Abstract
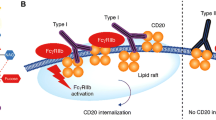
Over the last few years, new generations of anti-CD20 monoclonal antibodies (mAbs) have been developed for potential benefits over the classical, first-generation mAb rituximab. Compared with rituximab, new mAbs have enhanced antitumor activity resulting from increased complement-dependent cytotoxicity (CDC) and/or antibody-dependent cellular cytotoxicity (ADCC) and increased Fc binding affinity for the low-affinity variants of the FcγRIIIa receptor (CD16) on immune effector cells. The second-generation mAbs, which include ofatumumab, veltuzumab, and ocrelizumab, are humanized or fully human to reduce immunogenicity, but with an unmodified Fc region. Ofatumumab is a fully human anti-CD20 IgG1 mAb in clinical development for hematological malignancies and autoimmune diseases. Ofatumumab specifically recognizes an epitope encompassing both the small and large extracellular loops of CD20 molecule, and is more effective than rituximab at CDC induction and killing target cells. Veltuzumab (IMMU-106, hA20) is a humanized anti-CD20 mAb with complementarity-determining regions similar to rituximab. This antibody has enhanced binding avidities and a stronger effect on CDC compared with rituximab. Ocrelizumab is a humanized mAb with the potential for enhanced efficacy in lymphoid malignancies compared with rituximab due to increased binding affinity for the low-affinity variants of the FcγRIIIa receptor. The third-generation mAbs are also humanized mAbs, but in addition they have an engineered Fc to increase their binding affinity for the FcγRIIIa receptor. The third-generation mAbs include AME-133v, PRO131921 and GA-101. AME-133v (LY2469298) is a type I, humanized IgG1 mAb with enhanced affinity for FcγRIIIa receptor and an enhanced ADCC activity compared with rituximab. PRO131921 is a humanized anti-CD20 mAb engineered to have improved binding to FcγRIIIa and better ADCC compared with rituximab. GA-101 (RO5072759) is a fully humanized, type II, IgG1 mAb derived from humanization of the parental B-Ly1 mouse antibody and subsequent glycoengineering using GlycoMab® technology. GA-101 was designed for enhanced ADCC and superior direct cell-killing properties, in comparison with currently available type I antibodies. TRU-015 is a small modular immunopharmaceutical (SMIP) derived from key domains of an anti-CD20 antibody. TRU-015 represents a novel biological compound that retains Fc-mediated effector functions and is smaller than mAbs. In this article we review data on new anti-CD20 mAbs that are potentially useful in the treatment of lymphoid malignancies.


Similar content being viewed by others
References
Cragg MS, Walshe CA, Ivanov AO, et al. The biology of CD20 and its potential as a target for mAb therapy. Curr Dir Autoimmun 2005; 8: 140–74
Einfeld DA, Brown JP, Valentine MA, et al. Molecular cloning of the human B cell CD20 receptor predicts a hydrophobic protein with multiple trans-membrane domains. EMBO J 1988; 7: 711–7
Stashenko P, Nadler LM, Hardy R, et al. Expression of cell surface markers after human B lymphocyte activation. Proc Natl Acad Sci U S A 1981; 78: 3848–52
Beers SA, Chan CH, French RR, et al. CD20 as a target for therapeutic type I and II monoclonal antibodies. Semin Hematol 2010; 47: 107–14
Li H, Ayer LM, Lytton J, et al. Store-operated cation entry mediated by CD20 in membrane rafts. J Biol Chem 2003; 278: 42427–34
Beers SA, French RR, Chan HT, et al. Antigenic modulation limits the efficacy of anti-CD20 antibodies: implications for antibody selection. Blood 2010; 115: 5191–201
Giles FJ, Vose JM, Do KA, et al. Circulating CD20 and CD52 in patients with non-Hodgkin's lymphoma or Hodgkin's disease. Br J Haematol 2003; 123: 850–7
Manshouri T, Do KA, Wang X, et al. Circulating CD20 is detectable in the plasma of patients with chronic lymphocytic leukemia and is of prognostic significance. Blood 2003; 101(7): 2507–13
Taylor RP, Lindorfer MA. Immunotherapeutic mechanisms of anti-CD20 monoclonal antibodies. Curr Opin Immunol 2008; 20: 444–9
