Summary
Abstract
Tocilizumab (RoActemra® or Actemra®) is a recombinant humanized monoclonal antibody that acts as an interleukin (IL)-6 receptor antagonist. Intravenous tocilizumab 8 mg/kg (and no less than 480 mg), in combination with methotrexate, is approved in the EU for the treatment of moderate to severe active rheumatoid arthritis in adult patients with inadequate response to, or who are intolerant of, prior disease-modifying anti-rheumatic drug (DMARD) or tumour necrosis factor (TNF) antagonist therapy. It may also be administered as monotherapy in patients intolerant of methotrexate or in whom methotrexate therapy is inappropriate. Tocilizumab is also approved in Japan for the treatment of polyarticular-course juvenile idiopathic arthritis, systemic-onset juvenile idiopathic arthritis and Castleman’s disease.
Intravenous tocilizumab was effective and generally well tolerated when administered either as monotherapy or in combination with conventional DMARDs in several well designed clinical studies in adult patients with moderate to severe rheumatoid arthritis. Tocilizumab-based therapy was consistently more effective than placebo, methotrexate or other DMARDs in reducing disease activity, and some trials also showed significant benefits with tocilizumab in terms of reducing structural joint damage and improving health-related quality of life (HR-QOL). Notably, tocilizumab-based therapy was effective in patients with long-standing disease in whom anti-TNF therapy had previously failed. More data are required to determine the comparative efficacy and safety of tocilizumab versus other biological agents and to establish their relative cost effectiveness. However, the present data suggest that tocilizumab is an important emerging treatment option in adult patients with moderate to severe rheumatoid arthritis.
Pharmacological Properties
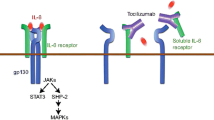
Elevated levels of IL-6 in the serum and synovial fluid of rheumatoid arthritis patients contribute to the chronic inflammatory process characterizing this disease and correlate positively with disease activity. Tocilizumab binds selectively and competitively to soluble and membrane-expressed IL-6 receptors, blocking IL-6 signal transduction. In vivo, maximum (>90%) IL-6 receptor saturation was achieved when serum tocilizumab concentrations were >1 μg/mL. In dose-ranging studies, serum levels of inflammatory markers were normalized in rheumatoid arthritis patients who had detectable levels of tocilizumab in the serum. Tocilizumab displays dose-dependent, non-linear pharmacokinetics and has a long elimination half-life, allowing administration every 4 weeks.
Therapeutic Efficacy
Several well designed studies in patients with early or long-standing rheumatoid arthritis, including those with treatment-refractory disease, demonstrated the efficacy of intravenous tocilizumab 8 mg/kg every 4 weeks in improving disease activity, structural joint damage and/or HR-QOL.
Monotherapy with tocilizumab 8 mg/kg was noninferior to methotrexate for achievement of a clinical response in patients who had not experienced any prior treatment failures in the AMBITION study; subsequent superiority analyses in the intent-to-treat population demonstrated that significantly more tocilizumab than methotrexate recipients achieved American College of Rheumatology (ACR) clinical responses at week 24. Tocilizumab recipients also demonstrated significant improvements at week 24 in HR-QOL measures.
Among patients with early (mean disease duration <3 years) rheumatoid arthritis whose previous treatment with conventional DMARDs had failed, monotherapy with tocilizumab 8 mg/kg for 1 year was significantly more effective than treatment with DMARDs for achieving an ACR20 response, preventing radiographic disease progression and improving HR-QOL.
