- 1Cancer Institute, University Hospital Fundacion Jimenez Diaz, Autonomous University of Madrid, Madrid, Spain
- 2Department of Clinical Oncology, St. Marianna University School of Medicine, Kawasaki, Japan
- 3Chaim Sheba Medical Center, Ramat Gan, Israel
- 4David Geffen School of Medicine at University of California, Los Angeles, CA, United States
- 5Merck Healthcare KGaA, Darmstadt, Germany
- 6Department of Hematology, Oncology, and Tumor Immunology (CCM) Charité Universitaetsmedizin, Berlin, Germany
Cetuximab and panitumumab are two distinct monoclonal antibodies (mAbs) targeting the epidermal growth factor receptor (EGFR), and both are widely used in combination with chemotherapy or as monotherapy to treat patients with RAS wild-type metastatic colorectal cancer. Although often considered interchangeable, the two antibodies have different molecular structures and can behave differently in clinically relevant ways. More specifically, as an immunoglobulin (Ig) G1 isotype mAb, cetuximab can elicit immune functions such as antibody-dependent cell-mediated cytotoxicity involving natural killer cells, T-cell recruitment to the tumor, and T-cell priming via dendritic cell maturation. Panitumumab, an IgG2 isotype mAb, does not possess these immune functions. Furthermore, the two antibodies have different binding sites on the EGFR, as evidenced by mutations on the extracellular domain that can confer resistance to one of the two therapeutics or to both. We consider a comparison of the properties of these two antibodies to represent a gap in the literature. We therefore compiled a detailed, evidence-based educational review of the known molecular, clinical, and functional differences between the two antibodies and concluded that they are distinct therapeutic agents that should be considered individually during treatment planning. Available data for one agent can only partly be extrapolated to the other. Looking to the future, the known immune activity of cetuximab may provide a rationale for this antibody as a combination partner with investigational chemotherapy plus immunotherapy regimens for colorectal cancer.
Introduction
The advent of targeted monoclonal antibodies (mAbs) brought a revolution in the field of oncology. With increased specificity, longer half-lives, and more predictable overall pharmacokinetic and pharmacodynamic behaviors than their small-molecule inhibitor counterparts, mAbs have become key components of standard-of-care treatments for multiple indications. Inevitably, sometimes several approved mAbs against the same target are available, requiring physicians to perform detailed research to understand which mAb is the optimal therapeutic agent for a given patient. In fact, more than half of the approved targeted mAbs in oncology (excluding the new wave of checkpoint inhibitors) are clustered around 5 targets: the epidermal growth factor receptor (EGFR), the human epidermal growth factor receptor 2 (HER2), tumor necrosis factor α, CD20, and vascular endothelial growth factor (VEGF) (1). Indeed, among treatment options for metastatic colorectal cancer (mCRC), in particular, are two anti-EGFR mAbs, cetuximab and panitumumab, currently indicated for the same subgroup of patients, those with RAS wild-type (wt) metastatic disease (2, 3). Approximately 40% of patients with CRC will eventually develop metastatic disease (4); per international guidelines, the majority of these patients should undergo RAS testing for suitability for an anti-EGFR mAb in combination with oxaliplatin- or irinotecan-based chemotherapy. Thus, clinicians must choose between prescribing cetuximab and panitumumab regularly.
In 2004, cetuximab was approved by both the US FDA and the EMA for use in EGFR-expressing (K)RAS-unselected chemorefractory mCRC. Panitumumab was approved by the US FDA for use in the same patient population in 2006. In 2007, the EMA rejected the use of panitumumab in an unselected chemorefractory population, but approved the use of panitumumab in a restricted population of KRAS exon 2 wt mCRC, and imposed a similar restriction on use of cetuximab in 2008. By 2009, the FDA followed the EMA by restricting use of either anti-EGFR agent to KRAS exon 2 wt chemorefractory mCRC patients.
In the first-line setting, panitumumab + CT was approved by the EMA in 2011, based on positive results from the randomized phase 3 PRIME trial. In 2012, cetuximab + CT was approved by the FDA following the phase 3 CRYSTAL trial. In 2013, extended RAS testing was required by the FDA and EMA for predicting response to anti-EGFR agents (5).
