Tertiary Lymphoid Structures Associate with Tumour Stage in Urothelial Bladder Cancer
Abstract
Background:
Urothelial bladder cancer (UBC) is a highly prevalent disease in North America, however its optimal management remains elusive. The contribution of B cell associated responses is poorly understood in bladder cancer. Lymphoid neogenesis is a hallmark of an active immune response at tumor sites that sometimes leads to formation of tertiary lymphoid structures (TLS) that resemble germinal centers formed in secondary lymphoid organs.
Objective:
This study was conducted with an aim to investigate the presence and characteristics of TLS in UBC with a focus to compare and contrast the TLS formation in treatment naive low grade non-muscle invasive (NMIBC) and muscle invasive bladder cancers (MIBC).
Methods:
The study cohort consisted of transurethral bladder resection tumour (TURBT) specimens from 28 patients. Sections showing lymphoid aggregates in hematoxylin and eosin (H&E) stained TURBT specimens were further subjected to multi-color immunohistochemistry using immune cell markers specific to CD20+ B cells, CD3+ and CD8+ T cells, PNAd+ high endothelial venules, CD208+ mature dendritic cells, CD21+ follicular dendritic cells to confirm the hallmarks of classical germinal centers.
Results:
Our pilot study investigating the presence of TLS in bladder cancer patients is the first to demonstrate that well-formed TLS are more common in aggressive high grade MIBC tumors compared to low grade NIMBC.
Conclusions:
These novel findings suggest B cell mediated anti-tumour humoral immune responses in bladder cancer progression.
INTRODUCTION
Urothelial bladder cancer (UBC) is the most common urinary tract malignancy and the fifth most common cancer worldwide with a high incidence in North America. In 2012, 330,400 new cases were diagnosed with UBC with an estimated 123,100 cases predicted to die of the disease. These statistics have only minimally changed over the past decade [1]. The prevalence of the disease, as well as its high recurrence rates and the need for close follow-up surveillance makes this one of the most management intensive and expensive cancers in North America. The majority of UBCs at initial presentation are superficial to the muscularis propria of the bladder (NMIBC) and are confined to the mucosa (stage Ta, Tis) or submucosa (stage T1) [2]. Intravesical instillation with attenuated Bacillus Calmette-Guerin (BCG) has been a gold standard and most effective adjuvant therapy in NMIBC to decrease recurrence and progression to muscle invasive disease for those at higher risk [3]. In contrast, high grade muscle invasive bladder cancer (MIBC) carries much worse prognosis despite aggressive treatment that optimally includes radical cystectomy with peri-operative chemotherapy.
Urothelial bladder cancer investigations have had a strong linkage to tumour immunology, especially given that BCG immunotherapy has been central to NMIBC management for over forty years. Significant efforts in cancer immunology have been directed towards the association of tumour infiltrating lymphocytes (TILs) with disease prognosis, a feature that is of both prognostic and predictive relevance across cancers [4, 5]. Towards this end recent reports on immunopathological evaluation of tumours have provided significant amount of evidence for similarities in specific spatiotemporal associations of TILs within the tumour microenvironment (TME) across different cancer types [6]. Given their key roles in anti-tumour immunity and therapeutic implications, most efforts have been made to evaluate the cytotoxic CD8+ TIL populations across cancers which has contributed to the development of prognostic indicators such as the “Immunoscore” in addition to the successful therapeutic exploitation by immune checkpoint blockade therapies [7, 8].
Chronic inflammation and/or persistent antigen exposure leads to cellular accumulations within non-lymphoid areas of inflammation where lymphoid neogenesis (or lymphoid neo-organogenesis) can initiate. In secondary lymphoid organs, germinal centers (GC) are specialized areas where B cells (primary mediators of humoral immune response) diversify themselves, post antigenic challenge, in order to achieve highest affinity towards an antigen [9]. Memory B cells and antigen specific high affinity antibody producing plasma cells are thus generated. Similar to these, persistent antigenic stimulation in non-lymphoid tissue leads to formation of structures called ‘ectopic’ or tertiary lymphoid structures (TLS), which resemble germinal center areas located in secondary lymphoid organs such as spleen and lymph nodes [10]. It is also established that these two forms of lymphoid aggregates bear significant similarities despite their distinct origins, location and programming. The canonical lymphoid aggregates arise during ontogeny whereas the ectopic aggregates arise due to factors such as chronic inflammation and persistent antigen exposure. These extra-nodal sites of adaptive immunity are most common in autoimmune (rheumatoid arthritis) or inflammatory conditions [10, 11].
