotherapy or another checkpoint inhibitor, CTLA4 blockage may be potentiated, which would induce a greater load of tumor-specific antigen leading to further response. Currently, a study with ipilumumab, humanized anti-CTLA-4 monoclonal antibody, and a PD-1 inhibitor is ongoing (details below).
PD-1
Currently, it is well known that MSI is a biomarker for PD-1 blockade (23). MMR-d CRC have an increased number of mutation-associated neoantigens as a result of mutations (i.e., frameshift), which have the potential to be recognized by the immune system. However, PD-L1 and PD-L2 on tumor cells suppress the immune response by binding to PD-1 receptor on effector T cells. Tumors upregulate PD-L1 to evade the host immune system. This very complex interaction serves as the rationale for MMR-d CRC having an enhanced anti-PD-1 responsiveness, which is not observed in MMR-p CRC (24).
Currently, two PD-1 inhibitors are the most advanced in development, nivolumab and pembrolizumab. Nivolumab (Opdivo, BMS-936558) is a fully human IgG4 monoclonal antibody directed against PD-1, currently FDA approved for several solid tumors such as melanoma, non-small cell lung, kidney, head and neck and bladder cancers. Pembrolizumab (Keytruda, MK-3475) is a humanized IgG4 monoclonal antibody that binds and prevents the interaction of PD-L1 and PD-L2, resulting in immune recognition and response. Pembrolizumab is currently FDA approved for melanoma, non-small cell lung, and head and neck cancers.
In initial phase I studies of PD-1 inhibitors, such as nivolumab, patients with CRC did not have objective responses (25,26). However, a long-term follow-up of a patient with a complete response was noted at 3 years. The patient had MSI with PDL1- expression on tumor-infiltrating macrophages, lymphocytes, and rare tumor cells, which were associated with PD-1 and CD-3 positive T cells (27). This was one of the first promises of immunotherapy in CRC.
The hypothesis of a phase II study was that MMR-d tumors are more responsive to PD-1 blockade with pembrolizumab than are MMR–p tumors (24). This study had 21 patients with MMR-p CRC, 11 patients with MMR-d CRC, and 9 patients with MMR-d non-CRC. The immune-related objective response rate (ORR) and immune-related progression-free survival (PFS) rate were 40% and 78%, respectively, for MMR-d tumors and 0% and 11% for MMR-p tumors (24). Updated results of CheckMate 142 show durable responses and overall survival (OS) rates were 83.4% (6 months) and 73.8% (12 months) (28). In this study, they identified a mean of 578 potential mutation-associated neoantigens from patients with MMR-d CRC versus 21 neoantigens from patients with MMR-d CRC (24). The uniqueness of the number and type of alterations are being investigated to determine efficacy of other targets in MMR-p cancers as well.
Nivolumab has also been studied with ipilumumab in a phase II study with advanced CRC patients, which is currently ongoing (NCT02060188). Preliminary results show the ORR for Nivolumab was 27% versus the combination was 15% (29). Median PFS for nivolumab 5.3 months versus combination is not reached. In non-MSI-H patient, median PFS was 1.4 months. This study is currently ongoing.
The scope of PD-1 inhibition is currently limited to patients with MSI/MMR-d tumors. Given the promise of these agents based on the data above, the NCCN guidelines version 1.2017 recommends pembrolizumab or nivolumab as treatment options for patients with MMR-d metastatic CRC that is refractory to chemotherapy. Larger studies are underway to confirm the benefit of these agents (see Table 1). Further, ongoing studies evaluate combinations of PD-1 inhibitors with other agents, which will induce the immunogenic environment that enables checkpoint inhibitors to be efficacious in MSS/MMR-p patients (see Tables 2,3). The challenge will be to develop a combination that has a tolerable safety profile.