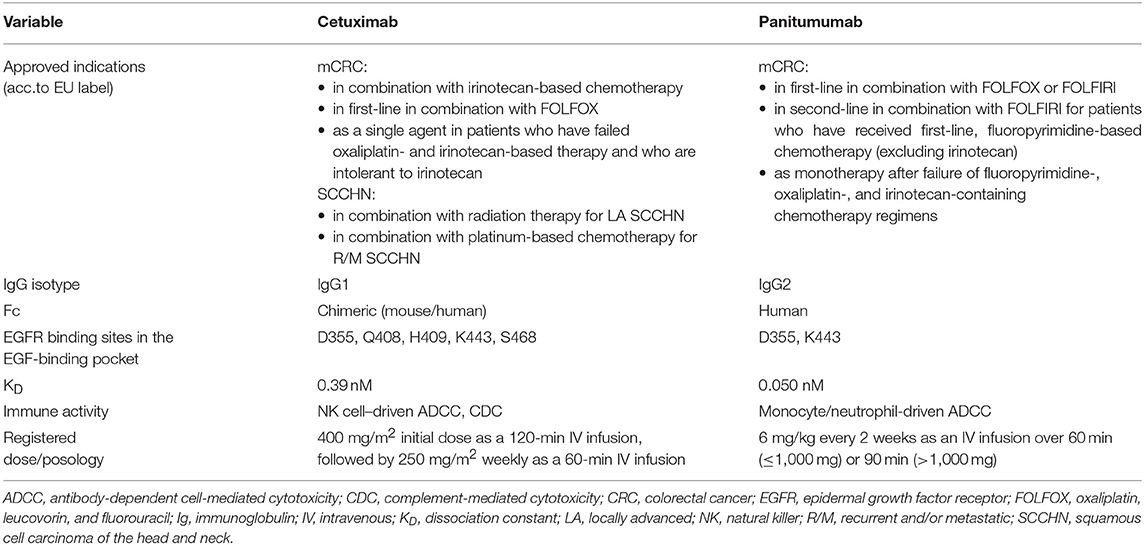
According to the EU SmPC, cetuximab is currently indicated for EGFR-expressing RAS wt mCRC as a monotherapy in patients who have failed oxaliplatin- and irinotecan-based therapy and who are intolerant to irinotecan, in combination with irinotecan-based therapy in any line, and in combination with FOLFOX in first-line. Cetuximab is also indicated for use in SCCHN, both in locally advanced disease (in combination with radiation therapy) and in recurrent/metastatic disease (in combination with platinum-based chemotherapy). Panitumumab is indicated for RAS wt mCRC as a monotherapy after failure of fluoropyrimidine-, oxaliplatin-, and irinotecan-containing chemotherapy regimens, in combination with FOLFOX or FOLFIRI in first-line, and in combination with FOLFIRI in second-line mCRC (6, 7).
To date, >480,000 patients with mCRC have received cetuximab-based therapy worldwide, and >240,000 patients with mCRC have been treated with panitumumab-containing therapy (8, 9). Although these two mAbs are considered to be very similar, important biological, molecular, and practical differences exist between them. Thus, there are uncertainties regarding whether they can be considered equivalent and whether it is prudent to ascribe conclusions gleaned from a study of one agent to the other and to pool data on the two in meta-analyses. In this article, we summarize and discuss these differences, primarily within the context of mCRC, but we also describe their differential activity in the treatment of squamous cell carcinomas of the head and neck (SCCHN). We then relate how these differences could impact the potential for anti-EGFR mAbs to be combined with emerging immunotherapies. The goal of this review is to provide a comprehensive discussion of the available data on the two mAbs and to highlight how they are distinct therapeutic agents with individual, clinically relevant properties.
Mode of Action Against EGFR
Dysregulation in the EGFR signaling pathway has long been associated with pro-oncogenic activities such as increased cell proliferation, reduced apoptosis, and increased angiogenesis and metastatic tendencies (1, 4, 10). The EGFR is activated when one of its many ligands (including the epidermal growth factor [EGF], transforming growth factor α, amphiregulin, or epiregulin) binds the receptor's extracellular domain, resulting in receptor dimerization, conformational change, and tyrosine autophosphorylation (4, 10, 11). Upon receptor binding, downstream signaling cascades including the MAPK/ERK (mitogen-activated protein kinase/extracellular signal-regulated kinase), JAK/STAT (Janus kinase/signal transducers and activators of transcription), and PI3K/Akt (phosphoinositide 3-kinase/protein kinase B) pathways become active. Constitutive activation of these pathways can lead to cancer cell survival and proliferation (1, 4, 5).
Cetuximab and panitumumab both function by binding to the extracellular domain III of the EGFR, thereby preventing ligand binding and locking the EGFR in the autoinhibitory monomeric conformation (1, 4, 11). The antibody-receptor construct is then internalized, ubiquitinated, and either degraded or recycled. This turnover is regulated by the ubiquitin proteasome system (12, 13). Briefly, after activation of the receptor tyrosine kinase through ligand binding and dimerization, the activated receptor is internalized by clathrin-dependent endocytosis and ubiquitinated. This process terminates the tyrosine kinase activity of activated EGFR and regulates the number of receptors expressed on the cell surface. The final step of degradation is performed by the proteasome; however, ubiquitinated receptors can be deubiquitinated by deubiquitinating enzymes and then recycled back to the cell membrane (12). Receptor ubiquitination has been identified as a mechanism of resistance to anti-EGFR therapy (12).