Recently TLS have gained attention due to their associations with prognosis in certain cancers [12–14]. Intra-tumoural TLS have been widely reported in colorectal and ovarian cancers and are suggestive of ongoing B cell expansion. In most cancers, TLS are associated with a favorable prognosis [15–17]. It must be noted that TLS are also speculated to be a mere bystander effect of inflammation within the TME and not as an active participant in mediating anti-tumour immune response in certain cancer sites [12, 18]. Interestingly, a recent study on hepatocellular carcinoma describes these TLS as micro niches supporting tumour cell growth and survival [19]. TLS mimicking features of a canonical GC reaction, within an inflamed tissue and cancer are evidence supporting active adaptive immunity triggered within the tumor microenvironment.
Bladder tumours are associated with extensive lymphocytic infiltration and, given the role of immunotherapy in both localized and systemic disease, it would appear to be a good model to study the immunopathological distributions of adaptive immune cells. It is widely accepted that lower grade NMIBC is biologically distinctive from higher grade MIBC, with significant differences in oncogenic drivers of the disease and with clinical outcomes. Therefore, it is of great interest to compare and contrast the tumor microenvironment, including the tertiary lymphoid structures that are representative of anti-tumour humoral responses, in these two divergent stages of UBC. This is the first study to investigate the presence and characteristics of TLS in UBC with a focus to compare and contrast the TLS formation in treatment naïve, low and high grade bladder tumours.
ETHICS AND PATIENT SAMPLES
Institutional ethics approval was obtained from the Queen’s University, Research Ethics Board for this study. Bladder cancer specimens derived from a retrospective cohort of patients who underwent transurethral resection of bladder tumour (TURBT) procedures at Kingston Health Sciences Centre between 2010 and 2014 with a pathology diagnosis of either low grade (TaLG) NMIBC or high grade (≥T2HG) MIBC from the TURBT specimens. Patients who had undergone intravesical BCG treatment or neoadjuvant chemotherapy treatment prior to resection were excluded. The cohort consisted of 12 patients in the NMIBC group (10 males, 2 females); and 16 patients in the MIBC group (15 males, 1 female). The pathologic diagnosis of all cases were confirmed via histopathological evaluation (A.X, K.R and D.B.). A representative unstained formalin fixed paraffin embedded (FFPE) section from each tumour was selected for further immunohistochemistry analysis. The average tissue volume per tissue block from which the FFPE section was calculated to normalize for the variations in sample size.
HISTOLOGY AND IMMUNOHISTOCHEMISTRY
Hematoxylin and Eosin (H&E) stained FFPE sections of the TURBT specimens were examined by a pathologist (A.X; K.R; DB) to identify lymphoid aggregates resembling TLS. We defined TLS as those that morphologically resembled lymphoid aggregates in the lymph nodes, which contain B cell rich areas with well-defined mantle zone and other associated immune cells. One representative slide was selected for immunohistochemistry (IHC). Multicolour IHC was performed as previously described by Kroeger and colleagues [20, 21] at the Deeley Research Center (BC cancer agency, Canada). IHC was performed on 5 μm thick FFPE sections as previously described [20, 21] on the Intellipath FLX Automated Staining system, using mouse anti-human CD20 (1 : 500 dilution, clone L26, Biocare Medical), rabbit anti-human CD3 (1 : 250 dilution, clone SP7, Spring Biosciences), mouse anti-human CD8 (1 : 300 dilution, C8/144B, Cell Marque), rat anti-human CD208 (1 : 100, clone 1010E1.01, Novus Biologicals), rat anti-human PNAd (1 : 100 dilution, MECA-79, Biolegend) and mouse anti-human CD21 (1 : 100 dilution, clone 2G9, Biocare Medical) antibodies. Multispectral images of stained sections were captured using the PerkinElmer Vectra system. The selected H&E slide were scanned with Aperio ScanScope software (Leica Biosystems) and analyzed by a pathologist.