Full table

Full table
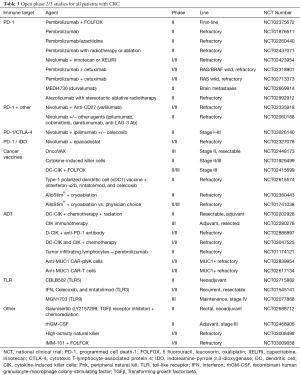
Full table
LAG-3
LAG-3 is expressed on activated T-cells and has various effects on the function of T cells. The primary ligand is MHC class II, and the interaction of LAG-3 with MHC class II results in a downregulation of antigen-dependent CD4+ T cell stimulation (30). Also, it leads to negative regulation of cell proliferation, activation, and homeostasis of T cells as well as suppression of Tregs (31). LAG-3 maintains tolerance to self and tumor antigens by directly effecting CD8+ T cells, resulting in a tolerogenic state, and also synergizes with PD-1 to induce CD8+ T cell exhaustion (32,33).
The role of LAG-3 has been associated with CRC progression (34). LAG-3 is also expressed at higher levels in MSI tumors compared to MSS, making it an ideal focus for immunotherapeutics in MSI CRC (21). Currently, most studies are early phase, with a phase 2 study of an anti-LAG-3 antibody alone and in combination with nivolumab in recurrent and mCRC open to recruitment (NCT 02060188).
TIM-3
TIM-3 plays a co-inhibitory role in the immune system, parallel to PD-1 and CTLA-4, and contributes to CD8+ T cell exhaustion (35). In colon cancer tissues, TIM-3 expression is higher than in normal tissues and TIM-3 expression correlates with lymphatic metastasis and TNM (P<0.0001) (35). In the mouse colon tumor model, TIM-3 is expressed on CD8+T cells, which leads to increased effector cytokine secretion and apoptosis when compared to TIM-3 negative cells (36). Galectin-9 is secreted by tumor cells, resulting in apoptosis of tumor-infiltrating CD8+ T cells (36). Anti-TIM-3 antibodies decrease apoptosis and inhibit tumor growth by disrupting the galectin-9/TIM-3 signaling pathway and also enhance therapeutic efficacy of chemotherapy (36). Currently, anti-TIM antibodies are in phase 1 development (NCT02817633).
IDO
IDO is an intracellular enzyme that results in tryptophan depletion to have an immunosuppressive effect and resulting in immune escape of tumors (37). IDO accomplishes this by promoting inflammation of the tumor microenvironment, developing immune tolerance to tumor antigens, suppressing T and NK cells, generating active Tregs and MDSCs, and promoting tumor angiogenesis (38). Colon adenocarcinoma cells are positive for IDO expression (39). Inhibition of IDO expression alters immune response in the colon tumor microenvironment in mice by increasing expression of pro-inflammatory cytokines and a decreasing Foxp3-positive Tregs, but in this study, this did not correlate with tumor reduction (40). It is possible that IMO may need to be given in combination with another immunomodulating agent to demonstrate efficacy. Anti-IDO agents, such as GDC-0919, are in phase 1 development (NCT 02048709). Epacadostat, an IDO-1 inhibitor, will be studied in a phase I/II study in combination with pembrolizumab and azacitidine, with an expansion cohort in MSS CRC (NCT02959437).
Cancer vaccines
Cancer vaccines can be utilized to facilitate the destruction of cancer cells by activing and maintaining the anti-tumor immune response by uncovering the hidden tumor-associated antigens.
Autologous vaccines
Autologous vaccines use the patient’s tumor cells that will contain all tumor-associated antigens specific to the patient; whereas whole cell vaccines include antigens from isolated cells, including normal tissue, and therefore, generating a non-specific response (41). However, there has been limited efficacy to date. A large phase III study of an autologous whole cell vaccine and BCG vaccine as adjuvant therapy versus observation for CRC did not show statistically significant differences between the groups in terms of disease-free and overall survival (42). Newcastle disease virus (NDV)-infected, an autologous tumor cell vaccine, is an irradiated whole cell tumor vaccine, which can up-regulate the innate and adaptive immune response and has been evaluated in CRC. A phase III study of patients with CRC with liver metastases who underwent metastasectomy compared NDV-infected to a control group, and there was no significant difference in overall survival (43).
