- Nyberg Human Cellular Therapy Laboratory, Department of Laboratory Medicine and Pathology, Mayo Clinic, Phoenix, AZ, United States
Recent successes in cancer immunotherapy have been tempered by sub-optimal clinical responses in the majority of patients. The impaired anti-tumor immune responses observed in these patients are likely a consequence of immune system dysfunction contributed to by a variety of factors that include, but are not limited to, diminished antigen presentation/detection, leukopenia, a coordinated network of immunosuppressive cell surface proteins, cytokines and cellular mediators. Monocytes that have diminished or no HLA-DR expression, called CD14+HLA-DRlo/neg monocytes, have emerged as important mediators of tumor-induced immunosuppression. These cells have been grouped into a larger class of suppressive cells called myeloid derived suppressor cells (MDSCs) and are commonly referred to as monocytic myeloid derived suppressor cells. CD14+HLA-DRlo/neg monocytes were first characterized in patients with sepsis and were shown to regulate the transition from the inflammatory state to immune suppression, ultimately leading to immune paralysis. These immunosuppressive monocytes have also recently been shown to negatively affect responses to PD-1 and CTLA-4 checkpoint inhibition, CAR-T cell therapy, cancer vaccines, and hematopoietic stem cell transplantation. Ultimately, the goal is to understand the role of these cells in the context of immunosuppression not only to facilitate the development of targeted therapies to circumvent their effects, but also to potentially use them as a biomarker for understanding disparate responses to immunotherapeutic regimens. Practical aspects to be explored for development of CD14+HLA-DRlo/neg monocyte detection in patients are the standardization of flow cytometric gating methods to assess HLA-DR expression, an appropriate quantitation method, test sample type, and processing guidances. Once detection methods are established that yield consistently reproducible results, then further progress can be made toward understanding the role of CD14+HLA-DRlo/neg monocytes in the immunosuppressive state.
Introduction
Exquisitely and carefully modulated immune responses coordinate the balance between preventing microbial onslaught and preventing autoimmune attack. Too little immune activation results in insufficient clearance of foreign invaders, and too much immune activation results in the targeting of self-antigens and potentially devastating autoimmune syndromes. This finely choreographed tightrope act is accomplished, in part, by a specialized array of immune cells which patrol the body and exert immunomodulatory roles. While in the larger context these cells exert the beneficial tempering of immune over-responsiveness, they can also by similar mechanisms negatively impact anti-cancer immunotherapy efficacy.
The goal of cancer immunotherapy is to successfully stimulate anti-tumor responses and overcome tumor-mediated immunosuppression. Generation of anti-tumor immunity has been accomplished through different modalities including cellular immunotherapy, vaccines, monoclonal antibodies, cytokine administration, and oncolytic virotherapy. These multi-faceted approaches have yielded tremendous clinical successes in the past few years. Even so, there have been significant difficulties in generating durable responses in a majority of cancer patients. As such, further understanding of immune dysfunction and the identification of predictive biomarkers are required so that methods may be developed to increase the efficacy of immunotherapeutic agents.
Recent data reveal that the potential exists to utilize the assessment of CD14+HLA-DRlo/neg monocyte abundance as a biomarker to predict which patients may or may not respond to immunotherapy regimens. For CD14+HLA-DRlo/neg monocytes in particular, an extensive array of studies involving immunotherapy demonstrate that high baseline levels of these immunosuppressive monocytes were associated with diminished anti-tumor responses and/or poor clinical outcomes. As CD14+ monocytes lose HLA-DR expression and thus convert from an inflammatory to an anti-inflammatory phenotype, they play a role in subverting effective anti-tumor responses, and their abundance in patient blood inversely correlates with favorable outcomes.
The purpose of this review is to highlight the documented findings which demonstrate this correlation. In the context of cancer immunotherapy, the abundance of these cells may guide patient selection and/or provide patient monitoring capabilities for understanding clinical responses on the individual level. Since there are considerable differences in the biology of these cells in animal models compared to human studies, this review will focus mainly on published data from human studies and clinical trials. However, in some cases, examples may be provided from animal models where the observations appear to be congruent with human data.
CD14+HLA-DRlo/neg Monocytes are Immunosuppressive Cells That Respond to Systemic Pro-Inflammatory Conditions
Myeloid cells that suppress the immune system have been described by a variety of names including myeloid derived suppressor cells (MDSCs), M2 monocytes/macrophages, tumor associated macrophages/myeloid cells, and regulatory myeloid cells (1–3). They are a heterogeneous population comprised of precursors of granulocytes, macrophages, and dendritic cells (DC). However, their characterization and classification into different subsets remains to be resolved as there are considerable inconsistencies in the way these subsets are defined and reported (4). Monocytes that have low or no HLA-DR expression have been most commonly referred to as CD14+HLA-DRlo/neg monocytes or monocytic MDSCs. HLA-DR is one of three MHC class II glycoproteins expressed on antigen-presenting cells whose function is to present peptides derived from antigens ingested by the cell to T-cell receptors (TCR) resulting in T-cell activation. As such, these cells have a diminished capacity to present antigens to T cells and a large body of work has demonstrated these cells to be immunosuppressive. Since the functional capacities related to the immunosuppressive mechanisms of these cells have been reviewed elsewhere (5–7), this subject will not be discussed here. Although, there is still considerable debate over the origins of human MDSCs (8), several lines of evidence that will be discussed in this review suggest that CD14+HLA-DRlo/neg monocytes should be best understood in terms of arising from the normal circulating monocyte pool and not from an early precursor cell independent of monocytes. As for other MDSCs, lineage negative (CD3−CD19−CD56−CD14−) LIN−CD33+HLA-DR− cells have been described as immature MDSCs (iMDSCs) and CD33+CD15+HLA-DR− cells as polymorphonuclear MDSCs (PMN-MDSCs). CD14+HLA-DRlo/neg monocytes also express high levels of CD33 and CD11b on their surface. As will be discussed later, CD33 expression is greater on monocytes compared to other myeloid cells. CD11b is expressed on nearly all myeloid cells but also is expressed on human natural killer NK cells (9) and therefore is not an appropriate marker for human MDSCs. For the sake of brevity, CD33+CD11b+CD14+HLA-DRlo/neg immunosuppressive monocytes throughout this review will be referred to as CD14+HLA-DRlo/neg monocytes/cells. It should also be noted that there has been another type of immunosuppressive monocyte described as CD1c/BDCA1+CD14+ (10). These CD1c+ monocytes are a mix of classical and intermediate monocytes and are functionally distinct from CD1c+ dendritic cells (11). These cells express HLA-DR but not to the same extent as dendritic cells (10, 11).
Monocytes play a critical role in the response to infection. Sepsis results when the initial strong pro-inflammatory phase [referred to as systemic inflammatory response syndrome (12, 13)] then switches to an anti-inflammatory phase. At first glance, it appears that these conditions act sequentially, but there are likely elements of both pro- and anti- inflammatory mediators throughout the entire process. Monocytes are highly sensitive in the transition to the immunosuppressive state and become deactivated, resulting in a phase known as “immunoparalysis” (14, 15) or compensatory anti-inflammatory response syndrome (16, 17). Immunoparalysis is defined by a decrease in the level of HLA-DR expression on monocytes during the course of sepsis.
One group has defined immunoparalysis in patients with septic shock as having occurred when < 30% of the monocyte pool expresses HLA-DR (14). In the early stage of sepsis in these patients, pro-inflammatory cytokines such as TNFα, IL-1, GM-CSF, and IL-6, drove the deactivation of monocytes by down regulating HLA-DR through IL-10 and TGF-β mediated pathways and diminished capacity for pro-inflammatory cytokine production (16, 18). These observations have been confirmed by many other studies. For example, in patients with injuries from blunt trauma, those that had low levels of HLA-DR on monocytes after the second day of admission were significantly more likely to develop sepsis than those patients that had high levels (19). Monneret et al. demonstrated the relationship of low HLA-DR levels to survival in patients with sepsis (20). In the early stages of septic shock, the expression of HLA-DR on monocytes was not different between survivors and non-survivors. However, after 48 h post onset, survivors had significantly higher expression of HLA-DR on monocytes than those that did not survive the event.
In contrast, there are some reports that have not found a relationship between the loss of monocyte HLA-DR expression and septic shock (21, 22). These apparent contradictory reports perhaps may result from differences in the timing of sample procurement, clinical settings, and measurement parameters for HLA-DR expression or other uncharacterized variables. However, a recent review has confirmed the regulatory role of CD14+HLA-DRlo/neg monocytes in both normal and pathological responses to a diverse array of microbial infections (23).
The loss of HLA-DR on monocytes has been reported in other non-malignant conditions with an inflammatory component. CD14+HLA-DRlo/neg monocytes have been described in patients with severe burns (24, 25), acute and chronic liver inflammation (26–28), pancreatitis (29–31), amyotrophic lateral sclerosis (32), and immediately after surgical procedures (33). Although the precise mechanisms of monocyte deactivation and HLA-DR loss have yet to be elucidated in each of these diseases, a familiar pattern of either acute or chronic inflammation tends to be an initial event triggering the development of immunosuppressive monocytes. Taken together, the overall data in non-malignant conditions demonstrate that the loss of HLA-DR is a well-established marker of functional deactivation of monocytes and that it associates with poor clinical outcomes in critically ill patients.
HLA-DR can be down regulated through a variety of mechanisms. Under normal physiological conditions, HLA-DR is under the transcriptional control of the MHC Class II transactivator (CIITA) (34, 35). HLA-DR expression can be induced by IFN-γ through transcriptional activation via CIITA (36) and also by GM-CSF possibly through post-transcriptional mechanisms (33). Conversely, several cytokines can down-regulate monocytic HLA-DR expression. IL-1β and TGF-β directly down-regulate transcription of HLA-DR through CIITA and/or prevent IFN-γ induction of HLA-DR (16, 37, 38). IL-10 also strongly decreases surface HLA-DR expression but it's mechanism of action is to increase intracellular sequestration of MHC Class II molecules (39) via ubiquitination by inducing the membrane-associated RING-CH (MARCH) ubiquitin ligase (40). Glucocorticoids and steroid hormones can also negatively regulate HLA-DR transcription by decreasing CIITA mRNA levels. Down-regulation of HLA-DR on monocytes has been observed in response to cortisol (41), prednisolone (42), and dexamethasone (43, 44). Overall, many of these mechanisms that regulate HLA-DR expression have been shown to be critical mediators of immune paralysis in both sepsis and in malignant settings.
Soon after the role of monocyte deactivation was observed in sepsis patients, reports began appearing in the literature that cancer patients also exhibit monocytes with low HLA-DR expression. Patients with glioblastoma (45), ovarian cancer (46), and melanoma (47) were some of the first cancer patients discovered to have low monocytic HLA-DR expression. In previous studies with our colleagues at Mayo Clinic Rochester, we found high levels of these cells in a variety of different cancer patient groups including those with glioblastoma (43), non-Hodgkin lymphoma (48), chronic lymphocytic leukemia (49), and renal cell carcinoma (50). In order to understand the severity of immunosuppression in cancer patients, we compared the presence of CD14+HLA-DRlo/neg monocytes in cancer patients to those patients with acute lung injury with or at risk for sepsis (51). Many of the cancer patients had levels of CD14+HLA-DRlo/neg monocytes equally high as patients with sepsis.