Uchida J, Hamaguchi Y, Oliver JA, et al. The innate mononuclear phagocyte network depletes B lymphocytes through Fc receptor-dependent mechanisms during anti-CD20 antibody immunotherapy. J Exp Med 2004; 199: 1659–69
Lefebvre ML, Krause SW, Salcedo M, et al. Ex vivo-activated human macrophages kill chronic lymphocytic leukemia cells in the presence of rit-uximab: mechanism of antibody-dependent cellular cytotoxicity and impact of human serum. J Immunother 2006; 29: 388–97
Boye J, Elter T, Engert A. An overview of the current clinical use of the anti-CD20 monoclonal antibody rituximab. Ann Oncol 2003; 14: 520–35
Robak T, Dmoszynska A, Solal-Celigny A, et al. Rituximab plus fludarabine and cyclophosphamide prolongs progression-free survival compared with fludarabine and cyclophosphamide alone in previously treated chronic lymphocytic leukemia. J Clin Oncol 2010; 28: 1756–65
Robak T, Jamroziak K, Robak P. Current and emerging treatments for chronic lymphocytic leukaemia. Drugs 2009; 69: 2415–49
Onrust SV, Lamb HM, Balfour JA. Rituximab. Drugs 1999; 58: 79–88
Golay J, Zaffaroni L, Vaccari T, et al. Biologic response of B lymphoma cells to anti-CD20 monoclonal antibody rituximab in vitro: CD55 and CD59 regulate complement-mediated cell lysis. Blood 2000; 95: 3900–8
Zhou X, Hu W, Qin X. The role of complement in the mechanism of action of rituximab for B-cell lymphoma: implications for therapy. Oncologist 2008; 13: 954–66
Byrd JC, Kitada S, Flinn IW, et al. The mechanism of tumor cell clearance by rituximab in vivo in patients with B-cell chronic lymphocytic leukemia: evidence of caspase activation and apoptosis induction. Blood 2002; 99: 1038–43
Pedersen IM, Buhl AM, Klausen P, et al. The chimeric anti-CD20 antibody rituximab induces apoptosis in B-cell chronic lymphocytic leukemia cells through a p38 mitogen activated protein-kinase-dependent mechanism. Blood 2002; 99: 1314–9
Teeling JL, Mackus WJ, Wiegman LJ, et al. The biological activity of human CD20 monoclonal antibodies is linked to unique epitopes on CD20. J Immunol 2006; 177: 362–71
Wilson WH. Chemotherapy sensitization by rituximab: experimental and clinical evidence. Semin Oncol 2000; 27Suppl. 12: 30–6
Stolz C, Schuler M. Molecular mechanisms of resistance to rituximab and pharmacologic strategies for its circumvention. Leuk Lymphoma 2009; 50: 873–85
Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgRIIIa gene. Blood 2002; 99: 754–8
Dall'Ozzo S, Tartas S, Paintaud G, et al. Rituximab-dependent cytotoxicity by natural killer cells: influence of FCGR3A polymorphism on the concentration-effect relationship. Cancer Res 2004; 64: 4664–9
Hatjiharissi E, Xu L, Santos DD, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the FcγRIIIa-158 V/V and V/F polymorphism. Blood 2007; 110: 2561–4
Kim DH, Jung HD, Kim JG, et al. FCGR3A gene polymorphisms may correlate with response to frontline R-CHOP therapy for diffuse large B-cell lymphoma. Blood 2006; 108: 2720–5
Racila E, Link BK, Weng WK, et al. A polymorphism in the complement component C1qA correlates with prolonged response following rituximab therapy of follicular lymphoma. Clin Cancer Res 2008; 14: 6697–703
van Meerten T, van Rijn RS, Hol S, et al. Complement-induced cell death by rituximab depends on CD20 expression level and acts complementary to antibody-dependent cellular cytotoxicity. Clin Cancer Res 2006; 12: 4027–35
Golay J, Lazzari M, Facchinetti V, et al. CD20 levels determine the in vitro susceptibility to rituximab and complement of B-cell chronic lymphocytic leukemia: further regulation by CD55 and CD59. Blood 2001; 98: 3383–9
Weitzman J, Betancur M, Boissel L, et al. Variable contribution of monoclonal antibodies to ADCC in patients with chronic lymphocytic leukemia. Leuk Lymphoma 2009; 50: 1361–8
Henry C, Deschamps M, Rohrlich PS, et al. Identification of an alternative CD20 transcript variant in B-cell malignancies coding for a novel protein associated to rituximab resistance. Blood 2010; 115: 2420–9