Peptide-based vaccines
Peptide-based vaccines target a particular unique portion of the cancer cell, such as a peptide. Due to the ability to modify the target tumor-specific antigens, there is a higher specificity; however, limitations include antigenic escape leading to recurrence and that it is limited to specific HLA haplotypes (44). Peptide vaccines targeting multiple epitopes may overcome these limitations (45).Tumor-associated antigens targeted by peptide vaccines in CRC, include carcinoembryonic antigen (CEA) and beta-human chorionic gonadotropin (hCG), but most have not been able to show survival benefit (46,47). Vaccines based on dendritic cells (DCs) and pox vectors encoding CEA and MUC1 (PANVAC) were evaluated in patients with resected mCRC, and recurrence-free survival at 2 years was similar (47% and 55%, respectively) (48). A randomized trial of such vaccines compared with standard follow-up after metastasectomy would evaluate efficacy further.
Dendritic cell vaccines
Dendritic cells are a natural agent for antigen delivery, and therefore, have an ability to mediate the immune response via cytokine release. This process can be exploited by obtaining host dendritic cells and then pulsing them with an antigen ex vivo, such as tumor associated antigens or portions of the tumor, and then after maturity, re-infusing them into the patient so that a immune response is generated (49). A phase II study in metastatic CRC with autologous tumor lysate dendritic cell vaccine plus best supportive care vs best supportive care was futile and terminated early (50).
Other vaccines
Prior studies with cancer vaccines have shown modest results and haven’t changed the practice for CRC. Viral and bacterial antigen vaccines and cytokine therapy are possible avenues to overcome the limitations of the vaccines described earlier. Investigations are still in early phases.
Adoptive cell transfer (ADT)
ADT is the treatment of patients with cell populations that have been expanded ex vivo, which enables the T cells to overcome tolerance and immune suppression that takes place in vivo (51). In a phase I/II study of adjuvant therapy, when sentinel lymph node (SLN)-T lymphocytes were expanded ex vivo and then transfused to the patient, the median OS was 28 versus 14 months (control) and was well tolerated (52). This shows promise for adoptive T cell therapy and warrants further investigation.
Toll-like receptor (TLR) agonists
TLRs are present on the innate immune cells and present damage-associated pattern molecules (DAMPs) after tumor cell death. Of the ten human TLRs, TLR-9 can be protective against transformation of normal colorectal mucosa to malignancy (53). TLRs agonists potentiate this process in the innate immune system. TLR9 agonist PF-3512676 was not effective with chemotherapy in lung cancer (54). This may be due to TLR9 agonists first require a release of tumor-associated antigens for an effective immune response, with a lower disease burden and after immune recovery, and therefore, would be more successful given after chemotherapy (54). MGN1703, which is a TLR-9 agonist, in mice colon cancer models showed tumor growth inhibition by 28% and with combination PD-1 inhibitor by 48%, resulting in prolonged survival of the mice (55). MGN1703 was given to patients with mCRC as a maintenance treatment and showed a modest, but significant, improvement in PFS from 2.6 months with placebo to 2.8 months (56). Future studies evaluating these agents alone and in combination with other checkpoint inhibitors may show activity in all mCRC patients.
Microbiome
In the era of precision medicine, the complex interplay of the immune system with other pathways such as the microbiome needs to be better understood. Microbial cometabolism impacts the microenvironment, which may ultimately influence the efficacy of immunotherapeutics as well as cancer survival (57). Future studies with immunotherapy should evaluate the interplay between bacteria-metabolite-cancer, so that we can maximize modulation of the immune system by these agents. Clinical trials are underway where the effects of chemotherapy and immunotherapy on the microbiome are investigated (NCT02960282).