Patients with long-standing treatment-refractory disease achieved significantly better clinical responses with tocilizumab 8 mg/kg monotherapy than with placebo or methotrexate. These beneficial effects were maintained for up to 5 years with tocilizumab in a noncomparative extension of a placebo-controlled trial. Five studies of tocilizumab-based combination therapy for up to 52 weeks in patients with long-standing, treatment-refractory disease demonstrated better clinical responses with tocilizumab 8 mg/kg plus methotrexate or other DMARDs than with methotrexate or other DMARDs alone. Improvements in the levels of inflammatory markers were also observed, including serum C-reactive protein levels, which reduced as early as week 2. The mean changes from baseline in HR-QOL outcomes also favoured patients receiving tocilizumab-based combination therapy. The RADIATE trial found that generally better clinical responses were achieved with tocilizumab 8 mg/kg plus methotrexate than methotrexate alone in patients whose therapy with at least one anti-TNF agent had failed. In LITHE, significantly less radiographic disease progression was seen with tocilizumab plus methotrexate than with methotrexate alone.
Tolerability
Intravenous tocilizumab was generally well tolerated in adult patients with early or long-standing rheumatoid arthritis. The rates of withdrawal because of treatment-emergent adverse events among patients receiving tocilizumab 8 mg/kg monotherapy or in combination with DMARDs were generally low (≤12% of patients) and similar to those observed in active comparator groups. Most treatment-emergent adverse events were mild to moderate in intensity; upper respiratory tract infections, nasopharyngitis, headache, hypertension and ALT elevations were the most frequently reported adverse events in patients receiving tocilizumab monotherapy or combination therapy for up to 52 weeks. Mild infusion reactions were also common, but were generally transient.
Serious adverse events generally occurred with similar incidence among patients receiving tocilizumab, alone or in combination (with methotrexate or other DMARDs), and patients receiving methotrexate or other DMARDs alone. The incidence of serious treatment-emergent infections was generally low with tocilizumab (1–8% of patients) and similar to the incidence observed with methotrexate or other DMARDs.
Moderate, reversible increases in mean serum levels of total cholesterol, low-and high-density lipoprotein cholesterol and triglycerides occurred in tocilizumab recipients. No corresponding increases in vascular adverse events were observed with tocilizumab plus methotrexate versus methotrexate alone during 24 weeks of therapy and few changes were observed in the atherogenic index in patients receiving tocilizumab for up to 1 year. Dose-dependent increases in serum ALT and AST levels were also frequently observed in patients receiving tocilizumab, with elevations in ALT and AST generally occurring more frequently with tocilizumab monotherapy than combination therapy (with methotrexate or other DMARDs). Hypersensitivity reactions and the development of antibodies are uncommon with tocilizumab.







Similar content being viewed by others
References
Helmick CG, Felson DT, Lawrence RC, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Arthritis Rheum 2008 Jan; 58(1): 15–25
Arthritis Foundation. Rheumatoid arthritis fact sheet [online]. Available from URL: http://www.arthritis.org/disease-center.php?disease_id=31 [Accessed 2009 Mar 23]
Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of non-biologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum 2008 Jun 15; 59(6): 762–84
Luqmani R, Hennell S, Estrach C, et al. British Society for Rheumatology and British Health Professionals in Rheumatology guideline for the management of rheumatoid arthritis (the first two years). Rheumatology (Oxford) 2006 Sep; 45(9): 1167–9