RESULTS
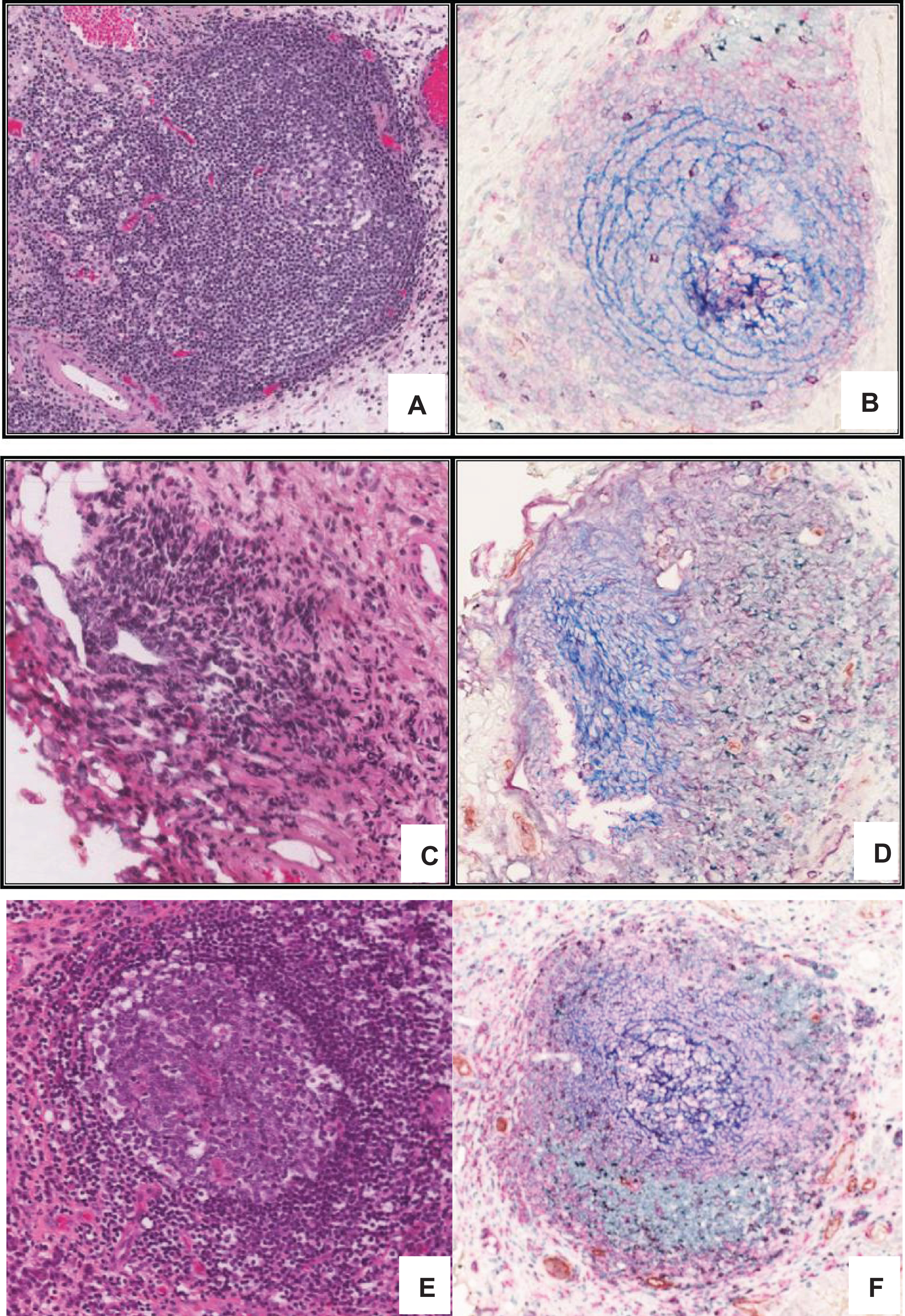
Evaluation of TLS in a cohort of 28 bladder cancer cases using multicolor IHC revealed their distinct organizational and developmental stages (Fig. 1). We followed the recently reported classification criteria for lymphoid aggregates based on size, cellular composition and degree of organization to define the types of TLS in the cohort under study [21]. Type 1 aggregates are small, compact, T-cell predominant lymphoid aggregates without apparent follicular structures. Type 2 aggregates are larger, and also T cell predominant with few CD20+ B cells lacking follicular structures. Type 3 aggregates contain well-formed TLSs containing CD20+ B cells and a network of CD21+ interdigitating follicular dendritic cells (fDCs), in addition to an abundant population of T cells surrounding the GCs. Type 4 aggregates contain very few T-cells, but have well-formed GCs containing CD20+ B cells and CD21+ fDCs. In our study, we defined TLS as lymphoid aggregates with well-formed GCs, which included Type 3 and Type 4 lymphoid aggregates similar to those described in ovarian cancer by Kroeger and colleagues [21].
Fig.1
Developmental continuum of TLS in bladder tumours. Multi-color IHC performed on 5 μm thick sections from formalin fixed paraffin embedded bladder tumour tissue to reveal features associated with the TLS architecture. Different Types of aggregates and immune cell distributions were defined as previously described by Kroeger et al., [20], A) Type I B) Type 2 C) Type 3 and D) Type 4 aggregates. E) Well formed tertiary lymphoid structure in high grade muscle invasive bladder tumour showing CD3+ (green) and CD8+ T lymphocytes (purple), CD20+ B lymphocytes (pink), CD21+ follicular dendritic cells (blue), CD208+ mature dendritic cells (black), PNAd+ high endothelial venule (brown).