Other treatment modalities
Immunotherapy may potentiate the immunogenicity induced by other treatment modalities, such as radiation. Ionizing radiation induces anti-tumor immune responses that may lead to controlled tumor growth, and therefore, may elicit a more robust immune response when combined with other immunotherapies (58). Radiation induces immunity by inducing tumor immunogenicity, triggers immune cell infiltration, induces immunogenic cell death and changes the tumor-associated effector to Treg ratio (58). Such investigations are currently in early development in colon cancer. Currently, we await the results of a phase I/II clinical trial evaluating neoadjuvant, low dose radiotherapy’s effect on T cell connected anti-tumor immune response in CRC liver metastases, with the primary endpoint of number of tumor infiltrating T cells (NCT01191632). Another phase I study is evaluating pembrolizumab in combination stereotactic body radiotherapy (SBRT) to the liver (NCT02837263).
Radiofrequency ablation (RFA) is an established treatment for CRC liver metastases, and by heating the tissue, RFA induces coagulation necrosis, leading to a strong inflammatory response (59). One study has investigated the efficacy and safety of the combination of RFA and cytokine-induced killer (CIK) cell transfusion for patients with CRC liver metastases (60). Compared to RFA alone, the median PFS is 23 months with the combination versus 18.5 months with RFA alone. The study showed that RFA and CIK cells augment CEA-specific T cell responses and RFA with CIK cell transfusion has clinical efficacy. Early studies demonstrate that immunotherapeutics have the potential to enhance the immunogenicity of other treatment modalities, which may improve survival for CRC patients. Further studies with other agents are ongoing (see Table 3).
Immunotherapy in treating CRC patients today
While investigations are underway, the current role of immunotherapy in the treatment of resectable or metastatic CRC is limited. PD-1 inhibitors, nivolumab and pembrolizumab, are now the new standard of care as treatment of chemotherapy-refractory MSI-high/MMR-d CRC, which provides us now with an additional line of treatment for these patients. These agents are now included in the latest version of NCCN guidelines (January 2017). As further immunotherapeutic agents are under development, we should continue to enroll patients onto these clinical trials and also identify biomarkers of efficacy. Predictive markers of relapse and prognostic markers will help us identify which patients will most benefit from these new therapies. For example, the “immunoscore” technique quantifies tumor features (including MSI status), tumor immune microenvironment features, and systemic disorders to better prognosticate and predict responses to treatments (NCT02274753).
Conclusions
Immunotherapy does have a role in MSI/MMR-d CRC and is now part of the treatment algorithm for advanced refractory patients. Newer agents either alone or in combination with PD-1 inhibitors show promise in all patients with CRC, regardless of microsatellite or mismatch repair status. Given the efficacy in early phase trials and now ongoing studies investing these agents in the first-line setting and in combination with other modalities such as radiation, these new agents will have a significant impact on survival of these patients. These agents give us hope that we will be able to treat all patients with CRC with immunotherapy, not just the select MSI/MMR-d CRC. To continue rapid development of these agents, as clinicians, we should continue to enroll patients onto these studies and also identify biomarkers of efficacy.