Maloney DG. Follicular NHL: from antibodies and vaccines to graft-versus-lymphoma effects. In: Hematology. Washington,DC: American Society of Hematology, 2007: 226–32
Robak T. Novel drugs for chronic lymphoid leukemias: mechanism of action and therapeutic activity. Curr Med Chem 2009; 16: 2212–34
Robak T. Ofatumumab, a human monoclonal antibody for lymphoid malignancies and autoimmune disorders. Curr Opin Mol Ther 2008; 10: 294–309
Lim SH, Beers SA, French RR, et al. Anti-CD20 monoclonal antibodies: historical and future perspectives. Haematologica 2010; 95: 135–43
Ruuls SR, Lammerts van Bueren JJ, van de Winkel JG, et al. Novel human antibody therapeutics: the age of the Umabs. Biotechnol J 2008; 3: 1157–71
Hutas G. Ocrelizumab, a humanized monoclonal antibody against CD20 for inflammatory disorders and B-cell malignancies. Curr Opin Investig Drugs 2008; 9: 1206–15
Robak T. GA-101, a third-generation, humanized and glyco-engineered anti-CD20 mAb for the treatment of B-cell lymphoid malignancies. Curr Opin Investig Drugs 2009; 10: 588–96
van Meerten T, Hagenbeek A. CD20-targeted therapy: the next generation of antibodies. Semin Hematol 2010; 47: 199–210
Beers SA, Chan CH, James S, et al. Type II (tositumomab) anti-CD20 monoclonal antibody out performs type I (rituximab-like) reagents in B-cell depletion regardless of complement activation. Blood 2008; 112: 4170–7
Hammadi M, Pers JO, Berthou C, et al. A new approach to comparing anti-CD20 antibodies: importance of the lipid rafts in their lytic efficiency. Onco Targets Ther 2010; 3: 99–109
Coiffier B, Lepretre S, Pedersen LM, et al. Safety and efficacy of ofatumumab, a fully human monoclonal anti-CD20 antibody, in patients with relapsed or refractory B-cell chronic lymphocytic leukemia: a phase 1-2 study. Blood 2008; 111: 1094–100
Coiffier B, Losic N, Rønn BB, et al. Pharmacokinetics and pharmacokinetic/pharmacodynamic associations of ofatumumab, a human monoclonal CD20 antibody, in patients with elapsed or refractory chronic lymphocytic leukaemia: a phase 1-2 study. Br J Haematol 2010; 150: 58–71
Wierda WG, Kipps TJ, Mayer J, et al. Ofatumumab as single-agent CD20 immunotherapy in fludarabine-refractory chronic lymphocytic leukemia. J Clin Oncol 2010; 28: 1749–55
Hagenbeek A, Gadeberg O, Johnson P, et al. First clinical use of ofatumumab, a novel fully human anti-CD20 monoclonal antibody in relapsed or refractory follicular lymphoma: results of a phase I/II trial. Blood 2008; 111: 5486–95
Hagenbeek A, Fayad L, Delwail V, et al. Evaluation of ofatumumab, a novel human CD20 monoclonal antibody, as single agent therapy in rituximab-refractory follicular lymphoma [abstract]. Blood (ASH Annual Meeting Abstracts) 2009; 114: 935
Salles GA, Morschhauser F, Cartron G, et al. A phase I/II study of RO5072759 (GA101) in patients with relapsed/refractory CD20+ malignant disease [abstract]. Blood (ASH Annual Meeting Abstracts) 2008; 93: 234
Morschhauser F, Cartron G, Lamy T, et al. Phase I study of RO5072759 (GA101) in relapsed/refractory chronic lymphocytic leukemia [abstract]. Blood (ASH Annual Meeting Abstracts) 2009; 114: 884
Salles G, Morschhauser F, Lamy T, et al. Phase I study of RO5072759 (GA101) in patients with relapsed/refractory CD20+ non-Hodgkin lymphoma (NHL) [abstract]. Blood (ASH Annual Meeting Abstracts) 2009; 114: 1704
Morschhauser F, Leonard JP, Fayad L, et al. Humanized anti-CD20 antibody, veltuzumab, in refractory/recurrent non-Hodgkin's lymphoma: phase I/II results. J Clin Oncol 2009; 27: 3346–53
Negrea OG, Allen SL, Rai KR, et al. Subcutaneous injections of low doses of humanized anti-CD20 veltuzumab for treatment of indolent B-cell malignancies [abstract]. Blood (ASH Annual Meeting Abstracts) 2009; 114: 3757
Morschhauser F, Marlton P, Vitolo U, et al. Results of a phase I/II study of ocrelizumab, a fully humanized anti-CD20 mAb, in patients with relapsed/refractory follicular lymphoma. Ann Oncol 2010; 21: 1870–6