In vitro experiments demonstrated that monocytes isolated from healthy volunteers can lose HLA-DR expression through co-culture with tumor-derived exosomes (47), exposure to conditioned media from cultured tumor cells (52, 53), or even incubation with cytokines like TGF-β (37). Furthermore, Ribechini et al. have identified a potentially unique pathway in which GM-CSF can license CD14+ monocytes such that upon later exposure to INF-γ, the monocytes would switch to an immunosuppressive phenotype through the upregulation of indolamine 2,3-dioxygenase (IDO) (54). Bergenfeltz et al. found that monocytes isolated from breast cancer patients exhibited gene expression profiles similar to monocytes isolated from sepsis patients (55). Specifically, TNFα, IL-1β, HLA-DR, and CD86 genes were significantly down-regulated in monocytes from breast cancer patients compared to controls suggesting that some of the mechanisms that convert monocytes to the immunosuppressive state are identical in both septic and malignant conditions.
The implications of these findings for cancer immunotherapy are significant. The presence of high levels of CD14+HLA-DRlo/neg monocytes suggests that many of these cancer patients had reached a point of immunoparalysis prior to treatment and thus may not be very responsive to immunotherapeutic approaches. On the other hand, many cancer patients have been observed with normal levels of CD14+HLA-DRlo/neg monocytes. The timing of onset, progression and intensity of immunoparalysis in cancer patients compared to patients with sepsis will certainly involve both similar and unique mechanisms. As such, further work is needed to understand how these cells respond and contribute to tumor development.
Impact on Immunotherapy
Checkpoint Inhibitors
The impact of CD14+HLA-DRlo/neg monocytes on CTLA-4 inhibition with ipilimumab has most clearly been demonstrated in melanoma patients with advanced disease. Meyer et al. reported that CD14+HLA-DRlo/neg monocytes were elevated in melanoma patients. While CD14+HLA-DRlo/neg monocyte populations were not affected by ipilimumab treatment, patients that responded to ipilimumab treatment had significantly less pre-treatment frequencies of CD14+HLA-DRlo/neg monocytes than those patients that did not respond to treatment (56). In another study, lower pre-treatment frequencies of CD14+HLA-DRlo/neg monocytes were associated with overall patient survival (57). The percentages of CD14+HLA-DRlo/neg cells of total monocytes appeared to be more predictive of survival than absolute cell counts (cells/μl). The authors also reported that after 6 weeks of ipilimumab treatment, lower percentages of CD14+HLA-DRlo/neg cells were associated with higher changes in absolute T cell counts, suggesting that the CD14+HLA-DRlo/neg monocytes restricted CD8+ T cell response. These data were confirmed to some extent by Tarhini et al. (58), Martens et al. (59) and Gebhardt et al. (60). Gebhardt et al. found that decreased CD14+HLA-DRlo/neg monocytes were related to declines in nitric oxide production in response to ipilimumab treatment. Finally, de Coaña et al. found that in melanoma patients PMN-MDSCs decreased upon ipilimumab treatment whereas CD14+HLA-DRlo/neg monocytes did not change (61). However, in patients who received a clinical benefit, CD14+HLA-DRlo/neg monocytes decreased after treatment whereas this was not the case in patients who progressed. While the frequency of CD14+HLA-DRlo/neg monocytes was not compared to healthy volunteers, baseline levels of these cells were similar between patients with progressive disease and those that had a clinical benefit. Taken together, the results from these studies present an interesting dynamic. Clearly, lower baseline frequencies of CD14+HLA-DRlo/neg monocytes are predictive of outcome and therefore these monocytes may interfere with the efficacy of ipilimumab treatment. However, in some patients, particularly for those who do respond to treatment, there is evidence that immunosuppressive monocytes decline after treatment. Further studies are needed to confirm and delineate the mechanisms behind these observations.
Data are also emerging that demonstrate the role of monocytes in altered responses to anti-PD-1 therapy. In a study of stage IV melanoma patients receiving anti-PD-1 therapy, single cell mass cytometry was used to investigate peripheral blood biomarkers (62). The pre-treatment frequency of classical monocytes (CD14+CD16−) that express high levels of HLA-DR was predictive of overall survival in these patients. The majority of CD14+HLA-DRlo/neg monocytes are classical monocytes so the higher expression of HLA-DR in these patients likely reflects lower levels of immunosuppressive monocytes. The authors of this study did report that a population similar to CD33loCD11b+HLA-DRlo cells was not different between responders and non-responders. However, the gating strategy for isolating CD33loCD11b+HLA-DRlo cells could very well include both monocytic and granulocytic populations. In mixed lymphocyte reaction proliferation assays using PBMCs from healthy donors, it was shown that proliferation of nivolumab-treated T cells improved in the absence of monocytes (63). Additionally, blockade of CSF-1R signaling on monocytes improved T cell proliferation. Interestingly, the authors of this study also report that activation of T cells by nivolumab treatment induced the release of M-CSF from T cells thereby increasing the immunosuppressive functions of monocytes through adenosine production and upregulation of PD-L1 on monocytes. PD-1 and CSF-1R blockade in combination was also found to augment the cytolytic capacity of tumor infiltrating lymphocytes (TILs) in co-cultures of CD3+ TILs and CD11b+ tumor infiltrating myeloid cells from patients with glioblastoma (64). CD14+HLA-DRlo/neg monocyte levels also appear to distinguish responders from non-responders in nivolumab treated metastatic melanoma patients who progressed after ipilimumab therapy (65). Since there have not been many mechanistic insights garnered from these human studies, further investigation is needed to determine whether the impact of CD14+HLA-DRlo/neg monocytes on checkpoint inhibition reflects a general immunosuppressive environment or to what degree the expression of monocytic PD-1 and/or PD-L1 disrupts the efficacy of checkpoint blockade (66–68).
Cancer Vaccines
Data from studies and clinical trials demonstrate that immunosuppressive monocytes impact cancer vaccines through several mechanisms that limit optimal anti-tumor responses. They have been shown to adversely affect responses to direct injection of peptides/whole tumor cells as well as to ex-vivo differentiated dendritic cell (DCs) vaccines. In a therapeutic cancer vaccine study using multiple tumor-associated peptides (TUMAPs) in renal cell carcinoma (RCC) patients, Walter et al. looked at six populations of MDSCs (among other immune parameters) to determine whether these cells influenced the survival outcome of patients treated with the vaccine (69). Although five of the six types of MDSCs were elevated in RCC patients prior to treatment, CD14+HLA-DRlo/neg monocytes demonstrated the strongest association with overall survival whereby their presence in high numbers was inversely correlated with survival. In non-small cell lung cancer (NSCLC) patients receiving a telomerase peptide vaccine, high levels of CD14+HLA-DRlo/neg monocytes were found to be associated with poorer progression free survival (70). Prostate cancer patients with low frequencies of CD14+HLA-DRlo/neg monocytes prior to receiving prostate/GVAX vaccine in combination with ipilimumab had a mean survival time of 52 months compared to 20 months mean survival for those with high pre-treatment frequencies (71). These cells did increase during the course of treatment but the increase did not correlate with response to treatment. In a trial testing DCs for patients with primary recurrent glioblastoma, both CD15+SSClo cells and CD14+HLA-DRlo/neg monocytes were found to be elevated in patients that progressed but only the CD14+HLA-DRlo/neg monocytes were found to be both higher as a percent of parent populations and also in cell counts (cells/μl) (72). Poschke et al. demonstrated in stage IV melanoma patients that the presence of CD14+HLA-DRlo/neg cells purified with elutriated monocytes negatively impacted the maturation, migration, antigen uptake, and cytokine production of DCs (73). In another trial with melanoma patients, BDCA1+CD14+ cells were shown to inhibit T cell proliferation in an antigen-dependent fashion resulting in impaired responses to monocyte-derived DCs (10). In pre-clinical studies, our group has found that monocytes purified by immunomagnetic selection from a variety of cancer patients have deficiencies in DC maturation and that the method of cell culture can influence their maturation (7). Additionally, we found that CD14+HLA-DRlo/neg cells inversely correlated with the maturation marker CD83 on dendritic cells. Taken together, the data from these studies and clinical trials demonstrate that immunosuppressive monocytes impact cancer vaccines through several mechanisms that limit optimal anti-tumor responses.
Hematopoietic Progenitor Cell Transplantation
The functional consequences of immunosuppressive monocytes in hematopoietic progenitor cell transplantation appear to be dependent on the type of transplant. In autologous transplants, higher levels of CD14+HLA-DRlo/neg cells in leukapheresis products were independent predictors of adverse outcomes both in terms of overall survival and progression free survival in patients with non- Hodgkin lymphoma (74). However, in the allogeneic setting, CD14+HLA-DRlo/neg cells may confer protection against acute graft vs. host disease (aGVHD). Myeloid cells, including CD14+HLA-DRlo/neg cells, are some of the first cells to recover after transplantation (75, 76). Mougiakakos et al. demonstrated that transplant patients had both elevated monocytes and CD14+HLA-DRlo/neg cells at 1–3 months post-transplant (77). They also demonstrated that higher frequencies in peripheral blood also associated with higher grades of aGVHD. The induction of these cells was likely in response to circulating levels of G-CSF and IL-6, along with other pro-inflammatory cytokines. The administration of G-CSF alone to human donors was sufficient to expand both CD14+HLA-DRlo/neg monocytes and PMN-MDSCs in both phenotype and function (78). Similarly, the presence of high CD14+HLA-DRlo/neg monocyte cell counts in the G-CSF mobilized graft was associated with lower risks of developing aGVHD in recipients without affecting the relapse rate or the transplant-related mortality rate (79). So whereas immunosuppressive monocytes negatively affect outcomes in autologous transplant patients, they conversely may provide a benefit of a reduced risk of aGVHD in allogeneic transplant recipients.