Voll RE, Kalden JR. Do we need new treatment that goes beyond tumor necrosis factor blockers for rheumatoid arthritis? Ann N Y Acad Sci 2005; 1051: 799–810
Siddiqui MAA. The efficacy and tolerability of newer biologics in rheumatoid arthritis: best current evidence. Curr Opin Rheumatol 2007 May; 19(3): 308–13
Cronstein BN. Interleukin-6: a key mediator of systemic and local symptoms in rheumatoid arthritis. Bull NYU Hosp Jt Dis 2007; 65 Suppl. 1: S11–5
Nishimoto N, Kishimoto T. Humanized antihuman IL-6 receptor antibody, tocilizumab. Handb Exp Pharmacol 2008; (181): 151-60
European Medicines Agency. RoActemra (tocilizumab): summary of product characteristics [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/RoActemra/H-955-PI-en.pdf [Accessed 2009 Mar 23]
Shimizu T. Product overview of Actemra [online]. Available from URL: http://www.chugai-pharm.co.jp [Accessed 2008 Jul 9]
Chugai Pharmaceutical Co. Actemra®, a humanized anti-human IL-6 receptor monoclonal antibody obtained approval for indications of rheumatoid arthritis, polyarticular-course juvenile idiopathic arthritis and systemic-onset juvenile idiopathic arthritis [media release]. Available from URL: http://www.chugai-pharm.co.jp [Accessed 2008 Sep 24]
Levi M, Frey N, Grange S, et al. Effect of tocilizumab exposure on IL-6 and IL-6 receptor levels in patients with rheumatoid arthritis: graphical analysis of pooled data from four phase 3 clinical trials [poster no. THU0176]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
Levi M, Frey N, Grange S, et al. Exposure to tocilizumab, an inhibitor of IL-6, is associated with reductions in disease activity score in patients with rhematoid arthritis: a population PK/PD analysis [poster no. THU0174]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
Frey N, Grange S, Woodworth T. Relationship between serum concentrations of the interleukin-6 receptor inhibitor tocilizumab and C-reactive protein reduction in RA patients: 6 months’ data from a phase 3 study [abstract no. 259]. Arthritis Rheum 2007 Sep; 56 (9 Suppl.): 148–9
Garnero P, Mareau E, Thompson E, et al. The anti-interleukin-6 receptor inhibitor tocilizumab combined with methotrexate has beneficial effects on bone and cartilage metabolism in patients with rheumatoid arthritis: results of a phase III 24-week randomised placebo-controlled study [abstract no. THU0178 plus poster]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
Terao K, Tsuru T, Suzaki M, et al. Drug-disease interaction study of tocilizumab in patients with rheumatoid arthritis: IL-6 signal inhbition normalized cytochrome P-450 enzymes expression which was reduced by inflammation [abstract no. FRI0166]. Ann Rheum Dis 2008; 67 Suppl. II: 339
Nishimoto N, Miyasaka N, Yamamoto K, et al. Relationship between serum IL-6 levels after tocilizumab treatment and clinical remission in active rheumatoid arthritis patients [abstract no. OP-0135]. Ann Rheum Dis 2008; 67 Suppl. II: 90
Castell JV, Gómez-Lechón MJ, David M, et al. Recombinant human interleukin-6 (IL-6/BSF-2/HSF) regulates the synthesis of acute phase proteins in human hepatocytes. FEBS Lett 1988 May; 232(2): 347–50
Tamura T, Udagawa N, Takahashi N, et al. Soluble interleukin-6 receptor triggers osteoclast formation by interleukin-6. Proc Natl Acad Sci U S A 1993 Dec; 90: 11924–8
Nishimoto N. Interleukin-6 in rheumatoid arthritis. Curr Opin Rheumatol 2006 May; 18(3): 277–81
Houssiau FA, Devogelaer J-P, Van Damme J, et al. Interleukin-6 in synovial fluid and serum of patients with rheumatoid arthritis and other inflammatory arthritides. Arthritis Rheum 1988 Jun; 31(6): 784–8