![Developmental continuum of TLS in bladder tumours. Multi-color IHC performed on 5 μm thick sections from formalin fixed paraffin embedded bladder tumour tissue to reveal features associated with the TLS architecture. Different Types of aggregates and immune cell distributions were defined as previously described by Kroeger et al., [20], A) Type I B) Type 2 C) Type 3 and D) Type 4 aggregates. E) Well formed tertiary lymphoid structure in high grade muscle invasive bladder tumour showing CD3+ (green) and CD8+ T lymphocytes (purple), CD20+ B lymphocytes (pink), CD21+ follicular dendritic cells (blue), CD208+ mature dendritic cells (black), PNAd+ high endothelial venule (brown).](https://content.iospress.com:443/media/blc/2017/3-4/blc-3-4-blc170120/blc-3-blc170120-g001.jpg)
TLS were present in only 1 (8%) of the 12 NMIBC tumours. In the remaining 11 of these tumours without TLS, we observed focal lymphoid aggregates without well-defined GC (Fig. 2) in 7 cases and no lymphoid aggregates in the remaining 4. In contrast, we observed TLS in 10 (62%) of the 16 MIBC cases (Fig. 3). Three of the remaining 6 MIBC cases contained focal lymphoid aggregates.
Fig.2
Lymphoid aggregates in low grade MIBC. H & E stained 5 μm thick FFPE section of a low grade NMIBC tumour showing lymphoid aggregation (A, C and E) further confirmed by staining with multi-color IHC (B, D and F) markers specific to TLS including showing CD20+ B cells (pink) and CD8+ T cells (purple).
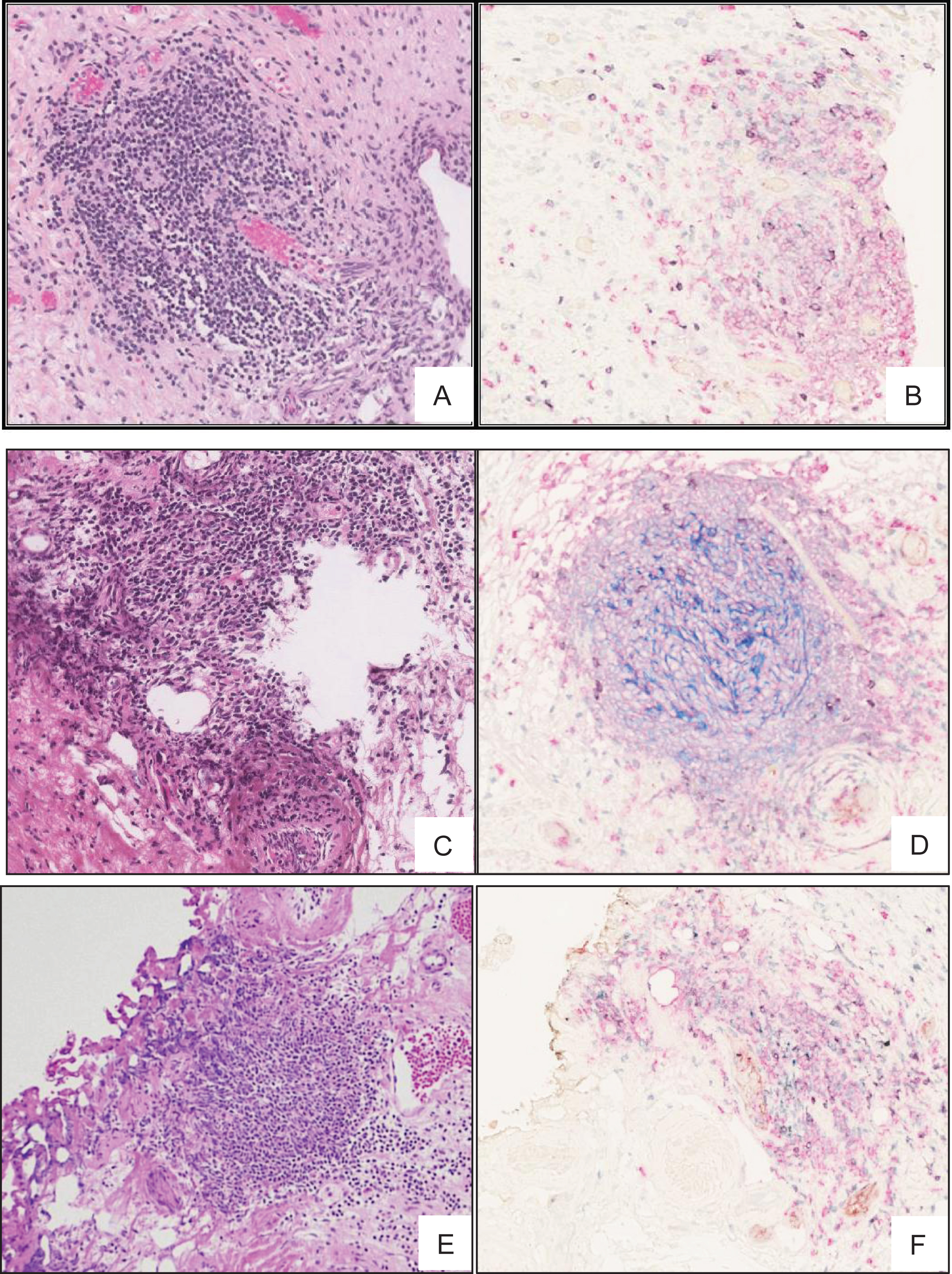
Fig.3
TLS in high grade MIBC. H & E stained 5 μm thick FFPE section of MIBC tumour showing TLS like lymphoid aggregation (A, C and E) further confirmed by staining with multi-color IHC (B, D and F) markers specific to TLS including, CD3+ (green) and CD8+ T cells (purple), CD20+ B cells (pink), CD21+ follicular dendritic cells (blue), CD208+ mature dendritic cells (black), PNAd+ high endothelial venule (brown).