Friedberg JW, Vose JM, Kahl BS, et al. A phase I study of PRO131921, a novel anti-CD20 monoclonal antibody in patients with relapsed/refractory CD20+ indolent NHL: correlation between clinical responses and AUC pharmacokinetics [abstract]. Blood (ASH Annual Meeting Abstracts) 2009; 114: 3742
Robak T. Ofatumumab for the treatment of chronic lymphocytic leukemia. Therapy 2009; 6: 577–87
Beum PV, Lindorfer MA, Beurskens F, et al. Complement activation on B lymphocytes opsonized with rituximab or ofatumumab produces substantial changes in membrane structure preceding cell lysis. J Immunol 2008; 181: 822–32
Du J, Yang H, Guo Y, et al. Structure of the Fab fragment of therapeutic antibody ofatumumab provides insights into the recognition mechanism with CD20. Mol Immunol 2009; 46: 2419–23
Teeling JL, French RR, Cragg MS, et al. Characterization of new human CD20 monoclonal antibodies with potent cytolytic activity against non-Hodgkin lymphomas. Blood 2004; 104: 1793–800
Cheson BD. Ofatumumab, a novel anti-CD20 monoclonal antibody for the treatment of B-cell malignancies. J Clin Oncol 2010; 28: 3525–30
Taylor RP, Beum PV, Stukenberg TP, et al. Complement (C) activation followed by penetration of the membrane attack complex (MAC) on B cells opsonized with CD20 mabs allows for calcium influx which induces streamers: generalization of streaming to include other mabs and target cells [abstract]. Blood (ASH Annual Meeting Abstracts) 2008; 112: 1577
Wierda WG, Kipps TJ, Dürig J, et al. Ofatumumab combined with fludarabine and cyclophosphamide (O-FC) shows high activity in patients with previously untreated chronic lymphocytic leukemia (CLL): results from a randomized, multicenter, international, two-dose, parallel group, phase II trial [abstract]. Blood (ASH Annual Meeting Abstracts) 2009; 114: 207
Robak T, Osterborg A, Kipps TJ, et al. High response rats and clinical improvements with single-agent ofatumumab in fludarabine: refractory chronic lymphocytic leukemia (CLL) also refractory to alemtuzumab or with bulky lymphadenopathy [abstract 9.7]. Haematologica 2009; 94Suppl. 3: 81
Wierda WG, Ronn BB, Jewell RC, et al. Circulating ofatumumab concentrations correlate with response and progression-free survivalin fludarabine-refractory chronic lymphocytic leukemia [abstract 10.28]. Haematologica 2009; 94Suppl. 3: 92
Milani C, Castillo J. Veltuzumab, an anti-CD20 mAb for the treatment of non-Hodgkin's lymphoma, chronic lymphocytic leukemia and immune throm-bocytopenic purpura. Curr Opin Mol Ther 2009; 11: 200–7
Goldenberg DM, Rossi EA, Stein R, et al. Properties and structure-function relationships of veltuzumab (hA20), a humanized anti-CD20 monoclonal antibody. Blood 2009; 113: 1062–70
Stein R, Qu Z, Chen S, et al. Characterization of a new humanized anti-CD20 monoclonal antibody, IMMU-106, and its use in combination with the humanized anti-CD22 antibody, epratuzumab, for the therapy of non-Hodgkin's lymphoma. Clin Cancer Res 2004; 10: 2868–78
Goldenberg DM, Morschhauser F, Wegener WA. Veltuzumab (humanized anti-CD20 monoclonal antibody): characterization, current clinical results, and future prospects. Leuk Lymphoma 2010; 51: 747–55
Kausar F, Mustafa K, Sweis G, et al. Ocrelizumab: a step forward in the evolution of B-cell therapy. Expert Opin Biol Ther 2009; 9: 889–95
Genovese MC, Kaine JL, Lowenstein MB, et al., ACTION Study Group. Ocrelizumab, a humanized anti-CD20 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: a phase I/II randomized, blinded, placebo-controlled, dose-ranging study. Arthritis Rheum 2008; 58: 2652–61
Robak T. Novel monoclonal antibodies for the treatment of chronic lymphocytic leukemia. Curr Cancer Drug Targets 2008; 8: 156–71
Martin P, Furman RR, Ruan J, et al. Novel and engineered anti-B-cell monoclonal antibodies for non-Hodgkin's lymphoma. Semin Hematol 2008; 45: 126–32
Campbell BJ, Barrett AMA, Ondek MD, et al. AME-133: A next-generation anti-CD20 engineered for enhanced killer function [abstract]. J Immunother 2004; 27: 52
Bowles JA, Wang SY, Link BK et al. Anti-CD20 monoclonal antibody with enhanced affinity for CD16 activates NK cells at lower concentrations and more effectively than rituximab. Blood 2006; 108: 2648–54