Kotake S, Sato K, Kim KJ, et al. Interleukin-6 and soluble interleukin-6 receptors in the synovial fluids from rheumatoid arthritis patients are responsible for osteoclast-like cell formation. J Bone Miner Res 1996; 11(1): 88–95
Mihara M, Kasutani K, Okazaki M, et al. Tocilizumab inhibits signal transduction mediated by both mIL-6R and sIL-6R, but not by the receptors of other members of IL-6 cytokine family. Int Immunopharmacol 2005 Nov; 5(12): 1731–40
Ohsugi Y, Kishimoto T. The recombinant humanized anti-IL-6 receptor antibody tocilizumab, an innovative drug for the treatment of rheumatoid arthritis. Expert Opin Biol Ther 2008 May; 8(5): 669–81
Nishimoto N, Terao K, Mima T, et al. Mechanisms and pathologic significances in increase in serum interleukin-6 (IL-6) and soluble IL-6 receptor after administration of an anti-IL-6 receptor antibody, tocilizumab, in patients with rheumatoid arthritis and Castleman disease. Blood 2008 Nov 15; 112(10): 3959–64
Nishimoto N, Yoshizaki K, Maeda K, et al. Toxicity, pharmacokinetics, and dose-finding study of repetitive treatment with the humanized anti-interleukin 6 receptor antibody MRA in rheumatoid arthritis: phase I/II clinical study. J Rheumatol 2003 Jul; 30(7): 1426–35
Choy EHS, Isenberg DA, Garrood T, et al. Therapeutic benefit of blocking interleukin-6 activity with an anti-interleukin-6 receptor monoclonal antibody in rheumatoid arthritis: a randomized, double-blind, placebo-controlled, dose-escalation trial. Arthritis Rheum 2002 Dec; 46(12): 3143–50
Levi M, Frey N, Grange S. Reduction in inflammatory biomarkers with increasing exposure to the IL-6 inhibitor, tocilizumab, in patients with rheumatoid arthritis: graphical analysis of pooled data [abstract no. THU0177]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
Nishimoto N, Yoshizaki K, Miyasaka N, et al. Treatment of rheumatoid arthritis with humanized anti-interleukin-6 receptor antibody: a multicenter, double-blind, placebo-controlled trial. Arthritis Rheum 2004 Jun; 50(6): 1761–9
Nishimoto N, Hashimoto J, Miyasaka N, et al. Study of active controlled monotherapy used for rheumatoid arthritis, an IL-6 inhibitor (SAMURAI): evidence of clinical and radiographic benefit from an x-ray reader-blinded randomised controlled trial of tocilizumab. Ann Rheum Dis 2007 Sep; 66(9): 1162–7
Nishimoto N, Miyasaka N, Yamamoto K, et al. Study of active controlled tocilizumab monotherapy for rheumatoid arthritis patients with an inadequate response to methotrexate (SATORI): significant reduction in disease activity and serum vascular endothelial growth factor by IL-6 receptor inhibition therapy. Mod Rheumatol 2009; 19(1): 12–9
Jones G, Sebba A, Gu J, et al. Comparison of tocilizumab monotherapy versus methotrexate monotherapy in patients with moderate to severe rheumatoid arthritis: The AMBITION study. Ann Rheum Dis Epub 2009 Mar 17
Sebba AI, Calvo A, Li X, et al. Tocilizumab monotherapy improvies quality of life compared with methotrexate monotherapy in patients with rheumatoid arthritis: the AMBITION study [abstract no. FRI0174 plus poster]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
Maini RN, Taylor PC, Szechinski J, et al. Double-blind randomized controlled clinical trial of the interleukin-6 receptor antagonist, tocilizumab, in European patients with rheumatoid arthritis who had an incomplete response to methotrexate [published erratum appears in Arthritis Rheum 2008 Mar; 58 (3): 887]. Arthritis Rheum 2006 Sep; 54(9): 2817–29
Smolen JS, Beaulieu A, Rubbert-Roth A, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. Lancet 2008 Mar 22; 371(9617): 987–97
Genovese MC, McKay JD, Nasonov EL, et al. Interleukin-6 receptor inhibition with tocilizumab reduces disease activity in rheumatoid arhtritis with inadequate response to disease-modifying antirheumatic drugs: the tocilizumab in combination with traditional disease-modifying antirheumatic drug therpy study. Arthritis Rheum 2008 Oct; 58(10): 2968–80