Adoptive and Chimeric Antigen Receptor (CAR) T Cell Therapies
CD14+HLA-DRlo/neg monocytes may negatively impact the effectiveness of adoptive and Chimeric Antigen Receptor (CAR) T cell therapies. While the data from clinical trials is too limited to show this conclusively, the ability of suppressive monocytes to inhibit T cells in human cancers has been well documented (43, 48, 80–83) and has been associated both with dysfunctional antigen-specific T cells and negative outcomes in melanoma patients (84). CD19-CAR T cell expansions from mononuclear cell collections that contained high percentages of monocytes produced poorer cell yields in children with acute lymphocytic leukemia and non-Hodgkin lymphoma (85). While they did not specifically measure CD14+HLA-DRlo/neg monocytes, when they depleted monocytes by adherence to plastic techniques, cell expansion improved, and typical expected yields were achieved. While data has yet to be published from clinical trials monitoring immunosuppressive monocytes in those receiving CAR-T cell therapies, reports from animal model studies suggest that rodent MDSCs are expanded after treatment with CAR-T cells in a GM-CSF dependent fashion, and that this limited the anti-tumor activity of the infused cells (86). Other data from animal models suggest that although transferred T cells likely induce myeloid derived suppressor cells (87), there may be some conditions in which T cell therapy can be successful despite immunosuppression caused by myeloid cells (88). As data emerges in the human setting, it is not unreasonable to expect that CD14+HLA-DRlo/neg monocytes will be shown to play some role in reducing the anti-tumor efficacy of CAR T cells because the nature of the cytokine release syndrome involves pro-inflammatory cytokines which have previously been demonstrated to induce these cells under other conditions.
Efficacy of Therapeutic Approaches Targeting Immunosuppressive Monocytes
As evidence for the role of immunosuppressive monocytes in inhibiting anti-tumor responses continues to build, it becomes readily apparent that therapeutically targeting these cells should improve responses to immunotherapy. Agents designed to interfere with MDSCs have generally been classified into four categories: (1) inhibition of the conversion, appearance and/or expansion of MDSCs, (2) inhibition of MDSC immunosuppressive functions, (3) interference of MDSC trafficking to tumors, and (4) direct removal and cytotoxic approaches (89–91). Several agents that interfere with these mechanisms have shown promise in pre-clinical animal studies and have been reviewed elsewhere (89–93). As these drugs move into clinical trials, it will be very important understand how they will affect each of the different MDSC populations as well as total myeloid cells.
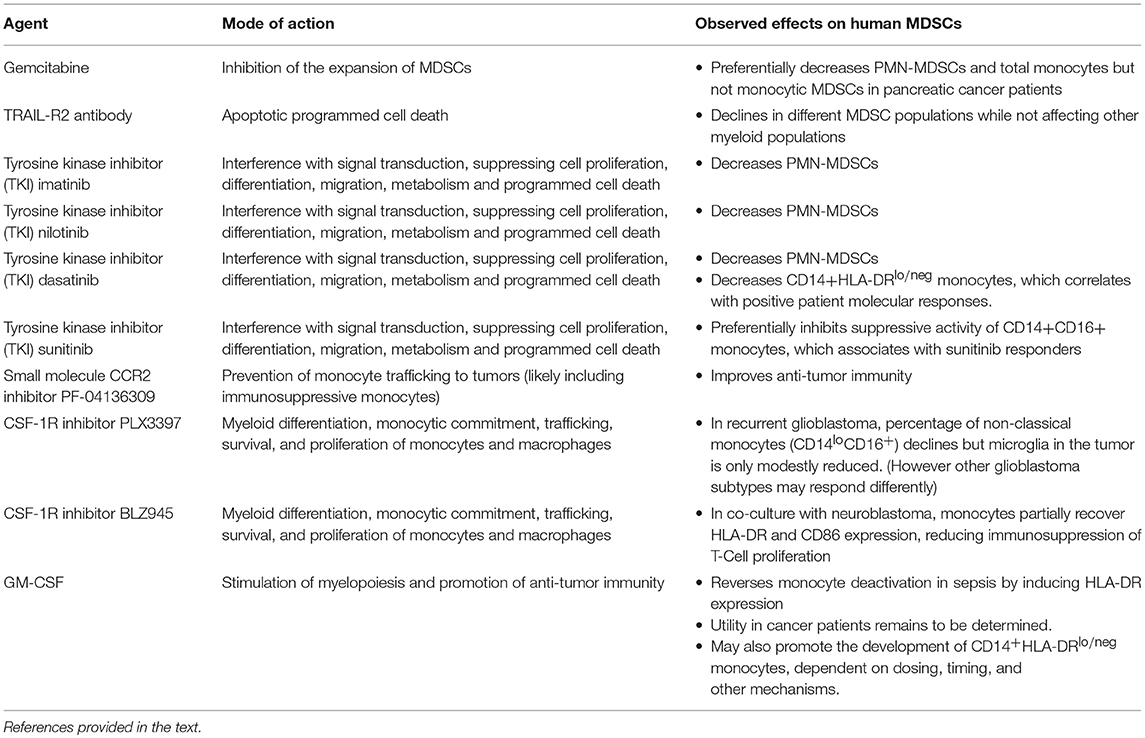
Several examples in the literature which are summarized in Table 1 highlight how drugs targeted to MDSCs affect the subpopulations in a differential manner. For example, gemcitabine has been shown to reduce MDSC accumulation in tumors in animal models (94) but appears to preferentially decrease PMN-MDSCs and total monocytes but not monocytic MDSCs when tested in pancreatic cancer patients (95). In another case, the treatment of solid tumor cancer patients with an agonistic TRAIL-R2 antibody resulted in the decline of different MDSC populations while not affecting other myeloid populations (96). Tyrosine kinase inhibitors (TKI) also demonstrate differential effects on MDSCs. In chronic myeloid leukemia patients treated with imatinib, nilotinib, or dasatinib, all three TKIs decreased PMN-MDSCs but only dasatinib reduced CD14+HLA-DRlo/neg monocytes (97). Furthermore, the decline in CD14+HLA-DRlo/neg monocytes correlated with positive patient molecular responses. Another TKI, sunitinib, was shown to preferentially inhibit the suppressive activity of CD14+CD16+ monocytes and reductions in these cells were associated with sunitinib responders (98).
There have been a few clinical trials that have specifically targeted monocytes and/or immunosuppressive monocytes. Nywening et al. hypothesized that pharmacological prevention of monocyte trafficking to tumors via a small molecule CCR2 inhibitor improves anti-tumor immunity (99). They tested the CCR2 inhibitor PF-04136309 in combination with FOLFIRINOX chemotherapy vs. chemotherapy alone in patients with pancreatic ductal adenocarcinoma. The CCR2 inhibitor prevented monocyte egress from bone marrow and subsequently reduced monocyte infiltration into tumors. The reduced monocyte infiltrate resulted in an increase of intra-tumoral lymphocytes and improved anti-tumor immunity. Patients receiving the combination of PF-04136309 and chemotherapy had higher than expected response rates. One potential caveat is that monocyte blockade may result in increased infiltration of granulocytes and, consequently, dual blockade of both monocytes and granulocytes has been proposed (100). While this study did not measure immunosuppressive monocytes per se, there is ample evidence that immunosuppressive monocytes also migrate to tumors via the CCL2/CCR2 pathway (43, 50, 101, 102).
In other clinical trials, investigators have tested the effectiveness of blocking CSF-1R signaling in cancer patients. Myeloid differentiation, monocytic commitment, trafficking, survival, and proliferation of monocytes/macrophages are all influenced by CSF-1R signaling (103). It is hypothesized that blocking the signaling function of this receptor will result in the reduction of monocyte/macrophage infiltration into tumors and consequently limit the immunosuppressive nature of the tumor microenvironment (104). In patients with recurrent glioblastoma treated with an oral CSF-1R inhibitor PLX3397, the percentage of non-classical monocytes (CD14loCD16+) declined after treatment but microglia in the tumor microenvironment were only modestly reduced (105). However, while taking this into account, it may be that different glioblastoma subtypes (i.e., pro-neural glioblastoma) may be more susceptible to the reprogramming of monocytes/macrophages from CSF-1R inhibition (106). Finally, CSF-1R+ myeloid cells are associated with negative outcomes in neuroblastoma patients (107). In monocytes co-cultured in the presence of neuroblastoma cells, the CSF-1R inhibitor BLZ945 partially restored HLA-DR and CD86 expression and reduced the immunosuppressive capacities of the monocytes on T cell proliferation.
GM-CSF has been used to support myelopoiesis and promote anti-tumor immunity as a stand-alone monotherapy and also to complement various immunotherapeutic approaches (108). Although GM-CSF has been shown to overcome monocyte deactivation in sepsis by inducing HLA-DR expression, the utility for use in cancer patients remains to be determined. In some cases, GM-CSF has been shown to provide a clinical benefit (109–112). But in several instances, GM-CSF has demonstrated neutral or negative results (113, 114). While it is clear that GM-CSF can act via pro- and anti- inflammatory pathways, emerging data, more prevalent in mouse models (115–119) but also from human studies (80, 120), indicate that GM-CSF strongly promotes the development of CD14+HLA-DRlo/neg monocytes. The data from Ribechini et al. suggest that the timing of GM-CSF administration may be critical in the transitioning of pro-inflammatory to anti-inflammatory pathways (54). Therefore, in order to optimize GM-CSF therapy, it is critical to further understand and define how dosing, timing, and other mechanisms contribute to CD14+HLA-DRlo/neg monocyte accumulation.
A common theme emerges from all studies cited. Therapies targeting immunosuppressive monocytes have a wide variety of effects on these cells, and underlying mechanisms are still not well understood. While many of these studies remain relatively limited in scope, and much work remains to better identify the optimal strategies and indications, these promising preliminary results clearly warrant further investigation into developing methods to target monocytes in cancer patients. The negative effect of immunosuppressive monocyte levels, particularly of the CD14+HLA-DRlo/neg phenotype, is clear. Therefore, rigorous and well-defined immune monitoring and phenotyping of patient myeloid cells in clinical trials is justified, as their measurement is critical for understanding the mechanism(s) of action of such therapies.
The Potential for Utilizing Immunosuppressive Monocytes as a Predictive Biomarker
The clinical significance of the broad class of MDSCs has been well documented and the pathway to utilizing these cells as biomarkers has recently been proposed (121). In many studies, the presence of CD14+HLA-DRlo/neg monocytes in circulation has been shown to be a systemic marker of immune suppression, and has been associated with the accumulation of these cells in tumors (50). Both their ability to impair anti-tumor immune responses and that they may be a promising therapeutic target make a compelling case for the development of standardized tools and/or assays to measure CD14+HLA-DRlo/neg monocytes in a manner that is useful for guiding therapeutic decisions for patients receiving immunotherapy.
Perhaps the simplest and most efficient way to measure CD14+HLA-DRlo/neg monocytes is by flow cytometry of peripheral blood; therefore, acquisition of tumor biopsies which may not always be available from patients is unnecessary. The small sample of blood that is required to measure these cells justifies further investigation into monitoring them as an informative biomarker. Significant variability currently exists in the way these cells have been measured and reported in the literature. We outline these variables in Table 2 and highlight areas of concern, including differences in flow cytometry gating strategies, cell enumeration methods, timing of sample procurement, and processing procedures. These differences in methodology have been problematic, creating variability in actual, and reported results. Mandruzzato et al. have shown that the lack of standardized gating strategies was one of the largest factors of variation when measuring the total group of MDSCs (122). Nonetheless, standardized gating strategies have been used to gain meaningful correlations to clinical outcomes. We and others (124) have shown that standardization of the measurement of these cells can result in consistent and robust assays.