Acknowledgements
Funding: SP Arora: NCI P30CA054174, NIA P30AG044271. D Mahalingam: NCI P30CA054174.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Benson AB 3rd, Bekaii-Saab T, Chan E, et al. Metastatic colon cancer, version 3.2013: featured updates to the NCCN Guidelines. J Natl Compr Canc Netw 2013;11:141-52. [Crossref] [PubMed]
- Patel SR, Karnad AB, Ketchum NS, et al. Should we move beyond VEGF inhibition in metastatic colorectal cancer? Lessons from early phase clinical trials. J Gastrointest Oncol 2014;5:99-103. [PubMed]
- Grothey A, Van Cutsem E, Sobrero A, et al. Regorafenib monotherapy for previously treated metastatic colorectal cancer (CORRECT): an international, multicentre, randomised, placebo-controlled, phase 3 trial. Lancet 2013;381:303-12. [Crossref] [PubMed]
- Hodi FS, O'Day SJ, McDermott DF, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med 2010;363:711-23. [Crossref] [PubMed]
- Ferris RL, Blumenschein G Jr, Fayette J, et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 2016;375:1856-67. [Crossref] [PubMed]
- Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus Docetaxel in Advanced Squamous-Cell Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:123-35. [Crossref] [PubMed]
- Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 2015;373:1627-39. [Crossref] [PubMed]
- Garon EB, Rizvi NA, Hui R, et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 2015;372:2018-28. [Crossref] [PubMed]
- Rosenberg JE, Hoffman-Censits J, Powles T, et al. Atezolizumab in patients with locally advanced and metastatic urothelial carcinoma who have progressed following treatment with platinum-based chemotherapy: a single-arm, multicentre, phase 2 trial. Lancet 2016;387:1909-20. [Crossref] [PubMed]
- Markman JL, Shiao SL. Impact of the immune system and immunotherapy in colorectal cancer. J Gastrointest Oncol 2015;6:208-23. [PubMed]
- Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell 2011;144:646-74. [Crossref] [PubMed]
- Grivennikov SI, Greten FR, Karin M. Immunity, inflammation, and cancer. Cell 2010;140:883-99. [Crossref] [PubMed]
- Zeng ZS, Huang Y, Cohen AM, et al. Prediction of colorectal cancer relapse and survival via tissue RNA levels of matrix metalloproteinase-9. J Clin Oncol 1996;14:3133-40. [Crossref] [PubMed]
- Wang X, Yang Y, Huycke MM. Microbiome-driven carcinogenesis in colorectal cancer: Models and mechanisms. Free Radic Biol Med 2017;105:3-15. [PubMed]
- Marx JL. Cancer immunotherapy: focus on the drug levamisole. Science 1976;191:57. [Crossref] [PubMed]
- Haller DG, Catalano PJ, Macdonald JS, et al. Phase III study of fluorouracil, leucovorin, and levamisole in high-risk stage II and III colon cancer: final report of Intergroup 0089. J Clin Oncol 2005;23:8671-8. [Crossref] [PubMed]
- Schippinger W, Jagoditsch M, Sorre C, et al. A prospective randomised trial to study the role of levamisole and interferon alfa in an adjuvant therapy with 5-FU for stage III colon cancer. Br J Cancer 2005;92:1655-62. [Crossref] [PubMed]
- Ward R, Meagher A, Tomlinson I, et al. Microsatellite instability and the clinicopathological features of sporadic colorectal cancer. Gut 2001;48:821-9. [Crossref] [PubMed]
- Popat S, Hubner R, Houlston RS. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol 2005;23:609-18. [Crossref] [PubMed]
- Xiao Y, Freeman GJ. The microsatellite instable subset of colorectal cancer is a particularly good candidate for checkpoint blockade immunotherapy. Cancer Discov 2015;5:16-8. [Crossref] [PubMed]
- Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov 2015;5:43-51. [Crossref] [PubMed]
- Chung KY, Gore I, Fong L, et al. Phase II study of the anti-cytotoxic T-lymphocyte-associated antigen 4 monoclonal antibody, tremelimumab, in patients with refractory metastatic colorectal cancer. J Clin Oncol 2010;28:3485-90. [Crossref] [PubMed]
- Dudley JC, Lin MT, Le DT, et al. Microsatellite Instability as a Biomarker for PD-1 Blockade. Clin Cancer Res 2016;22:813-20. [Crossref] [PubMed]
- Le DT, Uram JN, Wang H, et al. PD-1 Blockade in Tumors with Mismatch-Repair Deficiency. N Engl J Med 2015;372:2509-20. [Crossref] [PubMed]
- Topalian SL, Hodi FS, Brahmer JR, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 2012;366:2443-54. [Crossref] [PubMed]
- Brahmer JR, Tykodi SS, Chow LQ, et al. Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 2012;366:2455-65. [Crossref] [PubMed]
- Lipson EJ, Sharfman WH, Drake CG, et al. Durable cancer regression off-treatment and effective reinduction therapy with an anti-PD-1 antibody. Clin Cancer Res 2013;19:462-8. [Crossref] [PubMed]
- Overman MJ, Lonardi S, Leone F, et al. Nivolumab in patients with DNA mismatch repair deficient/microsatellite instability high metastatic colorectal cancer: Update from CheckMate 142. J Clin Oncol 2017;35:abstr 519.
