Abstract
A voluntary reporting system of adverse drug reactions (ADRs) is fundamental to drug safety surveillance but under-reporting is its major limitation. This bibliographic review sought to assess the influence of personal and professional characteristics on ADR reporting and to identify knowledge and attitudes associated with ADR reporting.
A systematic review was conducted using the MEDLINE and EMBASE databases. We included papers that were published in English, French and Spanish, and covered a study population made up of health professionals. In each case, the following data were extracted: study population; workplace; study type; sample size; type of questionnaire; type of scale for measuring knowledge; response rate; personal and professional factors; and knowledge and attitudes (based on Inman’s ‘seven deadly sins’) associated with reporting.
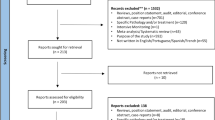
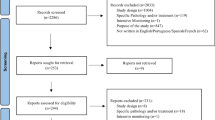
Based on a search of computerized databases, we identified a total of 657 papers in MEDLINE and 973 in EMBASE. In all, the review covered 45 papers that fulfilled the inclusion criteria. Medical specialty was the professional characteristic most closely associated with under-reporting in 76% of studies involving physicians. Other factors associated with under-reporting were ignorance (only severe ADRs need to be reported) in 95%; diffidence (fear of appearing ridiculous for reporting merely suspected ADRs) in 72%; lethargy (an amalgam of procrastination, lack of interest or time to find a report card, and other excuses) in 77%; indifference (the one case that an individual doctor might see could not contribute to medical knowledge) and insecurity (it is nearly impossible to determine whether or not a drug is responsible for a particular adverse reaction) in 67%; and complacency (only safe drugs are allowed on the market) in 47% of studies.
While personal and professional factors display a weak influence, the knowledge and attitudes of health professionals appear to be strongly related with reporting in a high proportion of studies. This result may have important implications in terms of public health, if knowledge and attitudes are viewed as potentially modifiable factors.




Similar content being viewed by others
References
Lazarou J, Pomeranz BH, Corey PN. Incidence of adverse drug reactions in hospitalized patients: a meta-analysis of prospective studies. JAMA 1998; 279: 1200–5
Smith CC, Bennett PM, Pearce HM, et al. Adverse drug reaction in a hospital general medical unit meriting notification to the Committee on Safety of Medicine. Br J Clin Pharmacol 1996; 42: 423–9
Ibanez L, Laporte JR, Carne X. Adverse drug reactions leading to hospital admission. Drug Saf 1991; 6: 450–9
Bates DW, Spell N, Cullen DJ, et al. The costs of adverse drug events in hospitalized patients: Adverse Drug Events Prevention Study Group. JAMA 1997; 277: 307–11
Edwards I, Olsson S. WHO: global monitoring. In: Mann RD, Andrews E, editors. Pharmacovigilance. Chichester: John Wiley & Sons, 2002: 169–82
Waller P, Bahri P. Regulatory pharmacovigilance in the EU. In: Mann RD, Andrews E, editors. Pharmacovigilance. Chichester: John Wiley & Sons, 2002: 183–94
Ahmad SR. Adverse drug event monitoring at the Food and Drug Administration. J Gen Intern Med 2003; 285: 437–43
Lexchin J. Is there a role for spontaneous reporting of adverse drug reactions? CMAJ 2006; 174: 191–2
Wysowsky DK, Swartz L. Adverse drug event surveillance and drug withdrawals in the United States, 1969–2002: the importance of reporting suspected reactions. Arch Intern Med 2005; 165: 1363–9