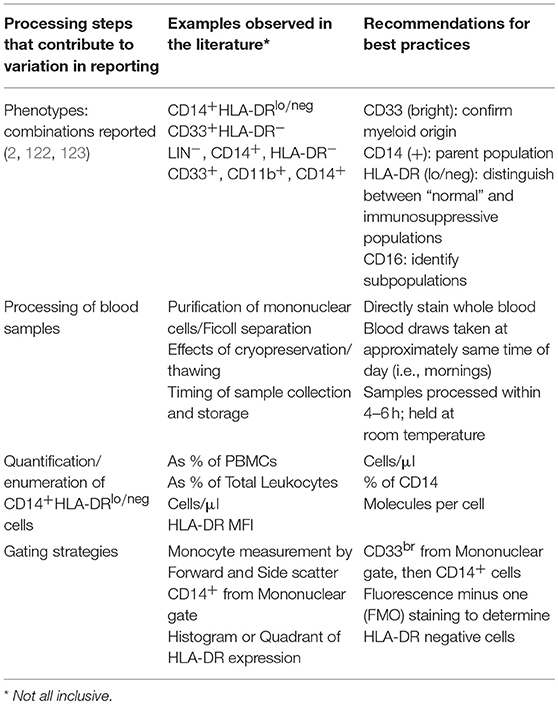
Table 2. Recommendations for consistent and reproducible reporting for immunosuppressive monocytes by flow cytometry.
Typically, CD14+HLA-DRlo/neg monocytes are measured by flow cytometry in blood samples collected from patients. These monocytes are phenotypically positive for CD14, CD33, and CD11b (125). The source of inconsistencies is often in the measurement of monocyte HLA-DR loss, as HLA-DR expression exhibits considerable variation and is not uniform across all subtypes of monocytes. Figure 1 displays a diagram of surface marker commonalities within the myeloid compartment. CD33 expression on human myeloid cells appears to be bi-modal as granulocytes and immature myeloid cells express moderate amounts of CD33 whereas monocytes exhibit strong CD33 expression (Figure 1A). MDSCs of both granulocytic and monocytic lineage reside within the CD33+ population of cells. CD33− cells comprise cells from the lymphoid lineage (Figure 1B). Often CD33 and HLA-DR are used to measure MDSCs but these two markers are not solely sufficient to distinguish the three types of MDSCs. To distinguish monocytic MDSCs by flow cytometry, CD33 positive cells are gated from total leukocytes and then monocytes are further gated based on CD14 expression. The combination of CD33++ and CD14+ distinguishes monocytes from all other myeloid cells (Figure 1D). CD33+ cells not expressing CD14 comprise a separate pool of CD33+ subtypes (Figure 1C) including CD15(+)/SSC(hi)/HLA-DR(−) granulocytes and granulocytic MDSCs (currently very few reproducible markers distinguish g-MDSCs by flow cytometry) (Figure 1E), CD15(−)/SSC(lo)/HLA-DR(−) immature MDSCs (Figure 1F), and LIN(−) HLA-DR(+++) dendritic cells or other myeloid cells (Figure 1G). Although the illustration is meant to visualize the hierarchy of myeloid cell populations, it is likely that some myeloid progenitors may become CD14+ and hence join the pool of monocytic cells.
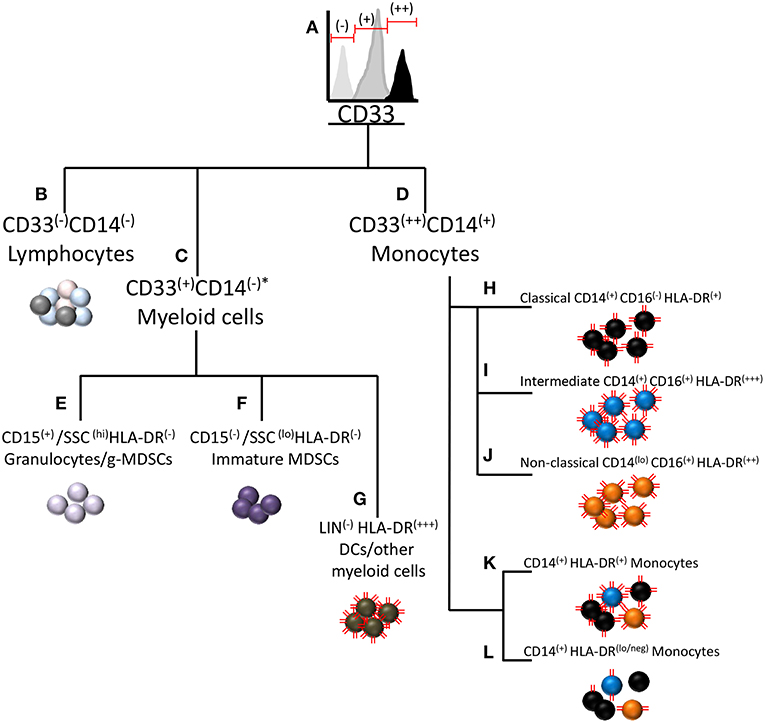
Figure 1. Relationship of immunosuppressive CD14+HLA-DRlo/neg monocytes to other myeloid cells and myeloid derived suppressor cells. A diagram of CD marker commonalities between cell types within the myeloid subclass including MDSCs and other cells with similar CD marker expression. (A) MDSCs of both granulocytic and monocytic lineage reside within the CD33+ population of cells. (B) Cells not expressing CD33 are considered to be of lymphoid lineage. (C) Cells not expressing CD14 comprise a separate pool of CD33+ subtypes. (D) CD14+ monocytes typically strongly express CD33, hereafter noted as CD33(++). (E) CD15(+)/SSC(hi)/HLA-DR(−) normal granulocytes and granulocytic MDSCs. (F) CD15(−)/SSC(lo)/HLA-DR(−) immature MDSCs. (G) LIN(−) HLA-DR(+++) dendritic cells or other myeloid cells. Three sub-populations of monocytes are (H) classical monocytes (CD14+CD16-) (I) intermediate monocytes (CD14+CD16+) (J) Non-classical monocytes (CD14loCD16+) (K) Representation of the combined pool of the three monocyte subtypes (L) The CD14+HLA-DRlo/neg cell most typically arises from the classical monocyte pool, but may also be derived from the intermediate and non-classical monocyte pools. The red lines on the cells represent relative HLA-DR expression on the cells. *Under some conditions, granulocytes can express CD14 (126).
The immunosuppressive monocyte of phenotype CD14+HLA-DRlo/neg resides within the CD33(++)/CD14(+) population of mononuclear cells (Figure 1D). This population may be further sub-divided based on CD16 expression into classical (CD14+CD16−), intermediate (CD14+CD16+), and non-classical (CD14loCD16+) monocytes [Figures 1H–J and Ziegler-Heitbrock et al.(127)]. Interestingly, HLA-DR expression varies between these subgroups in that intermediate monocytes express the highest amount of HLA-DR and classical monocytes expressing the least (125). Note that the HLA-DR expression level noted as HLA-DR(+), is not low enough to classify as the immunosuppressive HLA-DR(lo/neg) phenotype. From the combined pool of the three monocyte subtypes (Figure 1K), the immunosuppressive phenotype may arise by loss of HLA-DR expression (Figure 1L). The CD14+HLA-DRlo/neg cell most typically arises from the classical monocyte pool, but may also be derived from the intermediate and non-classical monocyte pools. For setting the HLA-DR(+) vs. HLA-DR(lo/neg) threshold in flow cytometry, a convenient internal negative control is available in the CD33−CD14−HLA-DR− mononuclear cell population. The threshold is set at the upper limit of HLA-DR in that population, thereby delineating the boundary to distinguish low or negative from high HLA-DR expression in the CD33+CD14+ monocyte population.
Further complicating the comparison of CD14+HLA-DRlo/neg monocyte levels between different studies is the output of how the cells are enumerated. Examples from the literature include reported cells as a percent of peripheral blood mononuclear cells (PBMCs), a percent of total leukocytes, a percent of monocytes, total cells per volume of blood (i.e., cells/μl), mean fluorescence intensity (MFI), molecules per cell, and based on other non-flow cytometry methods such as polymerase chain reaction assays. For flow cytometry based assays, reporting the abundance of CD14+HLA-DRlo/neg monocytes as a percent of PBMCs or total leukocytes is the least informative, particularly when measuring these cells from cancer patients where many patients exhibit severe leukopenia and/or lymphopenia. This phenomenon leads to artificially high percentages because the comparative denominator of total PBMCs or leukocytes can be much lower than in the control or healthy volunteer subject groups. Since it remains to be determined how much HLA-DR expression must be diminished before the monocyte becomes deactivated or immunosuppressive, it may be more appropriate to measure surface expression of HLA-DR on monocytes rather than measure cell abundance. While MFI is commonly used for measuring surface expression, it is difficult to standardize MFI values between different instruments within the same laboratory let alone between different laboratories. As such, we recommend the use of fluorescent beads such as Anti–HLA-DR/Anti-Monocyte Quantibrite™ (BD Biosciences) to better assess the quantity of surface protein expression on cells. Finally, computational approaches for reducing the effect of procedural and inter-user variability on assay results have been developed which use coefficient of variation to quantify the HLA-DR spread on monocytes in healthy subjects and patients with melanoma (57).
Another source of variation that contributes to inconsistent results is the method by which blood samples are processed. The most common processing steps for the isolation of peripheral blood mononuclear cells (PBMCs) include sucrose gradient centrifugation followed by subsequent cryopreservation of the purified cells. In direct comparisons of processing steps in samples from patients with gastrointestinal cancer, Duffy et al. found that although the processing steps yielded relatively consistent results when comparing cancer patients to healthy subjects, the absolute numbers of CD14+HLA-DRlo/neg monocytes were significantly different when comparing whole blood staining to freshly isolated PBMCs in the cancer patient cohort (128). Several groups have found that cryopreservation can negatively affect the immunosuppressive functions, enzymatic activity, and/or the abundance/distribution of MDSC subsets (61, 129, 130). Monneret et al. found that in blood samples collected in EDTA anti-coagulant tubes, HLA-DR expression was influenced both by storage time and temperature in their study of patients with sepsis and in control subjects (131). After sample collection, increased storage time at room temperature led to dramatically increased HLA-DR expression both in terms of percent positive monocytes and MFI. Higher storage temperatures also appeared to increase HLA-DR levels as well. Docke et al. also found that processing and transport steps can influence HLA-DR and thus recommended staining unprocessed blood within 4 h of the blood draw (132). Additionally, they found that the HLA-DR values for samples that were lysed/washed vs. lysed/no wash strongly correlated despite the slightly higher overall HLA-DR values reported in the lyse/no wash samples. In summary, there are many processing steps that affect the accurate measurement of CD14+HLA-DRlo/neg monocytes. Results from minimally processed samples appear to yield the most reliable results for HLA-DR quantification. Therefore, as whole blood staining of fresh blood is becoming more standard practice, this will no doubt improve the prospect of using CD14+HLADRlo/neg monocytes as a biomarker for understanding responses to cancer immunotherapy.