Bello C, Eduardo M. Monoclonal antibodies for B-cell lymphomas: rituximab and beyond. In: Hematology. Washington,DC: American Society of Hematology, 2007: 233–42
Weiner GJ. Rituximab: mechanism of action. Semin Hematol 2010; 47: 115–23
Safety and efficacy study of an anti-CD20 monoclonal antibody (AME-133v) to treat non-Hodgkin's lymphoma [online]. Available from URL: http://ClinicalTrials.gov [Accessed 2009 Jun 3]
Umana P, Moessner E, Bruenker P, et al. Novel 3rd generation humanized type II CD20 antibody with glycoengineered Fc and fodified elbow hinge for enhanced ADCC and superior apoptosis induction [abstract]. Blood (ASH Annual Meeting Abstracts) 2006; 108: 22
Mössner E, Brünker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood 2010; 115: 4393–402
Umana P, Ekkehard M, Peter B, et al. GA101, a novel humanized type IICD20 antibody with glycoengineered Fc and enhanced cell death induction, exhibits superior anti-tumor efficacy and superior tissue B cell depletion in vivo [abstract]. Blood (ASH Annual Meeting Abstracts) 2007; 110: 2348
Friess T, Gerdes C, Nopora A, et al. GA101, a novel humanized type II CD20 antibody with glycoengineered Fc and enhanced cell death induction, mediates superior efficacy in a variety of NHL xenograft models in comparison to rituximab [abstract]. Blood (ASH Annual Meeting Abstracts) 2007; 110: 2338
Niederfellner GJ, Lammens A, Schwaiger M, et al. Crystal structure analysis reveals that the novel type II anti-CD20 antibody GA101 interacts with a similar epitope as rituximab and ocrelizumab but in a fundamentally different way [abstract]. Blood (ASH Annual Meeting Abstracts) 2009; 114: 3726
Robak T, Robak P, Smolewski P. TRU-016, a humanized anti-CD37 IgG fusion protein for the potential treatment of B-cell malignancies. Curr Opin Investig Drugs 2009; 10: 1383–90
Hayden-Ledbetter M, Magaletti D, Mohler K, et al. CD16-mediated antibody dependent cellular cytotoxicity is required for B cell depletion by a small modular ImmunoPharmaceutical specific for CD20 [abstract]. Blood (ASH Annual Meeting Abstracts) 2003; 102: 2388
Barone D, Burge DJ, Baum P, et al. Prolonged depletion of circulating B cells in cynomolgus monkeys after a single dose of TRU-015, a novel CD20 directed therapeutic. Ann Rheum Dis 2005; 64Suppl. 3: 159–60
Burge D, Martin R, Wallace D, et al. Safety and pharmacodynamics of repeat administration of TRU-015, a CD20-directed SMIP(TM) therapeutic, in subjects with rheumatoid arthritis. Arthritis Rheum 2007; 56(9 Suppl.): S168–9
Barone D. TRU-015, a novel CD20-directed biologic therapy, demonstrates significant anti-tumor activity in human tumor xenograft models [abstract]. Proc Am Soc Clin Oncol 2005; 24: 2549
Hayden-Ledbetter MS, Cerveny CG, Espling E, et al. CD20-directed small modular immunopharmaceutical, TRU-015, depletes normal and malignant B cells. Clin Cancer Res 2009; 15: 2739–46
Rubbert-Roth A. TRU-015, a fusion protein derived from an anti-CD20 antibody, for the treatment of rheumatoid arthritis. Curr Opin Mol Ther 2010; 12: 115–23
Burge DJ, Bookbinder SA, Kivitz AJ, et al. Pharmacokinetic and pharmaco-dynamic properties of TRU-015, a CD20-directed small modular immunopharmaceutical protein therapeutic, in patients with rheumatoid arthritis: a phase I, open-label, dose-escalation clinical study. Clin Ther 2008; 30: 1806–16
Acknowledgements
This work was supported in part by a grant from the Medical University of Lodz (No. 503-1093-1) and by the Foundation for the Development of Diagnostics and Therapy, Warsaw, Poland. Professor T. Robak has worked as a consultant for F. Hoffman-La Roche Ltd, GlaxoSmithKline, and Celgene Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Robak, T., Robak, E. New Anti-CD20 Monoclonal Antibodies for the Treatment of B-Cell Lymphoid Malignancies. BioDrugs 25, 13–25 (2011). https://doi.org/10.2165/11539590-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11539590-000000000-00000