Hazell L, Shaki SA. Under-reporting of adverse drug reactions: a systematic review. Drug Saf 2006; 29: 385–6
Inman WHW. Assessment of drug safety problems. In: Gent M, Shigmatsu I, editors. Epidemiological issues in reported drug-induced illnesses. Honolulu (ON): McMaster University Library Press, 1976: 17–24
Inman WHW, Weber JCT. The United Kingdom. In: Inman WHW, editor. Monitoring for drug safety. 2nd ed. Lancaster: MTP Press Ltd, 1986: 13–47
Inman WHW. Attitudes to adverse drug-reaction reporting. Br J Clin Pharmacol 1996; 41: 433–5
van Grootheest K, Olsson S, Couper M, et al. Pharmacists’ role in reporting adverse drug reactions in an international perspective. Pharmacoepidemiol Drug Saf 2004 Jul; 13(7): 457–4
Milstien JB, Faich GA, Hsu JP, et al. Factors affecting physician reporting of adverse drug reactions. Drug Inf J 1986; 20: 157–64
Walker SR, Lumley CE. The attitudes of general practitioners to monitoring and reporting adverse drug reactions. Pharm Med 1986; 1: 195–203
Robins AH, Weir M, Biersteker EM. Attitudes to adverse drug reactions and their reporting among medical practitioners. S Afr Med J 1987; 72: 131–4
Rogers AS, Israel E, Smith CR, et al. Physician knowledge, attitudes, and behavior related to reporting adverse drug events. Arch Intern Med 1988; 148: 1596–600
Scott HD, Thacher-Renshaw A, Rosenbaum SE, et al. Physician reporting of adverse drug reactions: results of the rhode island adverse drug reaction reporting project. JAMA 1990; 263: 1785–8
Fincham JE. Hospital and nursing home pharmacists’ attitudes toward adverse drug reaction reporting. J Soc Admin Pharm 1990; 7: 117–22
Bateman DN, Sanders GL, Rawlins MD. Attitudes to adverse drug reaction reporting in the northern region. Br J Clin Pharmacol 1992; 34: 421–6
Lee KK, Chan TY, Raymond K, et al. Pharmacists’ attitudes toward adverse drug reaction reporting in Hong Kong. Ann Pharmacother 1994; 28: 1400–3
Gram LF. Attitude and considerations of Danish physicians towards reporting of adverse drug reactions: a questionnaire study under European direction. Ugeskr Laeger 1995; 157: 1692–4
Belton KJ, Lewis SC, Payne S, et al. Attitudinal survey of adverse drug reaction reporting by medical practitioners in the United Kingdom. Br J Clin Pharmacol 1995; 39: 223–6
Generali JA, Danish MA, Rosenbaum SE. Knowledge of and attitudes about adverse drug reaction reporting a mong Rhode Island pharmacists. Ann Pharmacother 1995; 29: 365–9
Pouget-Zago P, Lapeyre-Mestre M, Bagheri H, et al. Attitudinal survey about the drug surveillance system in general practitioners and residents in the south of France. Therapie 1995; 50: 459–2
Van Riemsdijk MM, Herings RMC, Rawlins MD, et al. Reasons for (not) reporting adverse reactions to drugs to The Netherlands centre for monitoring of adverse reactions to drugs. Ned Tijdschr Geneeskd 1995; 139: 2306–8
McGettigan P, Feely J. Adverse drug reaction reporting: opinions and attitudes of medical practitioners in Ireland. Pharmacoepidemiol Drug Saf 1995; 4: 355–8
Wallace SM, Suveges LG, Gesy KF. Adverse drug reaction reporting: part I. Survey of pharmacists and physicians in Saskatchewan. Drug Inf J 1995; 29: 571–9
Kurz X, Van Ermen A, Roisin T, et al. Knowledge and practice of adverse drug reaction reporting by Belgian physicians. Arch Public Health 1996; 54: 29–41
Belton KJ. Attitude survey of adverse drug-reaction reporting by health care professionals across the European 30 Union: the European Pharmacovigilance Research Group. Eur J Clin Pharmacol 1997; 52: 423–7
Cosentino M, Leoni O, Banfi F, et al. Attitudes to adverse drug reaction reporting by medical practitioners in a Northern Italian district. Pharmacol Res 1997; 35: 85–8