Conclusions
There is now a large body of evidence linking CD14+HLADRlo/neg monocytes to systemic immune suppression and paralysis and their negative affect on cancer immunotherapy. As new evidence suggests that systemic immunity plays an important role in optimal responses to cancer immunotherapy (51, 133), circulating monocytes likely contribute significantly to this phenomenon. From studies published to date, it appears that the various immunotherapeutic approaches do not drastically change the abundance of CD14+HLADRlo/neg monocytes but their pre-treatment levels correlate with poorer or more favorable outcomes in most settings. While deciphering the precise mechanisms of CD14+HLADRlo/neg monocyte-mediated suppression in humans will remain difficult, the established data warrant further efforts to investigate novel ways to counteract these cells. Finally, the immunosuppressive CD14+HLA-DRlo/neg monocyte not only may be a very good therapeutic target, but also may be a very good candidate for biomarker development. They are easy to quantify, likely to reflect general systemic immunosuppression, and may even reflect what is happening in the tumor microenvironment.
Author Contributions
AM contributed to the development, writing, and illustrations of the article. DG contributed to the study concept, development, and writing of the article. MG contributed to the study concept, development, writing, and illustrations of the article. All authors read and approved the final manuscript.
Funding
The study was supported by internal funds from the Mayo Clinic Department of Laboratory Medicine and Pathology and Center for Regenerative Medicine.
Conflict of Interest Statement
MG has intellectual property and two patents or pending patents associated with the enumeration of immune system profiles.
The remaining authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. VanGundy ZC, Guerau-de-Arellano M, Baker JD, Strange HR, Olivo-Marston S, Muth DC, et al. Continuous retinoic acid induces the differentiation of mature regulatory monocytes but fails to induce regulatory dendritic cells. BMC immunol. (2014) 15:8. doi: 10.1186/1471-2172-15-8
2. Bronte V, Brandau S, Chen SH, Colombo MP, Frey AB, Greten TF, et al. Recommendations for myeloid-derived suppressor cell nomenclature and characterization standards. Nat Commun. (2016) 7:12150. doi: 10.1038/ncomms12150
3. Manjili MH, Wang XY, Abrams S. Evolution of Our Understanding of Myeloid Regulatory Cells: From MDSCs to Mregs. Front Immunol. (2014) 5:303. doi: 10.3389/fimmu.2014.00303
4. Poschke I, Kiessling R. On the armament and appearances of human myeloid-derived suppressor cells. Clin Immunol. (2012) 144:250–68. doi: 10.1016/j.clim.2012.06.003
5. Veglia F, Perego M, Gabrilovich D. Myeloid-derived suppressor cells coming of age. Nat Immunol. (2018) 19:108–19. doi: 10.1038/s41590-017-0022-x
6. Condamine T, Gabrilovich DI. Molecular mechanisms regulating myeloid-derived suppressor cell differentiation and function. Trends Immunol. (2011) 32:19–25. doi: 10.1016/j.it.2010.10.002
7. Laborde RR, Lin Y, Gustafson MP, Bulur PA, Dietz AB. Cancer vaccines in the world of immune suppressive monocytes (CD14+HLA-DRlo/neg cells): the gateway to improved responses. Front Immunol. (2014) 5:147. doi: 10.3389/fimmu.2014.00147
8. Millrud CR, Bergenfelz C, Leandersson K. On the origin of myeloid-derived suppressor cells. Oncotarget. (2017) 8:3649–65. doi: 10.18632/oncotarget.12278
9. Werfel T, Witter W, Gotze O. CD11b and CD11c antigens are rapidly increased on human natural killer cells upon activation. J Immunol. (1991) 147:2423–7.
10. Bakdash G, Buschow SI, Gorris MA, Halilovic A, Hato SV, Skold AE, et al. Expansion of a BDCA1+CD14+ myeloid cell population in melanoma patients may attenuate the efficacy of dendritic cell vaccines. Cancer Res. (2016) 76:4332–46. doi: 10.1158/0008-5472.CAN-15-1695
11. Schroder M, Melum GR, Landsverk OJ, Bujko A, Yaqub S, Gran E, et al. CD1c-expression by monocytes - implications for the use of commercial CD1c+ dendritic cell isolation kits. PLoS ONE. (2016) 11:e0157387. doi: 10.1371/journal.pone.0157387
12. Balk RA. Systemic inflammatory response syndrome (SIRS): where did it come from and is it still relevant today? Virulence. (2014) 5:20–6. doi: 10.4161/viru.27135
13. Bone RC, Balk RA, Cerra FB, Dellinger RP, Fein AM, Knaus WA, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM consensus conference committee. American College of Chest Physicians/Society of Critical Care Medicine. Chest. (1992) 101:1644–55. doi: 10.1378/chest.101.6.1644
14. Volk HD, Reinke P, Krausch D, Zuckermann H, Asadullah K, Muller JM, et al. Monocyte deactivation–rationale for a new therapeutic strategy in sepsis. Intensive Care Med. (1996) 22 (Suppl. 4):S474–81. doi: 10.1007/BF01743727
15. Volk HD, Thieme M, Heym S, Docke WD, Ruppe U, Tausch W, et al. Alterations in function and phenotype of monocytes from patients with septic disease–predictive value and new therapeutic strategies. Behring Inst Mitt. (1991) 88:208–15.
16. Kox WJ, Bone RC, Krausch D, Docke WD, Kox SN, Wauer H, et al. Interferon gamma-1b in the treatment of compensatory anti-inflammatory response syndrome. A new approach: proof of principle. Arch Inter Med. (1997) 157:389–93. doi: 10.1001/archinte.157.4.389
17. Bone RC. Sir Isaac Newton, sepsis, SIRS, and CARS. Crit Care Med. (1996) 24:1125–8. doi: 10.1097/00003246-199607000-00010
18. Docke WD, Randow F, Syrbe U, Krausch D, Asadullah K, Reinke P, et al. Monocyte deactivation in septic patients: restoration by IFN-gamma treatment. Nat Med. (1997) 3:678–81. doi: 10.1038/nm0697-678
19. Ditschkowski M, Kreuzfelder E, Rebmann V, Ferencik S, Majetschak M, Schmid EN, et al. HLA-DR expression and soluble HLA-DR levels in septic patients after trauma. Ann Surg. (1999) 229:246–54. doi: 10.1097/00000658-199902000-00013
20. Monneret G, Lepape A, Voirin N, Bohe J, Venet F, Debard AL, et al. Persisting low monocyte human leukocyte antigen-DR expression predicts mortality in septic shock. Intens Care Med. (2006) 32:1175–83. doi: 10.1007/s00134-006-0204-8
21. Oczenski W, Krenn H, Jilch R, Watzka H, Waldenberger F, Koller U, et al. HLA-DR as a marker for increased risk for systemic inflammation and septic complications after cardiac surgery. Intens Care Med. (2003) 29:1253–7. doi: 10.1007/s00134-003-1826-8
22. Perry SE, Mostafa SM, Wenstone R, Shenkin A, McLaughlin PJ. Is low monocyte HLA-DR expression helpful to predict outcome in severe sepsis? Intens Care Med. (2003) 29:1245–52. doi: 10.1007/s00134-003-1686-2
23. Dorhoi A, Du Plessis N. Monocytic myeloid-derived suppressor cells in chronic infections. Front Immunol. (2017) 8:1895. doi: 10.3389/fimmu.2017.01895
24. Yang H-M, Yu Y, Chai J-K, Hu S, Sheng Z-Y, Yao Y-M. Low HLA-DR expression on CD14+ monocytes of burn victims with sepsis, and the effect of carbachol in vitro. Burns. (2008) 34:1158–62. doi: 10.1016/j.burns.2008.01.026
25. Venet F, Tissot S, Debard AL, Faudot C, Crampe C, Pachot A, et al. Decreased monocyte human leukocyte antigen-DR expression after severe burn injury: correlation with severity and secondary septic shock. Crit Care Med. (2007) 35:1910–7. doi: 10.1097/01.CCM.0000275271.77350.B6
26. Antoniades CG, Berry PA, Davies ET, Hussain M, Bernal W, Vergani D, et al. Reduced monocyte HLA-DR expression: a novel biomarker of disease severity and outcome in acetaminophen-induced acute liver failure. Hepatology. (2006) 44:34–43. doi: 10.1002/hep.21240
27. Wasmuth HE, Kunz D, Yagmur E, Timmer-Stranghoner A, Vidacek D, Siewert E, et al. Patients with acute on chronic liver failure display “sepsis-like” immune paralysis.[see comment]. J Hepatol. (2005) 42:195–201. doi: 10.1016/j.jhep.2004.10.019
28. Berres ML, Schnyder B, Yagmur E, Inglis B, Stanzel S, Tischendorf JJ, et al. Longitudinal monocyte human leukocyte antigen-DR expression is a prognostic marker in critically ill patients with decompensated liver cirrhosis. Liver Int. (2009) 29:536–43. doi: 10.1111/j.1478-3231.2008.01870.x
29. Kylanpaa M-L, Mentula P, Kemppainen E, Puolakkainen P, Aittomaki S, Silvennoinen O, et al. Monocyte anergy is present in patients with severe acute pancreatitis and is significantly alleviated by granulocyte-macrophage colony-stimulating factor and interferon-gamma in vitro. Pancreas. (2005) 31:23–7. doi: 10.1097/01.mpa.0000164449.23524.94
30. Richter A, Nebe T, Wendl K, Schuster K, Klaebisch G, Quintel M, et al. HLA-DR expression in acute pancreatitis. Eur J Surg Acta Chirurg. (1999) 165:947–51. doi: 10.1080/110241599750008053
31. Gotzinger P, Sautner T, Spittler A, Barlan M, Wamser P, Roth E, et al. Severe acute pancreatitis causes alterations in HLA-DR and CD14 expression on peripheral blood monocytes independently of surgical treatment. Eur J Surg Acta Chirurg. (2000) 166:628–32. doi: 10.1080/110241500750008286
32. Gustafson MP, Staff NP, Bornschlegl S, Butler GW, Maas ML, Kazamel M, et al. Comprehensive immune profiling reveals substantial immune system alterations in a subset of patients with amyotrophic lateral sclerosis. PLoS ONE. (2017) 12:e0182002. doi: 10.1371/journal.pone.0182002
33. Torrance HDT, Longbottom ER, Vivian ME, Lalabekyan B, Abbott TEF, Ackland GL, et al. Post-operative immune suppression is mediated via reversible, Interleukin-10 dependent pathways in circulating monocytes following major abdominal surgery. PLoS ONE. (2018) 13:e0203795. doi: 10.1371/journal.pone.0203795
34. Masternak K, Muhlethaler-Mottet A, Villard J, Zufferey M, Steimle V, Reith W. CIITA is a transcriptional coactivator that is recruited to MHC class II promoters by multiple synergistic interactions with an enhanceosome complex. Genes Dev. (2000) 14:1156–66. doi: 10.1011/gad.14.9.1156
35. LeibundGut-Landmann S, Waldburger JM, Krawczyk M, Otten LA, Suter T, Fontana A, et al. Mini-review: Specificity and expression of CIITA, the master regulator of MHC class II genes. Eur J Immunol. (2004) 34:1513–25. doi: 10.1002/eji.200424964
36. Steimle V, Siegrist CA, Mottet A, Lisowska-Grospierre B, Mach B. Regulation of MHC class II expression by interferon-gamma mediated by the transactivator gene CIITA. Science. (1994) 265:106–9. doi: 10.1126/science.8016643
37. Gonzalez-Junca A, Driscoll KE, Pellicciotta I, Du S, Lo CH, Roy R, et al. Autocrine TGFbeta is a survival factor for monocytes and drives immunosuppressive lineage commitment. Cancer Immunol Res. (2019) 7:306–20. doi: 10.1158/2326-6066.CIR-18-0310
38. Rohn W, Tang LP, Dong Y, Benveniste EN. IL-1 beta inhibits IFN-gamma-induced class II MHC expression by suppressing transcription of the class II transactivator gene. J Immunol. (1999) 162:886–96.