Emery P, Keystone E, Tony H-P, et al. IL-6 receptor inhibition with tocilizumab improves treatment outcomes in patients with rheumatoid arthritis refractory to anti-TNF biologics: results from a 24-week multicentre randomised placebo controlled trial. Ann Rheum Dis 2008; 67(11): 1516–23
Kremer JM, Fleischmann RM, Halland AM, et al. Tocilizumab inhibits structural joint damage in rheumatoid arthritis patients with inadequate response to methotrexate: the LITHE study [abstract no. L14]. 72nd Annual Scientific Meeting of the American College of Rheumatology and the 44th Annual Meeting of the Association of Rheumatology Health Professionals; 2008 Oct 24–29; San Francisco (CA)
Nishimoto N, Miyasaka N, Yamamoto K, et al. Long-term safety and efficacy of tocilizumab, an anti-interleukin-6 receptor monoclonal antibody, in monotherapy, in patients with rheumatoid arthritis (the STREAM study): evidence of safety and efficacy in a 5-year extension study. Ann Rheum Dis. Epub 2008 Nov 19
Smolen J, Martin-Mola E, Rubbert-Roth A, et al. Efficacy and safety of tocilizumab in rheumatoid arthritis in patients above and below 65 years of age with an inadequate response to DMARDs [abstract no. FRI0164 plus poster]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
Alten R, Shmidt E, Rovensky J. Tocilizumab provides rapid and significant improvement in patient-reported outcomes in the medical short form 36 questionnaire [abstract no. AB0345]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
Kremer J, Pope J, Dikranian A. Improvements in health related quality of life measures with tocilizumab treatment in patients with rheumatoid arthritis despite prior anti-TNF therapy: the RADIATE study [abstract no. AB0366]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
Ramos-Remus C, Nasonov E, Rovensky J, et al. Tocilizumab improves quality of life outcomes in rheumatoid arthritis patients regardless of age in patients with an inadequate response to DMARDs [abstract no. AB0381]. 2008 Annual European Congress of Rheumatology; 2008 Jun 11–14; Paris
European Medicines Agency. Assessment report for RoActemra (tocilizumab) [online]. Available from URL: http://www.emea.europa.eu/humandocs/PDFs/EPAR/RoActemra/H-955-en6.pdf [Accessed 2009 Feb 25]
Tsuru T, Suzaki M, Yoshio N, et al. Immune response to influenza vaccine in patients during the treatment with tocilizumab-comparison with conventional DMARDs and TNF inhibitors [abstract no. FRI0165]. Ann Rheum Dis 2008; 67 Suppl. II: 339
Yamanaka H, Nishimoto N, Inoue E, et al. Incidence of malignancies in Japanese rheumatoid arthritis patients treated with tocilizumab in comparison to those in an observational cohort of Japanese patients and a Japanese population database [abstract no. OP0228]. 2007 Annual European Congress of Rheumatology; 2007 Jun 13–16; Barcelona
Myllykangas-Luosujärvi RA, Aho K, Isomäki HA. Mortality in rheumatoid arthritis. Semin Arthritis Rheum 1995 Dec; 25(3): 193–202
Combe B, Landewe R, Lukas C, et al. EULAR recommendations for the management of early arthritis: report of a task force of the European Standing Committee for International Clinical Studies Including Therapeutics (ESCISIT). Ann Rheum Dis 2007; 66: 34–45
Furst DE, Breedveld FC, Kalden JR, et al. Updated consensus statement on biological agents for the treatment of rheumatic diseases, 2007. Ann Rheum Dis 2007; 66 Suppl. 3: iii2–22
Goekoop-Ruiterman YPM, de Vries-Bouwstra JK, Allaart CF, et al. Clinical and radiographic outcomes of four different treatment strategies in patients with early rheumatoid arthritis (the BeSt study): a randomised, controlled trial. Arthritis Rheum 2005 Nov; 52(11): 3381–90
Goldblatt F, Isenberg DA. New therapies for rheumatoid arthritis. Clin Exp Immunol 2005; 140: 195–204