McGettigan P, Golden J, Conroy RM, et al. Reporting of adverse drug reactions by hospital doctors and the response to intervention. Br J Clin Pharmacol 1997; 44: 98–100
Serrano Cozar G, Esteban Calvo C, Gijon Porta JA, et al. Adverse drug reactions and a program of voluntary notification: an opinion survey of primary care physicians. Aten Primaria 1997; 19: 307–12
Ball D, Tisocki T. Adverse drug reaction reporting by general medical practitioners and retail pharmacists in Harare: a pilot study. Cent Afr J Med 1998; 44: 190–5
Tubert-Bitter P, Haramburu F, Begaud B, et al. Spontaneous reporting of adverse drug reactions: who reports and what? Pharmacoepidemiol Drug Saf 1998; 7: 323–9
Williams D, Feely J. Underreporting of adverse drug reactions: attitudes of Irish doctors. Ir J Med Sci 1999; 168: 257–61
Eland IA, Belton KJ, van Grootheest AC, et al. Attitudinal survey of voluntary reporting of adverse drug reactions. Br J Clin Pharmacol 1999; 48: 623–7
Cosentino M, Leoni O, Oria C, et al. Hospital-based survey of doctors’ attitudes to adverse drug reactions and perception of drug-related risk for adverse reaction occurrence. Pharmacoepidemiol Drug Saf 1999; 8: S27–35
Figueiras A, Tato F, Fontainas J, et al. Influence of physicians’ attitudes on reporting adverse drug events: a case-control study. Med Care 1999; 37: 809–14
Green CF, Mottram DR, Brown AM, et al. Attitudes of hospital pharmacists to adverse drug reactions and the ‘yellow card’ scheme: a qualitative study. Int J Pharm Pract 1999; 7: 247–55
Houghton J, Woods F, Davis S, et al. Community pharmacist reporting of suspected ADRs: 2. Attitudes of community pharmacists and general practitioners in Wales. Pharm J 1999; 263: 788–91
Figueiras A, Tato F, Fontainas J, et al. Physicians’ attitudes towards voluntary reporting of adverse drug events. J Eval Clin Pract 2001; 7: 347–54
Backstrom M, Mjorndal T, Dahlqvist R, et al. Attitudes to reporting adverse drug reactions in northern Sweden. Eur J Clin Pharmacol 2000; 56: 729–32
Sweis D, Wong IC. A survey on factors that could affect adverse drug reaction reporting according to hospital pharmacists in Great Britain. Drug Saf 2000; 23: 165–72
Green CF, Mottram DR, Rowe PH, et al. Attitudes and knowledge of hospital pharmacists to adverse drug reaction reporting. Br J Clin Pharmacol 2001; 51: 81–6
Costantino D, Maione M, Piro B. Survey of spontaneous reporting in Calabria: physician’s attitude. G Ital Farm Clin 2001; 15: 291–95
Hasford J, Goettler M, Munter KH, et al. Physicians’ knowledge and attitudes regarding the spontaneous reporting system for adverse drug reactions. J Clin Epidemiol 2002; 55: 945–50
Perlik F, Slanar O, Smid M, et al. Attitude of Czech physicians to adverse drug reaction reporting. Eur J Clin Pharmacol 2002; 58: 367–9
Van Grootheest AC, Mes K, De Jong-Van Den Berg LTW. Attitudes of community pharmacists in the Netherlands towards adverse drug reaction reporting. Int J Pharm Pract 2002; 10: 267–72
Rehan HS, Vasudev K, Tripathi CD. Adverse drug reaction monitoring: knowledge, attitude and practices of medical students and prescribers. Natl Med J India 2002; 15: 24–6
Li Q, Zhang SM, Chen HT, et al. Awareness and attitudes of healthcare professionals in Wuhan, China to the reporting of adverse drug reactions. Chin Med J 2004; 117: 856–61
Kelly M, Kaye KI, Davis SR, et al. Factors influencing adverse drug reaction reporting in New South Wales teaching hospitals. J Pharm Res 2004; 34: 32–5
Milojevic K, Chassagnol I, Brion N, et al. Adverse drug reaction reporting in emergency medicine. Therapie 2004; 59: 611–4
Khoza S, Madungwe I, Nyambayo P, et al. Adverse drug reactions reporting at a referral hospital in Zimbabwe. Cent Afr J Med 2004; 50: 104–7