39. Koppelman B, Neefjes JJ, de Vries JE, de Waal Malefyt R. Interleukin-10 down-regulates MHC class II alphabeta peptide complexes at the plasma membrane of monocytes by affecting arrival and recycling. Immunity. (1997) 7:861–71. doi: 10.1016/S1074-7613(00)80404-5
40. Thibodeau J, Bourgeois-Daigneault MC, Huppe G, Tremblay J, Aumont A, Houde M, et al. Interleukin-10-induced MARCH1 mediates intracellular sequestration of MHC class II in monocytes. Eur J Immunol. (2008) 38:1225–30. doi: 10.1002/eji.200737902
41. Le Tulzo Y, Pangault C, Amiot L, Guilloux V, Tribut O, Arvieux C, et al. Monocyte human leukocyte antigen-DR transcriptional downregulation by cortisol during septic shock. Am J Respir Crit Care Med. (2004) 169:1144–51. doi: 10.1164/rccm.200309-1329OC
42. Haveman JW, van den Berg AP, van den Berk JM, Mesander G, Slooff MJ, de Leij LH, et al. Low HLA-DR expression on peripheral blood monocytes predicts bacterial sepsis after liver transplantation: relation with prednisolone intake. Trans Infect Dis. (1999) 1:146–52. doi: 10.1034/j.1399-3062.1999.010302.x
43. Gustafson MP, Lin Y, New KC, Bulur PA, O'Neill BP, Dietz AB. Systemic immune suppression in glioblastoma: the interplay between CD14+HLA-DRlo/neg monocytes, tumor factors, and dexamethasone. Neuro Oncol. (2010) 12:631–44. doi: 10.1093/neuonc/noq001
44. Schwiebert LM, Schleimer RP, Radka SF, Ono SJ. Modulation of MHC class II expression in human cells by dexamethasone. Cell Immunol. (1995) 165:12–9. doi: 10.1006/cimm.1995.1181
45. Woiciechowsky C, Asadullah K, Nestler D, Schoning B, Glockner F, Docke WD, et al. Diminished monocytic HLA-DR expression and ex vivo cytokine secretion capacity in patients with glioblastoma: effect of tumor extirpation. J Neuroimmunol. (1998) 84:164–71. doi: 10.1016/S0165-5728(97)00236-1
46. Loercher AE, Nash MA, Kavanagh JJ, Platsoucas CD, Freedman RS. Identification of an IL-10-producing HLA-DR-negative monocyte subset in the malignant ascites of patients with ovarian cancer that inhibits cytokine protien expression and proliferation of autologous T cells. J Immunology. (1999) 163:6251–60.
47. Valenti R, Huber V, Filipazzi P, Pilla L, Sovena G, Villa A, et al. Human tumor-released microvesicles promote the differentiation of myeloid cells with transforming growth factor-beta-mediated suppressive activity on T lymphocytes. Cancer Res. (2006) 66:9290–8. doi: 10.1158/0008-5472.CAN-06-1819
48. Lin Y, Gustafson MP, Bulur PA, Gastineau DA, Witzig TE, Dietz AB. Immunosuppressive CD14+HLA-DR(low)/- monocytes in B-cell non-Hodgkin lymphoma. Blood. (2011) 117:872–81. doi: 10.1182/blood-2010-05-283820
49. Gustafson MP, Abraham RS, Lin Y, Wu W, Gastineau DA, Zent CS, et al. Association of an increased frequency of CD14(+) HLA-DR(lo/neg) monocytes with decreased time to progression in chronic lymphocytic leukaemia (CLL). Br J Haematol. (2012) 156:674–6. doi: 10.1111/j.1365-2141.2011.08902.x
50. Gustafson MP, Lin Y, Bleeker JS, Warad D, Tollefson MK, Crispen PL, et al. Intratumoral CD14+ cells and circulating CD14+HLA-DRlo/neg monocytes correlate with decreased survival in patients with clear cell renal cell carcinoma. Clin Cancer Res. (2015) 21:4224–33. doi: 10.1158/1078-0432.CCR-15-0260
51. Gustafson MP, Lin Y, LaPlant B, Liwski CJ, Maas ML, League SC, et al. Immune monitoring using the predictive power of immune profiles. J Immunother Cancer. (2013) 1:7. doi: 10.1186/2051-1426-1-7
52. Rodrigues JC, Gonzalez GC, Zhang L, Ibrahim G, Kelly JJ, Gustafson MP, et al. Normal human monocytes exposed to glioma cells acquire myeloid-derived suppressor cell-like properties. Neuro-Oncology. (2010) 12:351–65. doi: 10.1093/neuonc/nop023
53. Okada SL, Simmons RM, Franke-Welch S, Nguyen TH, Korman AJ, Dillon SR, et al. Conditioned media from the renal cell carcinoma cell line 786.O drives human blood monocytes to a monocytic myeloid-derived suppressor cell phenotype. Cell Immunol. (2018) 323:49–58. doi: 10.1016/j.cellimm.2017.10.014
54. Ribechini E, Hutchinson JA, Hergovits S, Heuer M, Lucas J, Schleicher U, et al. Novel GM-CSF signals via IFN-gammaR/IRF-1 and AKT/mTOR license monocytes for suppressor function. Blood Adv. (2017) 1:947–60. doi: 10.1182/bloodadvances.2017006858
55. Bergenfelz C, Larsson AM, von Stedingk K, Gruvberger-Saal S, Aaltonen K, Jansson S, et al. Systemic monocytic-MDSCs are generated from monocytes and correlate with disease progression in breast cancer patients. PLoS ONE. (2015) 10:e0127028. doi: 10.1371/journal.pone.0127028
56. Meyer C, Cagnon L, Costa-Nunes CM, Baumgaertner P, Montandon N, Leyvraz L, et al. Frequencies of circulating MDSC correlate with clinical outcome of melanoma patients treated with ipilimumab. Cancer Immunol Immunother. (2014) 63:247–57. doi: 10.1007/s00262-013-1508-5
57. Kitano S, Postow MA, Ziegler CG, Kuk D, Panageas KS, Cortez C, et al. Computational algorithm-driven evaluation of monocytic myeloid-derived suppressor cell frequency for prediction of clinical outcomes. Cancer Immunol Res. (2014) 2:812–21. doi: 10.1158/2326-6066.CIR-14-0013
58. Tarhini AA, Edington H, Butterfield LH, Lin Y, Shuai Y, Tawbi H, et al. Immune monitoring of the circulation and the tumor microenvironment in patients with regionally advanced melanoma receiving neoadjuvant ipilimumab. PLoS ONE. (2014) 9:e87705. doi: 10.1371/journal.pone.0087705
59. Martens A, Wistuba-Hamprecht K, Geukes Foppen M, Yuan J, Postow MA, Wong P, et al. Baseline peripheral blood biomarkers associated with clinical outcome of advanced melanoma patients treated with ipilimumab. Clin Cancer Res. (2016) 22:2908–18. doi: 10.1158/1078-0432.CCR-15-2412
60. Gebhardt C, Sevko A, Jiang H, Lichtenberger R, Reith M, Tarnanidis K, et al. Myeloid cells and related chronic inflammatory factors as novel predictive markers in melanoma treatment with ipilimumab. Clin Cancer Res. (2015) 21:5453–9. doi: 10.1158/1078-0432.CCR-15-0676
61. de Coana YP, Wolodarski M, Poschke I, Yoshimoto Y, Yang Y, Nystrom M, et al. Ipilimumab treatment decreases monocytic MDSCs and increases CD8 effector memory T cells in long-term survivors with advanced melanoma. Oncotarget. (2017) 8:21539–53. doi: 10.18632/oncotarget.15368
62. Krieg C, Nowicka M, Guglietta S, Schindler S, Hartmann FJ, Weber LM, et al. High-dimensional single-cell analysis predicts response to anti-PD-1 immunotherapy. Nat Med. (2018) 24:144–53. doi: 10.1038/nm.4466
63. Eissler N, Mao Y, Brodin D, Reutersward P, Andersson Svahn H, Johnsen JI, et al. Regulation of myeloid cells by activated T cells determines the efficacy of PD-1 blockade. Oncoimmunology. (2016) 5:e1232222. doi: 10.1080/2162402X.2016.1232222
64. Antonios JP, Soto H, Everson RG, Moughon D, Orpilla JR, Shin NP, et al. Immunosuppressive tumor-infiltrating myeloid cells mediate adaptive immune resistance via a PD-1/PD-L1 mechanism in glioblastoma. Neuro-Oncology. (2017) 19:796–807. doi: 10.1093/neuonc/now287
65. Weber J, Gibney G, Kudchadkar R, Yu B, Cheng P, Martinez AJ, et al. Phase I/II study of metastatic melanoma patients treated with nivolumab who had progressed after ipilimumab. Cancer Immunol Res. (2016) 4:345–53. doi: 10.1158/2326-6066.CIR-15-0193
66. Chavan R, Salvador D, Gustafson MP, Dietz AB, Nevala W, Markovic SN. Untreated stage IV melanoma patients exhibit abnormal monocyte phenotypes and decreased functional capacity. Cancer Immunol Res. (2014) 2:241–8. doi: 10.1158/2326-6066.CIR-13-0094
67. Campbell DE, Tustin NB, Riedel E, Tustin R III, Taylor J, Murray J, et al. Cryopreservation decreases receptor PD-1 and ligand PD-L1 coinhibitory expression on peripheral blood mononuclear cell-derived T cells and monocytes. Clin Vaccine Immunol. (2009) 16:1648–53. doi: 10.1128/CVI.00259-09
68. Said EA, Dupuy FP, Trautmann L, Zhang Y, Shi Y, El-Far M, et al. Programmed death-1-induced interleukin-10 production by monocytes impairs CD4+ T cell activation during HIV infection. Nat Med. (2010) 16:452–9. doi: 10.1038/nm.2106
69. Walter S, Weinschenk T, Stenzl A, Zdrojowy R, Pluzanska A, Szczylik C, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. (2012) 18:1254–61. doi: 10.1038/nm.2883
70. Hansen GL, Gaudernack G, Brunsvig PF, Cvancarova M, Kyte JA. Immunological factors influencing clinical outcome in lung cancer patients after telomerase peptide vaccination. Cancer Immunol Immunother. (2015) 64:1609–21. doi: 10.1007/s00262-015-1766-5
71. Santegoets SJ, Stam AG, Lougheed SM, Gall H, Jooss K, Sacks N, et al. Myeloid derived suppressor and dendritic cell subsets are related to clinical outcome in prostate cancer patients treated with prostate GVAX and ipilimumab. J Immunother Cancer. (2014) 2:31. doi: 10.1186/s40425-014-0031-3