Between 60 and 80% of colorectal tumors overexpress the EGFR; although this characteristic was historically thought to be predictive of response to cetuximab and panitumumab, in more recent years this notion has not held up in practice (1, 4, 14). Alternative explanations for the efficacy of cetuximab and panitumumab in colorectal tumors regardless of EGFR overexpression status focus on the ligands to EGFR and potential dysregulation of the amount of ligands produced and released into the extracellular space (5). Indeed, both cetuximab and panitumumab compete with EGF for its binding site on EGFR. Mutational studies have demonstrated that the two mAbs have different binding sites on EGFR, but the binding epitopes are in close physical proximity and have some key residues in common (15) (Table 1). Panitumumab's binding epitope includes EGFR residues P349, P362, D355, F412, and I438, all of which are individually necessary for ≥50% binding affinity (15). In contrast, binding residues on EGFR critical for cetuximab binding are Q384, Q408, H409, K443, K465, I467, and S468, as well as F352, D355, and P387 (15). D355 is likely a source of competition between the mAbs and EGF because it is within the binding site of all three molecules (15). Notably, panitumumab's binding epitope overlaps with the EGF binding site in two locations (D355 and K443), whereas cetuximab overlaps with EGF's binding site in 5 locations (D355, Q408, H409, K443, and S468).
Furthermore, cetuximab and panitumumab have different binding affinities for EGFR, with dissociation constants (KD) of 0.39 nM vs. 0.05 nM, respectively (4). Cetuximab binds EGFR with ~2-fold greater affinity than EGF (16). Panitumumab binds EGFR with an ~8-fold greater affinity than that of cetuximab. However, it is unclear whether this characteristic is favorable. From one standpoint, a higher affinity for EGFR should translate into a greater proportion of mAb-bound EGFR; conversely, however, studies have observed that a KD between 1 and 10 nM is optimal for anti-EGFR mAb tumor targeting, accumulation, and retention (11). Although the KD of cetuximab is closer, neither mAb is within the optimal range. Cetuximab and panitumumab administration schedules are very different from each other (Table 1). Cetuximab is administered based on body surface area, and is usually given as a 400-mg/m2 initial dose by a 120-min intravenous (IV) infusion, followed by a weekly dose of 250 mg/m2 by 60-min IV infusion (6). However, Q2W doses of 500 mg/m2 have been investigated; this dosing schedule is frequently used, and is recommended based on NCCN guidelines but not approved by regulatory authorities (3). Maintenance cetuximab can be administered on the same weekly or Q2W schedule (17) and treatment with cetuximab is recommended to be given until progression of disease (6). Indeed, in pharmacokinetic studies, a 250-mg/m2 weekly cetuximab dose has a mean half-life of 4.19 days and a minimum recorded mean concentration of 49.6 μg/mL (17). By comparison, panitumumab is administered by weight at a dose of 6 mg/kg every 2 weeks; a 60-min infusion time is recommended for total doses ≤ 1,000 mg, and a 90-min infusion time is recommended for total doses > 1,000 mg (7). At this administration schedule, panitumumab's mean half-life is 7.5 days, with a minimum recorded mean serum concentration of 39 μg/mL (18). Studies have indicated that it takes 3 infusions of panitumumab to reach steady state (19), although similar information has not been published for cetuximab. Overall, administration of cetuximab and panitumumab per their standard schedules results in comparable pharmacokinetic behaviors and overall drug exposures. One final structural difference between the two mAbs is found in their respective backbones. Panitumumab is a human mAb and cetuximab is a mouse/human chimeric mAb. Although this distinction can sometimes lead to differences in the rates of infusion-related reactions between the two agents, these can be managed with the appropriate pre-medication prior to infusion.