To further characterize the lymphoid structures observed in H&E stained sections, we used a previously described [21] 6-colour IHC panel that enabled simultaneous detection of CD20+ B cells, CD3+ and CD8+ T cells, CD21+ fDC, CD208+ activated conventional DC, and PNAd+ high endothelial venule (HEV)-like vessels. All TLS identifiable as lymphoid aggregates by H&E were confirmed by IHC to contain well-defined follicular structures with GC highlighted by CD20+ B cells and CD21+ fDC networks surrounded by a varying number of CD3+ and CD8+ T-cells. In addition, IHC revealed follicular structures highlighted by CD21+ fDC networks that were not apparent by H&E stain in 2 patients from the MIBC group, and 3 patients from the NMIBC group (Fig. 3 A-E). Integrating the findings by H&E histology and immunohistochemistry, TLS was present in 3 of the 12 patients (25%) from the NMIBC group, in contrast to 12 of 16 patients (75%) from the MIBC group (Table 1).
Table 1
Distribution and characteristics of tertiary lymphoid structures in a representative test cohort of bladder tumours
| Overall | Non-muscle invasive low | Muscle invasive high | |
| grade (NMIBC) TaLG | grade (MIBC) T2HG | ||
| Number of patients | N = 28 | N = 12 | N = 16 |
| Median Age (Range) | 68.5 (54–82) | 71 (54–80) | 66.5 (54–82) |
| Presence of TLS identifiable by H&E | 11 (39%) | 1 (8%) | 10 (62%) |
| Presence of TLS identifiable by IHC | 15 (54%) | 3 (25%) | 12 (75%) |
| Presence of B-cell clusters that do | 22 (79%) | 9 (75%) | 13 (81%) |
| not qualify as TLS | |||
| Concurrent carcinoma in situ | 6 (21%) | 0 (0%) | 6 (38%) |
| in the TURBT specimen | |||
| Lymphovascular invasion | 0 | 0 | 0 |
Furthermore, IHC also helped to highlight the organization of CD20+ B cells amongst the lymphoid aggregates and lymphocytic infiltrates reflecting a GC like distribution. Clusters of B cells were identified in 13 of 16 tumours (81%) from the MIBC group, and in 9 of 12 tumours (75%) from the NMIBC group. In the MIBC group, all 13 tumours with CD20+ B cell clusters are associated with IHC identifiable follicular structures. In contrast, of the 9 from the NMIBC group with CD20+ B cell clusters, only 3 had associated follicular structures.
Following confirmation of classical architectural characteristics of a TLS we performed histomorphometric analysis, to determine the qualitative features, such size and number of lymphoid aggregates. The average tissue volume per tissue block is 2.4cc in the T2HG cases and is 3.0cc in the TaLG cases. In the T2HG tumours the average TLS number was of 9.1 compared to 2.4 in TaLG tumours. The average size of these aggregates was 0.44 mm in T2HG compared to 0.30 mm in TaLG tumours.
In summary, in a cohort of 28 cases we were able to identify different stages of TLS formation representing their wide prevalence in bladder tumours, specifically their well-defined architecture in tumours with more aggressive MIBC.