Herdeiro MT, Figueiras A, Polonia J, et al. Physicians’ attitudes and adverse drug reaction reporting: a case-control study in Portugal. Drug Saf 2005; 28: 825–33
Vallano A, Cereza G, Pedros C, et al. Obstacles and solutions for spontaneous reporting of adverse drug reactions in the hospital. Br J Clin Pharmacol 2005; 60: 653–8
Nita Y, Batty KT, Plumridge RJ. Adverse drug reaction reporting: attitudes of Australian hospital pharmacists and doctors. J Pharm Res 2005; 35: 9–14
Bhatia A, Kapoor U, Tayal G. A survey of issues regarding ADR and ADR reporting amongst doctors in Delhi. Int J Risk Saf Med 2005; 17: 39–46
Herdeiro MT, Figueiras A, Polonia J, et al. Influence of pharmacists’ attitudes on adverse drug reaction reporting: a case-control study in Portugal. Drug Saf 2006; 29: 331–40
Aziz Z, Siang TC, Badarudin NS. Reporting of adverse drug reactions: predictors of under-reporting in Malaysia. Pharmacoepidemiol Drug Saf 2006; 16: 223–8
Chatterjee S, Lyle N, Ghosh S. A survey of the knowledge, attitude and practice of adverse drug reaction reporting by clinicians in eastern India. Drug Saf 2006; 29: 641–2
Granas AG, Buajordet M, Stenberg-Nilsen H, et al. Pharmacists’ attitudes towards the reporting of suspected adverse drug reactions in Norway. Pharmacoepidemiol Drug Saf 2006; 16: 429–34
Bawazir SA. Attitude of community pharmacists in Saudi Arabia towards adverse drug reaction reporting. Saudi Pharm J 2006; 14: 75–83
Herdeiro MT, Polonia J, Gestal-Otero JJ, et al. Factors that influence spontaneous reporting of adverse drug reactions: a model centralized in the medical professional. J Eval Clin Pract 2004; 10: 483–9
Feely J, Moriarty S, O’Connor P. Stimulating reporting of adverse drug reactions by using a fee. BMJ 1990; 300: 22–3
Gilroy GW, Scollins MJ, Gay CA, et al. Pharmacycoordinated program that encourages physician reporting 31 of adverse drug reactions. Am J Hosp Pharm 1990; 47: 1327–33
McBride WG. Thalidomide and congenital abnormalities. [letter]. Lancet 1961; II: 1358
Castel JM, Figueiras A, Pedros C, et al. Stimulating adverse drug reaction reporting: effect of a drug safety bulletin and of including yellow cards in prescription pads. Drug Saf 2003; 26: 1049–55
Figueiras A, Herdeiro MT, Polonia J, et al. An educational intervention to improve physician reporting of adverse drug reactions: a cluster-randomized controlled trial. JAMA 2006; 296: 1086–93
Herdeiro MT, Polonia J, Gestal-Otero JJ, et al. Improving the reporting of adverse drug reactions: a cluster-randomized trial among pharmacists in Portugal. Drug Saf 2008; 31(4): 335–44
Acknowledgements
The authors wish to express their sincere thanks to Angel Salgado for his invaluable comments on the previous versions of this manuscript and to Michael Benedict for his help in reviewing and revising the English version. Dr Adolfo Figueiras’ work on this project was funded by CIBERESP (AC08_008). Professor Dr Maria Teresa Herdeiro’s work on this project was in part funded by Science and Technology Fundo (Fundação para a Ciência e a Tecnologia) grants SFRH/BPD/35746/2007 from the Portuguese Ministry of Science, Technology and Superior Education.
The authors have no conflicts of interest that are directly relevant to the content of this study.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Rights and permissions
About this article
Cite this article
Lopez-Gonzalez, E., Herdeiro, M.T. & Figueiras, A. Determinants of Under-Reporting of Adverse Drug Reactions. Drug-Safety 32, 19–31 (2009). https://doi.org/10.2165/00002018-200932010-00002
Published:
Issue Date:
DOI: https://doi.org/10.2165/00002018-200932010-00002