Molecular Structure and Associated Immune Activity
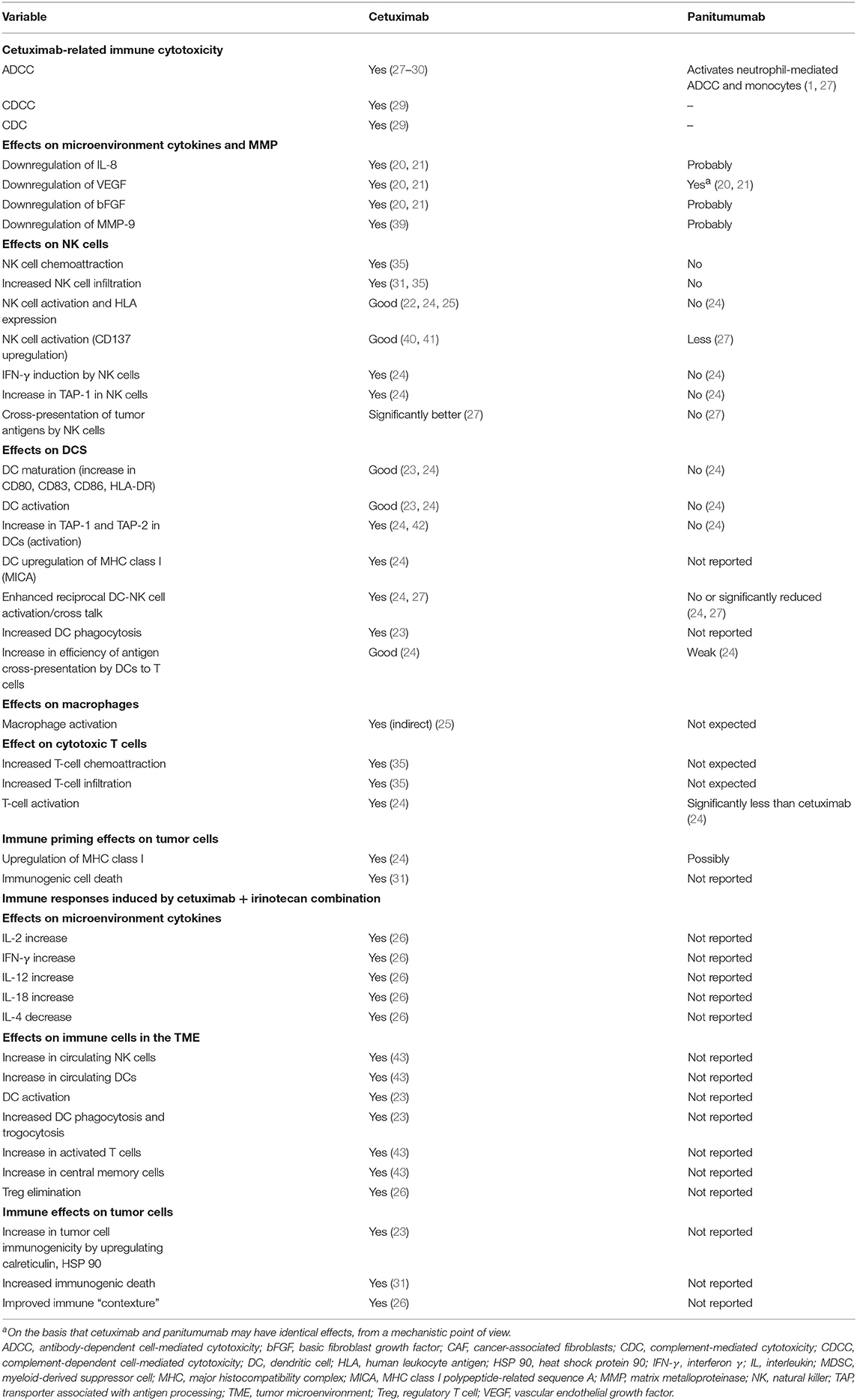
One of the most hotly debated topics is the functional implication of the differing immunoglobulin (Ig) G subtypes of cetuximab and panitumumab—namely, that cetuximab is an IgG1 isotype mAb, whereas panitumumab has the IgG2 backbone (Figure 1, Table 1) (4, 38). The two Ig isotypes differ in their ability to mobilize innate and adaptive immune cells against tumor cells (Figure 1, Table 2). For example, it has been demonstrated in preclinical models and ex vivo studies that target-bound cetuximab and other IgG1 isotype mAbs (e.g., rituximab, necitumumab, trastuzumab) stimulate natural killer (NK) cell–driven cytotoxicity against tumor cells coated in mAbs via the interaction of the constant region and the CD16 receptor on NK cells (38, 44–47). This antibody-dependent cell-mediated cytotoxicity (ADCC) is specifically carried out by NK cells of the innate immune system against tumor cells, resulting in antigen release into the intratumoral space (16). By secreting cytokines and interferon γ, active NK cells are further able to stimulate dendritic cell (DC) maturation and DC-NK cell cross talk (24, 27, 38) and use increased expression of CD137 to recruit anti-EGFR CD8+ cytotoxic T cells to the intratumoral space for additional cell-killing activity (40, 41, 48). In turn, mature DCs can mobilize a number of additional immunogenic processes, including antigen presentation to cytotoxic T cells and further activation of NK cells (24, 27, 38, 48). Collectively, NK cell–mediated ADCC and other immunogenic activity of IgG1 mAbs is thought to contribute to their antitumor activity, provided that sufficient target is available for the mAbs to dually bind to CD16 and their intended epitope (46, 49–51). This sequence of immune events initiated by cetuximab can be viewed as a chain reaction reminding of a domino effect (Figure 1). Furthermore, some clinical evidence has suggested that patients with higher baseline ADCC activity or specific CD16 polymorphisms that increase NK cell–binding affinity might be likelier to experience favorable outcomes with IgG1-based therapy (28, 52–55). By contrast, the Fc region of the IgG2 backbone of panitumumab has very low binding affinity for CD16; thus, panitumumab is unable to induce NK cell–driven ADCC or cytotoxic T-cell tumor infiltration (16, 48), although evidence suggests that panitumumab instead induces some immunostimulatory action via neutrophil-driven ADCC and monocytes (1, 27). However, its immunogenic properties are not considered to actively contribute to panitumumab's antitumor activity (47, 48). A final difference in the immunostimulatory capabilities of IgG1 and IgG2 mAbs concerns the C1 complex of complement, which can be induced by clusters (hexamers) of IgG1 mAbs but has not been shown to be induced to the same degree by IgG2 mAbs (47, 56, 57).
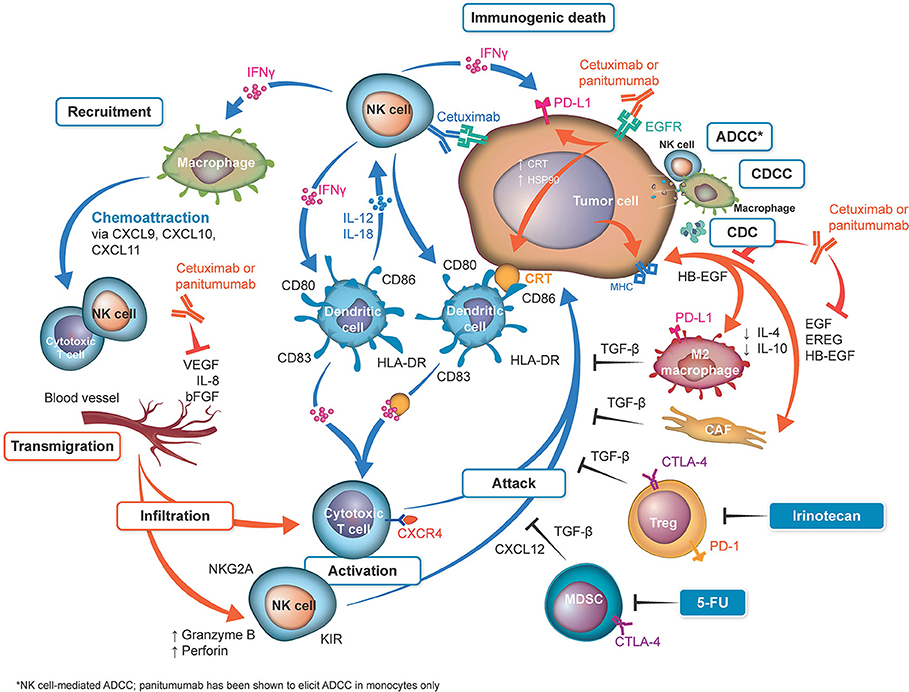