72. Olin MR, Low W, McKenna DH, Haines SJ, Dahlheimer T, Nascene D, et al. Vaccination with dendritic cells loaded with allogeneic brain tumor cells for recurrent malignant brain tumors induces a CD4(+)IL17(+) response. J Immunother Cancer. (2014) 2:4. doi: 10.1186/2051-1426-2-4
73. Poschke I, Mao Y, Adamson L, Salazar-Onfray F, Masucci G, Kiessling R. Myeloid-derived suppressor cells impair the quality of dendritic cell vaccines. Cancer Immunol Immunother. (2012) 61:827–38. doi: 10.1007/s00262-011-1143-y
74. Kansagra A, Inwards DJ, Ansell SM, Micallef IN, Johnston PB, Hogan WJ, et al. Infusion of autograft natural killer cell/CD14(+)HLA-DR(DIM) cell ratio predicts survival in lymphoma post autologous stem cell transplantation. Bone Marrow Transplant. (2018) 53:146–54. doi: 10.1038/bmt.2017.225
75. Guan Q, Blankstein AR, Anjos K, Synova O, Tulloch M, Giftakis A, et al. Functional myeloid-derived suppressor cell subsets recover rapidly after allogeneic hematopoietic stem/progenitor cell transplantation. Biol Blood Marrow Transplant. (2015) 21:1205–14. doi: 10.1016/j.bbmt.2015.04.015
76. Storek J, Geddes M, Khan F, Huard B, Helg C, Chalandon Y, et al. Reconstitution of the immune system after hematopoietic stem cell transplantation in humans. Sem Immunopathol. (2008) 30:425–37. doi: 10.1007/s00281-008-0132-5
77. Mougiakakos D, Jitschin R, von Bahr L, Poschke I, Gary R, Sundberg B, et al. Immunosuppressive CD14+HLA-DRlow/neg IDO+ myeloid cells in patients following allogeneic hematopoietic stem cell transplantation. Leukemia. (2013) 27:377–88. doi: 10.1038/leu.2012.215
78. Luyckx A, Schouppe E, Rutgeerts O, Lenaerts C, Fevery S, Devos T, et al. G-CSF stem cell mobilization in human donors induces polymorphonuclear and mononuclear myeloid-derived suppressor cells. Clin Immunol. (2012) 143:83–7. doi: 10.1016/j.clim.2012.01.011
79. Vendramin A, Gimondi S, Bermema A, Longoni P, Rizzitano S, Corradini P, et al. Graft monocytic myeloid-derived suppressor cell content predicts the risk of acute graft-versus-host disease after allogeneic transplantation of granulocyte colony-stimulating factor-mobilized peripheral blood stem cells. Biol Blood Marrow Transplant. (2014) 20:2049–55. doi: 10.1016/j.bbmt.2014.09.011
80. Filipazzi P, Valenti R, Huber V, Pilla L, Canese P, Iero M, et al. Identification of a new subset of myeloid suppressor cells in pheripheral blood of melanoma patients with modulation by a granulocyte-macrophage colony-stimulation factor-based antitumor vaccine. J Clin Oncol. (2007) 25:2546–53. doi: 10.1200/JCO.2006.08.5829
81. Hoechst B, Ormandy LA, Ballmaier M, Lehner F, Kruger C, Manns MP, et al. A new population of myeloid-derived suppressor cells in hepatocellular carcinoma patients induces CD4+CD25+Foxp3+ T cells. Gastroenterology. (2008) 135:234–43. doi: 10.1053/j.gastro.2008.03.020
82. Jitschin R, Braun M, Buttner M, Dettmer-Wilde K, Bricks J, Berger J, et al. CLL-cells induce IDOhi CD14+HLA-DRlo myeloid-derived suppressor cells that inhibit T-cell responses and promote TRegs. Blood. (2014) 124:750–60. doi: 10.1182/blood-2013-12-546416
83. Poschke I, Mougiakakos D, Hansson J, Masucci GV, Kiessling R. Immature immunosuppressive CD14+HLA-DR-/low cells in melanoma patients are Stat3hi and overexpress CD80, CD83, and DC-sign. Cancer Res. (2010) 70:4335–45. doi: 10.1158/0008-5472.CAN-09-3767
84. Weide B, Martens A, Zelba H, Stutz C, Derhovanessian E, Di Giacomo AM, et al. Myeloid-derived suppressor cells predict survival of patients with advanced melanoma: comparison with regulatory T cells and NY-ESO-1- or melan-A-specific T cells. Clin Cancer Res. (2014) 20:1601–9. doi: 10.1158/1078-0432.CCR-13-2508
85. Stroncek DF, Ren J, Lee DW, Tran M, Frodigh SE, Sabatino M, et al. Myeloid cells in peripheral blood mononuclear cell concentrates inhibit the expansion of chimeric antigen receptor T cells. Cytotherapy. (2016) 18:893–901. doi: 10.1016/j.jcyt.2016.04.003
86. Burga RA, Thorn M, Point GR, Guha P, Nguyen CT, Licata LA, et al. Liver myeloid-derived suppressor cells expand in response to liver metastases in mice and inhibit the anti-tumor efficacy of anti-CEA CAR-T. Cancer Immunol Immunother. (2015) 64:817–29. doi: 10.1007/s00262-015-1692-6
87. Hosoi A, Matsushita H, Shimizu K, Fujii S, Ueha S, Abe J, et al. Adoptive cytotoxic T lymphocyte therapy triggers a counter-regulatory immunosuppressive mechanism via recruitment of myeloid-derived suppressor cells. Int J Cancer. (2014) 134:1810–22. doi: 10.1002/ijc.28506
88. Arina A. Rethinking the role of myeloid-derived suppressor cells in adoptive T-cell therapy for cancer. Oncoimmunology. (2014) 3:e28464. doi: 10.4161/onci.28464
89. Liu Y, Wei G, Cheng WA, Dong Z, Sun H, Lee VY, et al. Targeting myeloid-derived suppressor cells for cancer immunotherapy. Cancer Immunol Immunother. (2018) 67:1181–95. doi: 10.1007/s00262-018-2175-3
90. Anani W, Shurin MR. Targeting myeloid-derived suppressor cells in cancer. Adv Exp Med Biol. (2017) 1036:105–28. doi: 10.1007/978-3-319-67577-0_8
91. Draghiciu O, Lubbers J, Nijman HW, Daemen T. Myeloid derived suppressor cells-An overview of combat strategies to increase immunotherapy efficacy. Oncoimmunology. (2015) 4:e954829. doi: 10.4161/21624011.2014.954829
92. Najjar YG, Finke JH. Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol. (2013) 3:49. doi: 10.3389/fonc.2013.00049
93. Weber R, Fleming V, Hu X, Nagibin V, Groth C, Altevogt P, et al. Myeloid-derived suppressor cells hinder the anti-cancer activity of immune checkpoint inhibitors. Front Immunol. (2018) 9:1310. doi: 10.3389/fimmu.2018.01310
94. Le HK, Graham L, Cha E, Morales JK, Manjili MH, Bear HD. Gemcitabine directly inhibits myeloid derived suppressor cells in BALB/c mice bearing 4T1 mammary carcinoma and augments expansion of T cells from tumor-bearing mice. Int Immunopharmacol. (2009) 9:900–9. doi: 10.1016/j.intimp.2009.03.015
95. Eriksson E, Wenthe J, Irenaeus S, Loskog A, Ullenhag G. Gemcitabine reduces MDSCs, tregs and TGFbeta-1 while restoring the teff/treg ratio in patients with pancreatic cancer. J Trans Med. (2016) 14:282. doi: 10.1186/s12967-016-1037-z
96. Dominguez GA, Condamine T, Mony S, Hashimoto A, Wang F, Liu Q, et al. Selective targeting of myeloid-derived suppressor cells in cancer patients using DS-8273a, an agonistic TRAIL-R2 antibody. Clin Cancer Res. (2017) 23:2942–50. doi: 10.1158/1078-0432.CCR-16-1784
97. Giallongo C, Parrinello NL, La Cava P, Camiolo G, Romano A, Scalia M, et al. Monocytic myeloid-derived suppressor cells as prognostic factor in chronic myeloid leukaemia patients treated with dasatinib. J Cell Mol Med. (2018) 22:1070–80. doi: 10.1111/jcmm.13326
98. Chen HM, Ma G, Gildener-Leapman N, Eisenstein S, Coakley BA, Ozao J, et al. Myeloid-derived suppressor cells as an immune parameter in patients with concurrent sunitinib and stereotactic body radiotherapy. Clin Cancer Res. (2015) 21:4073–85. doi: 10.1158/1078-0432.CCR-14-2742
99. Nywening TM, Wang-Gillam A, Sanford DE, Belt BA, Panni RZ, Cusworth BM, et al. Targeting tumour-associated macrophages with CCR2 inhibition in combination with FOLFIRINOX in patients with borderline resectable and locally advanced pancreatic cancer: a single-centre, open-label, dose-finding, non-randomised, phase 1b trial. Lancet Oncol. (2016) 17:651–62. doi: 10.1016/S1470-2045(16)00078-4
100. Nywening TM, Belt BA, Cullinan DR, Panni RZ, Han BJ, Sanford DE, et al. Targeting both tumour-associated CXCR2(+) neutrophils and CCR2(+) macrophages disrupts myeloid recruitment and improves chemotherapeutic responses in pancreatic ductal adenocarcinoma. Gut. (2018) 67:1112–23. doi: 10.1136/gutjnl-2017-313738
101. Chang AL, Miska J, Wainwright DA, Dey M, Rivetta CV, Yu D, et al. CCL2 Produced by the glioma microenvironment is essential for the recruitment of regulatory T cells and myeloid-derived suppressor cells. Cancer Res. (2016) 76:5671–82. doi: 10.1158/0008-5472.CAN-16-0144
102. Eruslanov E, Neuberger M, Daurkin I, Perrin GQ, Algood C, Dahm P, et al. Circulating and tumor-infiltrating myeloid cell subsets in patients with bladder cancer. Int J Cancer. (2012) 130:1109–19. doi: 10.1002/ijc.26123
103. Stanley ER, Chitu V. CSF-1 receptor signaling in myeloid cells. Cold Spring Harb Perspect Biol. (2014) 6:a021857. doi: 10.1101/cshperspect.a021857
104. Chitu V, Stanley ER. Colony-stimulating factor-1 in immunity and inflammation. Curr Opin Immunol. (2006) 18:39–48. doi: 10.1016/j.coi.2005.11.006