DISCUSSION
An integral component in most of the successful immune based therapies is a complete understanding of the pre-existing state of the TME [8]. Bladder cancers are understudied with regard to their immune cell distribution patterns and their association with prognosis. In the current study we evaluated the adaptive immune cell phenotypic distributions in low grade NMIBC and high grade MIBC. However, based on a limited cohort of 28 cases, our study revealed novel characteristics of TILs and thus establishes the presence of TLS in bladder tumours in addition to their significant association with MIBC. TLS containing well-defined B cell containing GCs were readily identifiable by H&E and enriched in high grade tumours relative to the low grade. Compared to H&E staining alone, a 6-colour IHC panel improved identification of TLS while also refining their architecture and cell type composition. Diverse association of B cells with disease outcome has been reported across cancers [22, 23]. In ovarian, colorectal, breast and lung cancer, intra-tumoural B cells or those located within organized TLS are associated with favorable outcomes suggesting there anti-tumour role whereas their increased numbers are negatively correlated with melanoma, prostate, renal cell and hepatocellular carcinoma prognosis [16, 19, 24]. In these cancers where TLS are associated with poor prognosis it is speculated that these could act as the microniches providing a source of the complex cytokine factors required for tumour cell survival [19]. The underlying mechanisms driving TLS formation in tumour sites are currently an area of active research.
Due the tissue fragmentation inherent to the TURBT procedure, it is difficult to determine the precise distribution of the lymphoid aggregates in this cohort. Qualitative observation showed that the lymphoid aggregates are present in fragments both within and without tumour. The differences in fragmented TURBT specimen size between T2HG and TaLG cases can be a potential source of confounding bias, and was adjusted by calculating the average tissue volume present in the FFPE tissue block from which the representative histology section was generated. T2HG cases more frequently contained well-formed TLS in higher numbers compared to TaLG cases despite containing lower tissue volumes on average. Future studies investigating cystectomy specimens would improve our understanding of the topographic distribution of TILs and TLS in UBC. The peri-tumoral as well as stromal locations of TLS seen in the current study suggest that factors intrinsic to the tumour might contribute to evasion of immune cell effector functions. This could represent a continuous spectrum of stages in TLS formation. Accordingly, the focal lymphoid aggregate present in 7 of the 12 TaLG NMIBC patients may represent an early, less developed form of TLS, a notion which is supported by the presence of CD20+ B cells in these cases. Furthermore, the degree of TLS formation and maturity may be associated with aggressiveness of the disease and the severity of stromal inflammatory response it evokes. It could be further also be speculated that the enrichment or evolution of tumour neo-epitopes during advancement to aggressive disease stages is permissive to maturation of B cell receptors within these intra-tumour TLS. These questions can be ideally addressed by characterizing the antibodies secreted from the B cells isolated from fresh tumour specimens, in conjunction with confirmation of other features associated with events such as somatic hypermutation and antibody affinity maturation.
A limitation of our study is the small number of cases. However, the identification of B cell infiltrates not associated with follicular structures in TaLG NMIBC is novel and provides a basis to further explore their possible associations with prognosis in NMIBC. An in depth analysis to understand the mechanistic basis of TLS formation in bladder tumours is necessary towards their therapeutic exploitation. It is also necessary to assess the spatiotemporal dynamics of other immune cells in the tumour microenvironment in the context of response to BCG immunotherapy with recent reports on their distinct association with poor prognosis in bladder tumours [25]. The prognostic relevance of B cells in NMIBC thus confirmed could lead to diagnostic and prognostic tools that can be incorporated at the time of TURBT with subsequent administration of additional therapies on the selected patients.
CONCLUSIONS AND FUTURE PERSPECTIVE
This is the first study reporting the presence of TLS in bladder tumours with novel insights into their stages associated with tumour grade. The features of stages associated with development of and well-formed TLS are not readily identifiable by H&E and could only be confirmed by a multicolor IHC. An in depth evaluation of B cells isolated from these tumours will reveal novel information on neo-epitopes that are putatively seen by these B cells to confirm their functional roles in UBC as well as their therapeutic implications. Future investigations in larger cohorts is warranted to confirm the association between TLS, disease recurrence and overall survival to evaluate their prognostic relevance in bladder cancer.
COMPETING INTERESTS
All authors have no competing interests to declare.
ACKNOWLEDGMENTS
The authors would like to thank Drs. Brad Nelson, Katy Milne and David Kroeger for the critical input in design and interpretation of findings of this study. This study was supported by the South Eastern Ontario Academic Medical Organization Innovation grant support to D. Robert Siemens.