Figure 1. Overview of differences in immune activation with cetuximab and panitumumab. Shown in orange: sites of activation by both anti–epidermal growth factor receptor (EGFR) monoclonal antibodies (mAbs). Both anti-EGFR mAbs neutralize the cross talk between the cancer cells and M2 monocytes and cancer-associated fibroblasts (CAFs) by neutralization of EGFR ligands. On the basis that cetuximab and panitumumab may have identical effects, from a mechanistic point of view, both antibodies reduce vascular endothelial growth factor (VEGF) production (20, 21). Cetuximab can upregulate calreticulin (CRT), heat shock protein (HSP) 90, and major histocompatibility complex (MHC) (22, 23), which may be theoretically upregulated by panitumumab (not reported). Shown in blue: sites activated by cetuximab. Natural killer (NK) cells are activated by their binding to the cetuximab loaded onto EGFR (22, 24, 25). The released interferon γ (IFN-γ) activates dendritic cells (DCs), which further activate the NK cells (26). Cetuximab-induced antibody-dependent cell-mediated cytotoxicity (ADCC), complement-dependent cell-mediated cytotoxicity (CDCC), complement-mediated cytotoxicity (CDC) (27–30), and immunogenic death (31) release tumor antigens, which are captured by the activated DC cells, to be presented to T cells (thus activating them). IFN-γ upregulates programmed cell death 1 ligand 1 (PD-L1) on tumor cells and activates macrophages to release chemoattraction substances for NK cells and T cells (25). Inhibition of the angiogenic factors VEGF, interleukin (IL) 8, and fibroblast growth factor (FGF) can be downregulated by both cetuximab and possibly by panitumumab (20, 21). Inhibition of these factors upregulates key homing adhesion molecules for the immune cells (intercellular adhesion molecule 1 [ICAM-1] and vascular cell adhesion protein 1 [VCAM-1]) (32, 33) and downregulates Fas antigen ligand (FasL) expression (34), which would be lethal for T cells. These effects enable the safe transmigration of T cells and NK cells into the tumor microenvironment (35). The T cells activated by DCs loaded with tumor cell antigens are then ready to attack the tumor cells. Shown in black: Immune suppressive mechanisms/prevention of the successful attack of activated cytotoxic T cells on tumor cells. These mechanisms include checkpoint inhibitory factors (programmed cell death 1 protein [PD-1], PD-L1, cytotoxic T-lymphocyte protein 4 [CTLA-4]) and TGF-β generated by tumor-associated cells (25). Notably, irinotecan and fluorouracil (5-FU) can eliminate tumor protective cells, such as regulatory T cells (Tregs) and myeloid-derived suppressor cells (MDSCs), from the tumor microenvironment (36, 37), reducing their immune suppressive effects and thus potentially facilitating the T-cell attack. bFGF, basic fibroblast growth factor; EREG, epiregulin; HB-EGF, heparin-binding EGF-like growth factor; HLA, human leukocyte antigen; KIR, killer cell immunoglobulin-like receptor; TGF-β, transforming growth factor β.
Biomarkers of Response, Target Populations, and Therapeutic Resistance