- Overman MJ, Kopetz S, McDermott RS, et al. Nivolumab ± ipilimumab in treatment (tx) of patients (pts) with metastatic colorectal cancer (mCRC) with and without high microsatellite instability (MSI-H): CheckMate-142 interim results. J Clin Oncol 2016;34:abstr 3501.
- Huard B, Tournier M, Hercend T, et al. Lymphocyte-activation gene 3/major histocompatibility complex class II interaction modulates the antigenic response of CD4+ T lymphocytes. Eur J Immunol 1994;24:3216-21. [Crossref] [PubMed]
- Huang CT, Workman CJ, Flies D, et al. Role of LAG-3 in regulatory T cells. Immunity 2004;21:503-13. [Crossref] [PubMed]
- Grosso JF, Kelleher CC, Harris TJ, et al. LAG-3 regulates CD8+ T cell accumulation and effector function in murine self- and tumor-tolerance systems. J Clin Invest 2007;117:3383-92. [Crossref] [PubMed]
- Blackburn SD, Shin H, Haining WN, et al. Coregulation of CD8+ T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2009;10:29-37. [Crossref] [PubMed]
- Chen J, Chen Z. The effect of immune microenvironment on the progression and prognosis of colorectal cancer. Med Oncol 2014;31:82. [Crossref] [PubMed]
- Zhou E, Huang Q, Wang J, et al. Up-regulation of Tim-3 is associated with poor prognosis of patients with colon cancer. Int J Clin Exp Pathol 2015;8:8018-27. [PubMed]
- Kang CW, Dutta A, Chang LY, et al. Apoptosis of tumor infiltrating effector TIM-3+CD8+ T cells in colon cancer. Sci Rep 2015;5:15659. [Crossref] [PubMed]
- Zou W. Immunosuppressive networks in the tumour environment and their therapeutic relevance. Nat Rev Cancer 2005;5:263-74. [Crossref] [PubMed]
- Prendergast GC, Smith C, Thomas S, et al. Indoleamine 2,3-dioxygenase pathways of pathogenic inflammation and immune escape in cancer. Cancer Immunol Immunother 2014;63:721-35. [Crossref] [PubMed]
- Uyttenhove C, Pilotte L, Theate I, et al. Evidence for a tumoral immune resistance mechanism based on tryptophan degradation by indoleamine 2,3-dioxygenase. Nat Med 2003;9:1269-74. [Crossref] [PubMed]
- Takamatsu M, Hirata A, Ohtaki H, et al. Inhibition of indoleamine 2,3-dioxygenase 1 expression alters immune response in colon tumor microenvironment in mice. Cancer Sci 2015;106:1008-15. [Crossref] [PubMed]
- Klebanoff CA, Acquavella N, Yu Z, et al. Therapeutic cancer vaccines: are we there yet? Immunol Rev 2011;239:27-44. [Crossref] [PubMed]
- Harris JE, Ryan L, Hoover HC Jr, et al. Adjuvant active specific immunotherapy for stage II and III colon cancer with an autologous tumor cell vaccine: Eastern Cooperative Oncology Group Study E5283. J Clin Oncol 2000;18:148-57. [Crossref] [PubMed]
- Schulze T, Kemmner W, Weitz J, et al. Efficiency of adjuvant active specific immunization with Newcastle disease virus modified tumor cells in colorectal cancer patients following resection of liver metastases: results of a prospective randomized trial. Cancer Immunol Immunother 2009;58:61-9. [Crossref] [PubMed]
- Bartnik A, Nirmal AJ, Yang SY. Peptide Vaccine Therapy in Colorectal Cancer. Vaccines (Basel) 2012;1:1-16. [Crossref] [PubMed]
- Hazama S, Nakamura Y, Tanaka H, et al. A phase IotaI study of five peptides combination with oxaliplatin-based chemotherapy as a first-line therapy for advanced colorectal cancer (FXV study). J Transl Med 2014;12:108. [Crossref] [PubMed]