105. Butowski N, Colman H, De Groot JF, Omuro AM, Nayak L, Wen PY, et al. Orally administered colony stimulating factor 1 receptor inhibitor PLX3397 in recurrent glioblastoma: an Ivy foundation early phase clinical trials consortium phase II study. Neuro-Oncology. (2016) 18:557–64. doi: 10.1093/neuonc/nov245
106. Pyonteck SM, Akkari L, Schuhmacher AJ, Bowman RL, Sevenich L, Quail DF, et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat Med. (2013) 19:1264–72. doi: 10.1038/nm.3337
107. Mao Y, Eissler N, Blanc KL, Johnsen JI, Kogner P, Kiessling R. Targeting Suppressive myeloid cells potentiates checkpoint inhibitors to control spontaneous neuroblastoma. Clin Cancer Res. (2016) 22:3849–59. doi: 10.1158/1078-0432.CCR-15-1912
108. Kaufman HL, Ruby CE, Hughes T, Slingluff CL, Jr. Current status of granulocyte-macrophage colony-stimulating factor in the immunotherapy of melanoma. J Immunother Cancer. (2014) 2:11. doi: 10.1186/2051-1426-2-11
109. Lipson EJ, Sharfman WH, Chen S, McMiller TL, Pritchard TS, Salas JT, et al. Safety and immunologic correlates of Melanoma GVAX, a GM-CSF secreting allogeneic melanoma cell vaccine administered in the adjuvant setting. J Trans Med. (2015) 13:214. doi: 10.1186/s12967-015-0572-3
110. Lacher MD, Bauer G, Fury B, Graeve S, Fledderman EL, Petrie TD, et al. SV-BR-1-GM, a clinically effective GM-CSF-secreting breast cancer cell line, expresses an immune signature and directly activates CD4(+) T lymphocytes. Front Immunol. (2018) 9:776. doi: 10.3389/fimmu.2018.00776
111. Lawson DH, Lee S, Zhao F, Tarhini AA, Margolin KA, Ernstoff MS, et al. Randomized, placebo-controlled, phase III trial of yeast-derived granulocyte-macrophage colony-stimulating factor (GM-CSF) versus peptide vaccination versus GM-CSF plus peptide vaccination versus placebo in patients with no evidence of disease after complete surgical resection of locally advanced and/or stage iv melanoma: a trial of the eastern cooperative oncology group-american college of radiology imaging network cancer research group (e4697). J Clin Oncol. (2015) 33:4066–76. doi: 10.1200/JCO.2015.62.0500
112. Le DT, Wang-Gillam A, Picozzi V, Greten TF, Crocenzi T, Springett G, et al. Safety and survival with GVAX pancreas prime and Listeria Monocytogenes-expressing mesothelin (CRS-207) boost vaccines for metastatic pancreatic cancer. J Clin Oncol. (2015) 33:1325–33. doi: 10.1200/JCO.2014.57.4244
113. Faries MB, Hsueh EC, Ye X, Hoban M, Morton DL. Effect of granulocyte/macrophage colony-stimulating factor on vaccination with an allogeneic whole-cell melanoma vaccine. Clin Cancer Res. (2009) 15:7029–35. doi: 10.1158/1078-0432.CCR-09-1540
114. Slingluff CL Jr, Petroni GR, Olson WC, Smolkin ME, Ross MI, Haas NB, et al. Effect of granulocyte/macrophage colony-stimulating factor on circulating CD8+ and CD4+ T-cell responses to a multipeptide melanoma vaccine: outcome of a multicenter randomized trial. Clin Cancer Res. (2009) 15:7036–44. doi: 10.1158/1078-0432.CCR-09-1544
115. Serafini P, Carbley R, Noonan KA, Tan G, Bronte V, Borrello I. High-dose granulocyte-macrophage colony-stimulating factor-producing vaccines impair the immune response through the recruitment of myeloid suppressor cells. Cancer Res. (2004) 64:6337–43. doi: 10.1158/0008-5472.CAN-04-0757
116. Morales JK, Kmieciak M, Knutson KL, Bear HD, Manjili MH. GM-CSF is one of the main breast tumor-derived soluble factors involved in the differentiation of CD11b-Gr1- bone marrow progenitor cells into myeloid-derived suppressor cells. Breast Cancer Res Treatment. (2010) 123:39–49. doi: 10.1007/s10549-009-0622-8
117. Thorn M, Guha P, Cunetta M, Espat NJ, Miller G, Junghans RP, et al. Tumor-associated GM-CSF overexpression induces immunoinhibitory molecules via STAT3 in myeloid-suppressor cells infiltrating liver metastases. Cancer Gene Therapy. (2016) 23:188–98. doi: 10.1038/cgt.2016.19
118. Strauss L, Sangaletti S, Consonni FM, Szebeni G, Morlacchi S, Totaro MG, et al. RORC1 regulates tumor-promoting “Emergency” granulo-monocytopoiesis. Cancer Cell. (2015) 28:253–69. doi: 10.1016/j.ccell.2015.07.006
119. Lesokhin AM, Hohl TM, Kitano S, Cortez C, Hirschhorn-Cymerman D, Avogadri F, et al. Monocytic CCR2(+) myeloid-derived suppressor cells promote immune escape by limiting activated CD8 T-cell infiltration into the tumor microenvironment. Cancer Res. (2012) 72:876–86. doi: 10.1158/0008-5472.CAN-11-1792
120. Kohanbash G, McKaveney K, Sakaki M, Ueda R, Mintz AH, Amankulor N, et al. GM-CSF promotes the immunosuppressive activity of glioma-infiltrating myeloid cells through interleukin-4 receptor-alpha. Cancer Res. (2013) 73:6413–23. doi: 10.1158/0008-5472.CAN-12-4124
121. Okla K, Wertel I, Wawruszak A, Bobinski M, Kotarski J. Blood-based analyses of cancer: Circulating myeloid-derived suppressor cells - is a new era coming? Crit Rev Clin Lab Sci. (2018) 55:376–407. doi: 10.1080/10408363.2018.1477729
122. Mandruzzato S, Brandau S, Britten CM, Bronte V, Damuzzo V, Gouttefangeas C, et al. Toward harmonized phenotyping of human myeloid-derived suppressor cells by flow cytometry: results from an interim study. Cancer Immunol Immunother. (2016) 65:161–9. doi: 10.1007/s00262-015-1782-5
123. Solito S, Marigo I, Pinton L, Damuzzo V, Mandruzzato S, Bronte V. Myeloid-derived suppressor cell heterogeneity in human cancers. Ann N Y Acad Sci. (2014) 1319:47–65. doi: 10.1111/nyas.12469
124. Demaret J, Walencik A, Jacob MC, Timsit JF, Venet F, Lepape A, et al. Inter-laboratory assessment of flow cytometric monocyte HLA-DR expression in clinical samples. Cytomet Part B Clin Cytomet. (2013) 84:59–62. doi: 10.1002/cyto.b.21043
125. Gustafson MP, Lin Y, Maas ML, Van Keulen VP, Johnston PB, Peikert T, et al. A method for identification and analysis of non-overlapping myeloid immunophenotypes in humans. PLoS ONE. (2015) 10:e0121546. doi: 10.1371/journal.pone.0121546
126. Wagner C, Deppisch R, Denefleh B, Hug F, Andrassay K, Hansch GM. Expression patterns of the lipopolysaccharide receptor CD14, and the FCg receptors CD16 and CD64 on polymorphonuclear neutrophils: data from patients with severe bacterial infections and lipopolysaccharide-exposed cells. Shock. (2003) 19:5–12. doi: 10.1097/00024382-200301000-00002
127. Ziegler-Heitbrock L, Ancuta P, Crowe S, Dalod M, Grau V, Hart DN, et al. Nomenclature of monocytes and dendritic cells in blood. Blood. (2010) 116:e74–80. doi: 10.1182/blood-2010-02-258558
128. Duffy A, Zhao F, Haile L, Gamrekelashvili J, Fioravanti S, Ma C, et al. Comparative analysis of monocytic and granulocytic myeloid-derived suppessor cell subsets in patients with gastrointestinal malignancies. Cancer Immunol Immunother. (2013) 62:299–307. doi: 10.1007/s00262-012-1332-3
129. Kotsakis A, Harasymczuk M, Schilling B, Georgoulias V, Argiris A, Whiteside TL. Myeloid-derived suppressor cell measurements in fresh and cryopreserved blood samples. J Immunol Methods. (2012) 381:14–22. doi: 10.1016/j.jim.2012.04.004
130. Idorn M, Kollgaard T, Kongsted P, Sengelov L, Thor Straten P. Correlation between frequencies of blood monocytic myeloid-derived suppressor cells, regulatory T cells and negative prognostic markers in patients with castration-resistant metastatic prostate cancer. Cancer Immunol Immunother. (2014) 63:1177–87. doi: 10.1007/s00262-014-1591-2
131. Monneret G, Elmenkouri N, Bohe J, Debard AL, Gutowski MC, Bienvenu J, et al. Analytical requirements for measuring monocytic human lymphocyte antigen DR by flow cytometry: application to the monitoring of patients with septic shock. Clin Chem. (2002) 48:1589–92.
132. Docke WD, Hoflich C, Davis KA, Rottgers K, Meisel C, Kiefer P, et al. Monitoring temporary immunodepression by flow cytometric measurement of monocytic HLA-DR expression: a multicenter standardized study. Clinical Chem. (2005) 51:2341–7. doi: 10.1373/clinchem.2005.052639
Keywords: immunosuppression, monocytes, MDSCs, cancer, immunotherapy, biomarker
Citation: Mengos AE, Gastineau DA and Gustafson MP (2019) The CD14+HLA-DRlo/neg Monocyte: An Immunosuppressive Phenotype That Restrains Responses to Cancer Immunotherapy. Front. Immunol. 10:1147. doi: 10.3389/fimmu.2019.01147
Received: 19 March 2019; Accepted: 07 May 2019;
Published: 22 May 2019.
Edited by:
Maija Hollmén, University of Turku, FinlandReviewed by:
Eyad Elkord, University of Salford, United KingdomRolf Kiessling, Karolinska Institute (KI), Sweden
Copyright © 2019 Mengos, Gastineau and Gustafson. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Michael P. Gustafson, gustafson.michael@mayo.edu
 April E. Mengos
April E. Mengos Dennis A. Gastineau
Dennis A. Gastineau Michael P. Gustafson
Michael P. Gustafson