Pucino Jr F, Harbus PT, Goldbach-Mansky R. Use of biologics in rheumatoid arthritis: where are we going? Am J Health Syst Pharm 2006 Sep 15; 63 (18 Suppl. 4): S19–41
Ledingham J, Deighton C. Update on the British Society for Rheumatology guidelines for prescribing TNFα blockers in adults with rheumatoid arthritis (update of previous guidelines of April 2001). Rheumatology (Oxford) 2005 Feb; 44(2): 157–63
Smolen JS, Maini RN. Interleukin-6: a new therapeutic target. Arthritis Res Ther 2006; 8 Suppl. 2: S5
Dhillon S, Lyseng-Williamson KA, Scott LJ. Etanercept: a review of its use in the management of rheumatoid arthritis. Drugs 2007; 67(8): 1211–41
Smolen JS, Aletaha D, Koeller M, et al. New therapies for treatment of rheumatoid arthritis. Lancet 2007 Dec 1; 370(9602): 1861–74
Dessein PH, Stanwix AE, Joffe BI. Cardiovascular risk in rheumatoid arthritis versus osteoarthritis: acute phase response related decreased insulin sensitivity and high-density lipoprotein cholesterol as well as clustering of metabolic syndrome features in rheumatoid arthritis. Arthritis Res 2002; 4(5): R5
Choy E, Sattar N. Interpreting lipid levels in the context of high-grade inflammatory states with a focus on rheumatoid arthritis: a challenge to conventional cardiovascular risk actions. Ann Rheum Dis 2009 Apr; 68(4): 460–9
Deighton CM, George E, Kiely PDW, et al. Updating the British Society for Rheumatology guidelines for anti-tumour necrosis factor therapy in adult rheumatoid arthritis (again). Rheumatology (Oxford) 2006 Jun; 45(6): 649–52
Acknowledgements
The preparation of this review was not supported by any external funding. During the peer review process, the manufacturers of the agent under review were offered an opportunity to comment on this article. Changes resulting from any comments received were made on the basis of scientific and editorial merit.
Author information
Authors and Affiliations
Corresponding author
Additional information
Various sections of the manuscript reviewed by: R. Alten, Department of Internal Medicine II, Rheumatology, Schlosspark-Klinik Teaching Hospital, Charité University Medicine Berlin, Berlin, Germany; H. Bird, Academic Unit of Musculoskeletal Disease, University of Leeds, Leeds, England; J. Gomez-Reino, Department of Medicine, University of Santiago de Compostela, Santiago de Compostela, Spain; A.I. Sebba, Arthritis Research of Florida, Palm Harbor, Florida, USA; H. Yamanaka, Institute of Rheumatology, Tokyo Women’s Medical University, Tokyo, Japan.
Data Selection
Sources: Medical literature published in any language since 1980 on ‘tocilizumab’, identified using MEDLINE and EMBASE, supplemented by AdisBase (a proprietary database of Wolters Kluwer Health | Adis). Additional references were identified from the reference lists of published articles. Bibliographical information, including contributory unpublished data, was also requested from the company developing the drug.
Search strategy: MEDLINE, EMBASE and AdisBase search terms were ‘tocilizumab’ or ‘R 1569’. Searches were last updated 2 April 2009.
Selection: Studies in patients with rheumatoid arthritis who received tocilizumab. Inclusion of studies was based mainly on the methods section of the trials. When available, large, well controlled trials with appropriate statistical methodology were preferred. Relevant pharmacodynamic and pharmacokinetic data are also included.
Index terms: Tocilizumab, rheumatoid arthritis, monoclonal antibody, pharmacodynamics, pharmacokinetics, therapeutic use, tolerability.
Rights and permissions
About this article
Cite this article
Oldfield, V., Dhillon, S. & Plosker, G.L. Tocilizumab. Drugs 69, 609–632 (2009). https://doi.org/10.2165/00003495-200969050-00007
Published:
Issue Date:
DOI: https://doi.org/10.2165/00003495-200969050-00007