Colorectal cancer is a highly heterogeneous disease (5), characterized by predictive and prognostic mutations (58, 59) as well as a tendency to undergo clonal selection under drug pressure and develop acquired resistance to certain therapies (60–62). For example, as recommended by the international guidelines, both cetuximab and panitumumab are suitable only for patients with RAS wt colorectal tumors, with genetic analysis of KRAS exon 2 (codons 12, 13), exon 3 (codons 59, 61), exon 4 (codons 117, 146) and NRAS exon 2 (codons 12, 13), exon 3 (codons 59, 61), and exon 4 (codons 117, 146) (“RAS wt”) (2, 3, 5, 63). Although several early retrospective RAS analyses (58, 64) provided evidence supporting testing beyond KRAS exon 2 (i.e., extended RAS analysis), the retrospective analysis of the PRIME study was the first phase 3 analysis to support refinement of the patient population by RAS status and the need for extended RAS analyses. In PRIME, panitumumab in combination with FOLFOX4 was shown to have greater benefit in a RAS wt-targeted patient population rather than in a patient population identified as KRAS wt, compared with FOLFOX4 alone (65). Additional post hoc analyses of several phase 3 trials involving cetuximab have also demonstrated improved responses and survival with cetuximab-based therapy with FOLFOX or FOLFIRI in patients with RAS wt mCRC compared with patients with KRAS wt tumors (66–68). Results from the TAILOR trial, the first phase 3 study to prospectively recruit a RAS wt patient population for first-line treatment of mCRC with cetuximab plus chemotherapy (specifically, FOLFOX), further confirmed the survival benefit with cetuximab-based treatment in RAS wt mCRC (69). Finally, KRAS amplification, although much rarer than and nearly always mutually exclusive with KRAS mutations (amplification is present in ~1–2% of cases of mCRC) (5), has been shown to confer resistance to cetuximab and panitumumab and is considered an emerging biomarker by current guidelines (2).
In addition to mutations existing in the predominant cell population of the tumor before treatment, overall resistance to therapy can arise during anti-EGFR therapy, as the drug can inhibit growth of sensitive clones, thereby allowing for expansion of initially rare RAS-mutant clones (10, 62). Indeed, there is preclinical and clinical evidence available demonstrating that RAS wt tumors can “switch” to RAS mutant after anti-EGFR treatment (with either cetuximab or panitumumab) (70), likely because of a significant reduction of the wt clone and an expansion of mutated clones. Finally, recent studies have suggested the possibility of a restoration of responsiveness to cetuximab after the development of resistance to previous cetuximab treatment (71, 72). The prospective CRICKET study, which evaluated third-line re-treatment with cetuximab plus irinotecan after an initial response followed by progression while patients had received the same regimen in the first line, showed that RAS wt status in circulating tumor DNA before start of third-line therapy was significantly associated with prolonged progression-free survival (PFS) compared with a RAS mutated status (73).