REFERENCES
[1] | Torre LA , Siegel RL , Ward EM , Jemal A . Global Cancer Incidence and Mortality Rates and Trends–An Update. Cancer Epidemiol Biomarkers Prev [Internet] (2015) ;25: (January):16–28. Available from: http://cebp.aacrjournals.org/cgi/doi/10.1158/1055-9965. EPI-15-0578 |
[2] | Babjuk M , Oosterlinck W , Sylvester R , Kaasinen E , Bohle A , Palou-Redorta J , et al. EAU guidelines on non-muscle-invasive urothelial carcinoma of the bladder, the 2011 update. Actas Urol EsServicio de Urologia, Hospital Motol, Segunda Facultad de Medicina, Universidad Carolina, Praga, Republica Checa. Marek.badjuk@ lfmotol.cuni.cz: AEU. Published by Elsevier Espana (2012) ;36: (7):389–402. |
[3] | Lima L , Dinis-Ribeiro M , Longatto-Filho A , Santos L . Predictive biomarkers of bacillus calmette-guerin immunotherapy response in bladder cancer: Where are we now? Adv Urol. Experimental Pathology and Therapeutics Group, Portuguese Institute of Oncology, 4200-072 Porto, Portugal (2012) ;2012: :232609. |
[4] | Schreiber RD , Old LJ , Smyth MJ . Cancer immunoediting: Integrating immunity’s roles in cancer suppression and promotion. Science. Department of Pathology and Immunology, Washington University School of Medicine, St. Louis, MO 63110, USA. [email protected] (2011) ;331: (6024):1565–70. |
[5] | Galon J , Mlecnik B , Bindea G , Angell HK , Berger A , Lagorce C , et al. Towards the introduction of the “Immunoscore” in the classification of malignant tumours. J Pathol. INSERM, U872, Laboratory of Integrative Cancer Immunology, Paris, France; Universite Paris Descartes, Paris, France; Centre de Recherche des Cordeliers, Universite Pierre et Marie Curie Paris 6, France.: The Authors. Journal of Pathology published by John Wiley & Sons Ltd on behalf of Pathological Society of Great Britain and Ireland (2014) ;232: (2):199–209. |
[6] | Bindea G , Mlecnik B , Tosolini M , Kirilovsky A , Waldner M , Obenauf AC , et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity (2013) ;39: (4):782–95. |
[7] | Galon J , Fox BA , Bifulco CB , Masucci G , Rau T , Botti G , et al. Immunoscore and Immunoprofiling in cancer: An update from the melanoma and immunotherapy bridge 2015. J Transl Med [Internet]. BioMed Central (2016) ;1–6. Available from: http://dx.doi.org/10.1186/s12967-016-1029-z |
[8] | Kyi C , Postow MA . Checkpoint blocking antibodies in cancer immunotherapy. FEBS Lett. Department of Medicine, New York Presbyterian Hospital Cornell, 525 E 68th St., New York, NY 10065, United States.; Department of Medicine, Memorial Sloan-Kettering Cancer Center, 300 E 66th St., New York, NY 10065, United States. Electronic address: Post: Federation of European Biochemical Societies. Published by Elsevier B.V (2014) ;588: (2):368–76. |
[9] | De Silva NS , Klein U . Dynamics of B cells in germinal centres. Nat Rev Immunol. Nature Publishing Group (2015) ;15: (3):137–48. |
[10] | Drayton DL , Liao S , Mounzer RH , Ruddle NH . Lymphoid organ development: From ontogeny to neogenesis. Nat Immunol [Internet] (2006) ;7: (4):344–53. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16550197 |
[11] | De Silva NS , Klein U . Dynamics of B cells in germinal centres. Nat Rev Immunol [Internet]. Nature Publishing Group (2015) ;15: (3):137–48. Available from: http://dx.doi.org/10.1038/nri3804n10.1038/nri3804 |
[12] | Pimenta EM , Barnes BJ . Role of tertiary lymphoid structures (TLS) in anti-tumor immunity: Potential tumor-induced cytokines/chemokines that regulate TLS formation in epithelial-derived cancers. Cancers (Basel) (2014) ;6: (2):969–97. |
[13] | Dieu-Nosjean MC , Goc J , Giraldo NA , Sautes-Fridman C , Fridman WH . Tertiary lymphoid structures in cancer and beyond. Trends Immunol. Laboratory “Cancer, Immune Control and Escape”, INSERM U1138 Cordeliers Research Centre, Paris, France; University Pierre and Marie Curie, UMRS 1138, Paris, France; University Paris Descartes, UMRS 1138, Paris, France.; Laboratory ’Cancer, Immune Control: Elsevier Ltd (2014) ;35: (11):571–80. |
[14] | Germain C , Gnjatic S , Tamzalit F , Knockaert S , Remark R , Goc J , et al. Presence of B cells in tertiary lymphoid structures is associated with a protective immunity in patients with lung cancer. Am J Respir Crit Care Med. 1 Laboratory “Immune Microenvironment and Tumors” and (2014) ;189: (7):832–44. |