- Moulton HM, Yoshihara PH, Mason DH, et al. Active specific immunotherapy with a beta-human chorionic gonadotropin peptide vaccine in patients with metastatic colorectal cancer: antibody response is associated with improved survival. Clin Cancer Res 2002;8:2044-51. [PubMed]
- Bilusic M, Heery CR, Arlen PM, et al. Phase I trial of a recombinant yeast-CEA vaccine (GI-6207) in adults with metastatic CEA-expressing carcinoma. Cancer Immunol Immunother 2014;63:225-34. [Crossref] [PubMed]
- Morse MA, Niedzwiecki D, Marshall JL, et al. A randomized phase II study of immunization with dendritic cells modified with poxvectors encoding CEA and MUC1 compared with the same poxvectors plus GM-CSF for resected metastatic colorectal cancer. Ann Surg 2013;258:879-86. [Crossref] [PubMed]
- Palucka K, Banchereau J. Dendritic-cell-based therapeutic cancer vaccines. Immunity 2013;39:38-48. [Crossref] [PubMed]
- Maurel J, Caballero-Baños M, Mila J, et al. Phase II randomized trial of autologous tumor lysate dendritic cell vaccine (ADC) plus best supportive care (BSC) compared with BSC, in pre-treated advanced colorectal cancer patients. J Clin Oncol 2015;33:abstr 3048.
- Restifo NP, Dudley ME, Rosenberg SA. Adoptive immunotherapy for cancer: harnessing the T cell response. Nat Rev Immunol 2012;12:269-81. [Crossref] [PubMed]
- Zhen YH, Liu XH, Yang Y, et al. Phase I/II study of adjuvant immunotherapy with sentinel lymph node T lymphocytes in patients with colorectal cancer. Cancer Immunol Immunother 2015;64:1083-93. [Crossref] [PubMed]
- Eiro N, Gonzalez L, Gonzalez LO, et al. Study of the expression of toll-like receptors in different histological types of colorectal polyps and their relationship with colorectal cancer. J Clin Immunol 2012;32:848-54. [Crossref] [PubMed]
- Hirsh V, Paz-Ares L, Boyer M, et al. Randomized phase III trial of paclitaxel/carboplatin with or without PF-3512676 (Toll-like receptor 9 agonist) as first-line treatment for advanced non-small-cell lung cancer. J Clin Oncol 2011;29:2667-74. [Crossref] [PubMed]
- Schmidt M, Kapp K, Volz B, et al. Combination of TLR9 agonist lefitolimod/MGN1703 with checkpoint inhibitors for cancer immunotherapy. J Clin Oncol 2017;35:abstr 634.
- Schmoll HJ, Wittig B, Arnold D, et al. Maintenance treatment with the immunomodulator MGN1703, a Toll-like receptor 9 (TLR9) agonist, in patients with metastatic colorectal carcinoma and disease control after chemotherapy: a randomised, double-blind, placebo-controlled trial. J Cancer Res Clin Oncol 2014;140:1615-24. [Crossref] [PubMed]
- Johnson CH, Spilker ME, Goetz L, et al. Metabolite and Microbiome Interplay in Cancer Immunotherapy. Cancer Res 2016;76:6146-52. [Crossref] [PubMed]
- Haikerwal SJ, Hagekyriakou J, MacManus M, et al. Building immunity to cancer with radiation therapy. Cancer Lett 2015;368:198-208. [Crossref] [PubMed]
- Rughetti A, Rahimi H, Rossi P, et al. Modulation of blood circulating immune cells by radiofrequency tumor ablation. J Exp Clin Cancer Res 2003;22:247-50. [PubMed]
- Li X, Dai X, Shi L, et al. Phase II/III Study of Radiofrequency Ablation Combined with Cytokine-Induced Killer Cells Treating Colorectal Liver Metastases. Cell Physiol Biochem 2016;40:137-45. [Crossref] [PubMed]