Resistance to anti-EGFR therapy can also be conferred through extracellular domain mutations in the EGFR itself, which have been observed in only EGFR therapy–experienced patients, suggesting that these mutations arise specifically as a mechanism of acquired resistance (13, 60, 74–76). Notably, different mutations in the extracellular domain can dictate resistance only to cetuximab, only to panitumumab, or to both mAbs, owing to their differential binding sites (15). For example, the S492R and S468R mutations in the extracellular domain of the EGFR confer resistance only to cetuximab (13, 75), whereas the G465R mutation that arises in 1 of every 6 patients who receive panitumumab confers resistance to both mAbs (77). Such observations may have implications for planning treatment sequencing, treatment continuation, and maintenance therapy designed to maximize the number of efficacious lines of therapy and the likelihood of response at each stage.
Clinical Impact of Cetuximab and Panitumumab in Colorectal Cancer
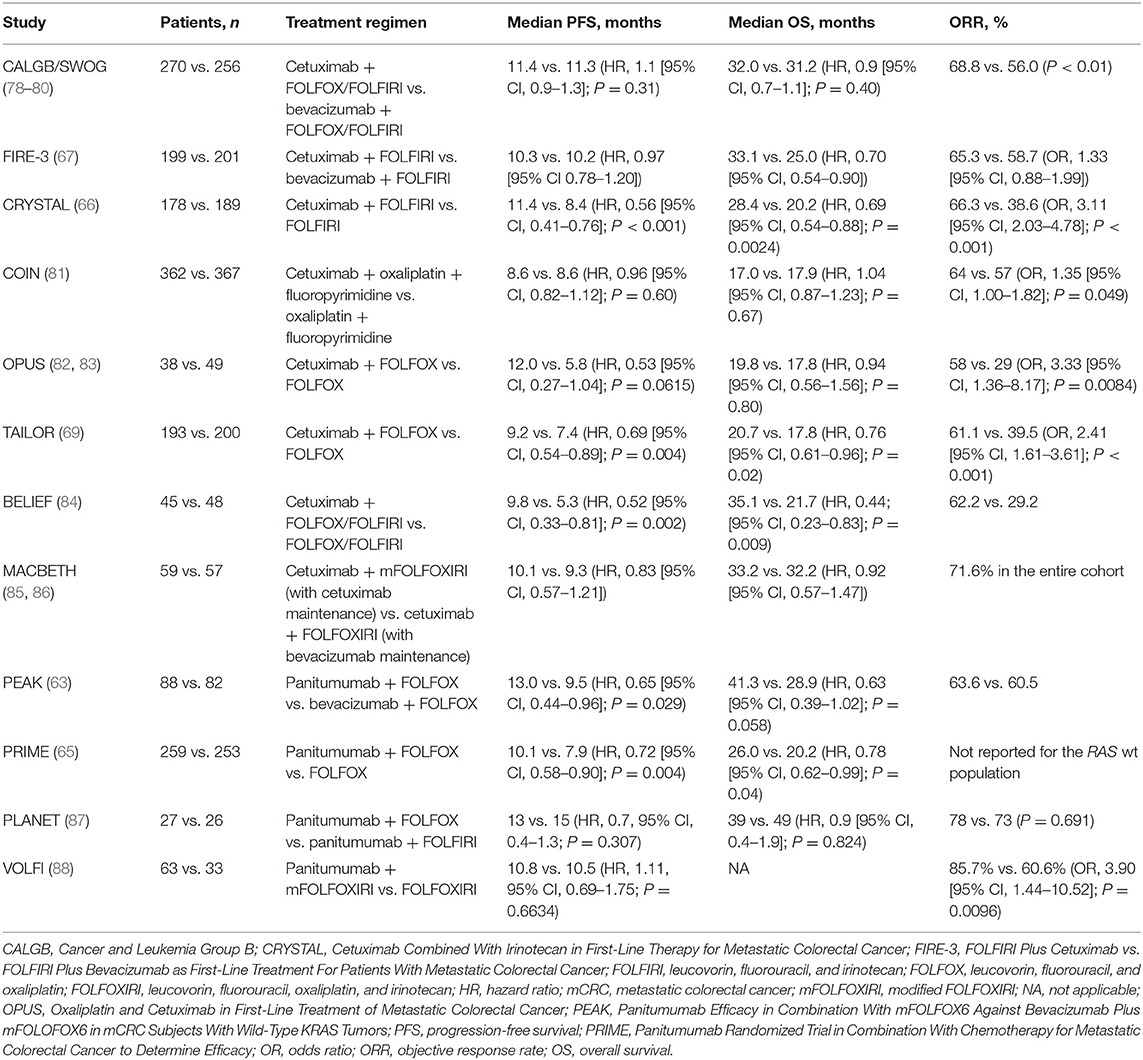
Over the last two decades, cetuximab and panitumumab have been evaluated for efficacy and safety in mCRC in many clinical trials. With approximately half a million patients treated with cetuximab, and close to a quarter of a million treated with panitumumab, the clinical impact of these two mAbs on the disease has been substantial. Currently, the median overall survival (OS) in patients who present with RAS wt metastatic disease is usually ≥ 30 months, with hazard ratios (HRs) for survival with first-line cetuximab-based therapy of 0.763 in combination with FOLFOX vs. FOLFOX alone, 0.69 in combination with FOLFIRI vs. FOLFIRI alone, and 0.70 to 0.90 in combination with either doublet chemotherapy vs. bevacizumab plus doublet chemotherapy, according to phase 3 trials (66, 67, 69, 78). Although panitumumab has not been extensively studied in combination with FOLFIRI chemotherapy in the first-line setting, first-line panitumumab plus FOLFOX vs. FOLFOX alone yielded an HR for survival of 0.78 in a retrospective analysis of the RAS wt population of the phase 3 PRIME trial (65). Additionally, a retrospective analysis of the phase 2 PEAK trial yielded an HR for survival of 0.63 with panitumumab plus FOLFOX vs. bevacizumab plus FOLFOX in the population with RAS wt disease; however, patient numbers were much lower in this phase 2 study than in the analogous cetuximab phase 3 CALGB/SWOG 80405 and FIRE-3 trials (63, 67, 78).