[15] | Coppola D , Nebozhyn M , Khalil F , Dai H , Yeatman T , Loboda A , et al. Unique ectopic lymph node-like structures present in human primary colorectal carcinoma are identified by immune gene array profiling. Am J Pathol. Anatomic Pathology Division, Moffitt Cancer Center, Tampa, FL 33612-9497, USA.: American Society for Investigative Pathology. Published by Elsevier Inc (2011) ;179: (1):37–45. |
[16] | Dieu-Nosjean MC , Antoine M , Danel C , Heudes D , Wislez M , Poulot V , et al. Long-term survival for patients with non-small-cell lung cancer with intratumoral lymphoid structures. J Clin Oncol. Laboratoire Microenvironnement, Immunitaire et Tumeurs, Institut National de la Sante et de la Recherche Medicale (INSERM) U872, Centre de Recherche des Cordeliers, Paris cedex 06, France. [email protected] (2008) ;26: (27):4410–7. |
[17] | Martinet L , Garrido I , Filleron T , Le Guellec S , Bellard E , Fournie JJ , et al. Human solid tumors contain high endothelial venules: Association with T- and B-lymphocyte infiltration and favorable prognosis in breast cancer. Cancer Res. CNRS, IPBS (Institut de Pharmacologie et de Biologie Structurale), INSERM, U563, Centre de Physiopathologie de Toulouse-Purpan, Toulouse, France.: AACR (2011) ;71: (17):5678–87. |
[18] | Pitzalis C , Jones GW , Bombardieri M , Jones SA . Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev. Centre for Experimental Medicine and Rheumatology, William Harvey Research Institute, Barts and The London, School of Medicine and Dentistry, Queen Mary University of London, Charterhouse Square, London EC1M 6BQ, UK.; Cardiff Institute for Infection and I (2014) ;14: (7):447–62. |
[19] | Finkin S , Yuan D , Stein I , Taniguchi K , Weber A , Unger K , et al. Ectopic lymphoid structures function as microniches for tumor progenitor cells in hepatocellular carcinoma. Nat Immunol [Internet]. Nature Publishing Group; (2015) ;(October):1–12. Available from: http://www.nature.com/doifinder/10.1038/ni.3290 |
[20] | Nielsen JS , Sahota RA , Milne K , Kost SE , Nesslinger NJ , Watson PH , et al. CD20+ tumor-infiltrating lymphocytes have an atypical CD27- memory phenotype and together with CD8+T cells promote favorable prognosis in ovarian cancer. Clin Cancer Res. Trev and Joyce Deeley Research Centre, British Columbia Cancer Agency, Department of Biochemistry and Microbiology, University of Victoria, Victoria, British Columbia, Canada. [email protected]: AACR (2012) ;18: (12):3281–92. |
[21] | Kroeger DR , Milne K , Nelson BH , Columbia B , Agency C , Nelson B , et al. Tumor infiltrating plasma cells are associated with tertiary lymphoid structures, cytolytic T cell responses, and superior prognosis in ovarian cancer (2016) ;9. |
[22] | Siliņa K , Rulle U , Kalniņa Z , Linē A . Manipulation of tumour-infiltrating B cells and tertiary lymphoid structures: A novel anti-cancer treatment avenue? Cancer Immunol Immunother [Internet] (2014) ;63: (7):643–62. Available from: http://link.springer.com/10.1007/s00262-014-1544-9 |
[23] | Matsumoto H , Koo SL , Dent R , Tan PH , Iqbal J . Role of inflammatory infiltrates in triple negative breast cancer. J Clin Pathol. Department of Pathology, Singapore General Hospital, Singapore, Singapore Department of Pathology, Ryukyu University Hospital, Okinawa, Japan.; Department of Medical Oncology, National Cancer Centre, Singapore, Singapore.; Department of Medical Oncology;; (2015) . |
[24] | Quinn DI , Shore ND , Egawa S , Gerritsen WR , Fizazi K . Immunotherapy for castration-resistant prostate cancer: Progress and new paradigms. Urol Oncol. Division of Medical Oncology, University of Southern California Norris Comprehensive Cancer, Los Angeles, CA.Carolina Urologic Research Center, Myrtle Beach, SC.; Department of Urology, Jikei University School of Med: The Authors. Published by Elsevier Inc; (2015) . |
[25] | Patschan O , Sjödahl G , Chebil G , Lövgren K , Lauss M , Gudjonsson S , et al. A Molecular Pathologic Framework for Risk Stratification of Stage T1 Urothelial Carcinoma. Eur Urol [Internet] (1839) ;68: (5):824–32. Available from: http://linkinghub.elsevier.com/retrieve/pii/S0302283815001839 |