A full summary of the available first-line data for cetuximab and panitumumab in combination with chemotherapy is presented in Table 3. Notably, however, while cetuximab has been shown to pair well with FOLFIRI, FOLFOX, and FOLFOXIRI (leucovorin, 5-FU, oxaliplatin, and irinotecan) chemotherapy backbones in multiple randomized studies (66, 67, 78, 85, 86, 89), almost all available data for panitumumab in the first-line RAS wt setting are in combination with FOLFOX and include only 1 phase 3 and 1 phase 2 study. Evidence for panitumumab plus FOLFIRI in mCRC comes from two studies. The first was a phase 2, single-arm study of panitumumab + FOLFIRI in first-line mCRC, which showed favorable efficacy of the combination in KRAS wt vs. KRAS mt mCRC (90). The second study was the phase 3 20050181 trial, which administered this combination in the second-line setting in patients with KRAS wt mCRC. The phase 3 second-line study reported a significant but modest improvement in PFS compared with FOLFIRI alone (median, 6.7 vs. 4.9 months; HR, 0.82), a trend toward improvement in OS (median, 14.5 vs. 12.5 months; HR, 0.92), and a significant improvement in objective response rate (ORR; 36 vs. 10%) (91). Recently, the phase 2 Gruppo Oncologico del Nord Ovest (GONO) and VOLFI trials provided published evidence for the first-line panitumumab combination with FOLFOXIRI in patients (N = 37 and N = 96, respectively) with non–liver-limited mCRC (88, 92).
In recent years, primary tumor location has gained importance as another characteristic of mCRC that impacts patient prognosis and treatment decision making. Primary tumor location (right vs. left, or proximal vs. distal, respectively) has been demonstrated to have significant implications for patient survival and response to available therapies (93). Specifically, patients diagnosed with left-sided tumors have appeared to have better responses with anti-EGFR therapy than with anti-VEGF therapy, with the bulk of tumor location subgroup analysis evidence coming from the available cetuximab-based phase 3 trials. In contrast, patients with right-sided tumors have appeared to derive less benefit from therapy in general (80, 94). In the populations of patients with RAS wt left-sided primary tumors in the CALGB/SWOG 80405 and FIRE-3 trials, the median OS approached 40 months with cetuximab plus chemotherapy (FOLFIRI or FOLFOX in CALGB/SWOG 80405 and FOLFIRI in FIRE-3) (80, 94). Indeed, a small retrospective study by Sagawa et al. demonstrated a median OS of over 50 months with cetuximab-based treatment in patients with RAS wt left-sided tumors (95). Furthermore, improvements in OS with cetuximab-based treatment were statistically significant compared with bevacizumab-based treatment in the population with RAS wt left-sided tumors (80, 94, 95). Efficacy data for first-line panitumumab- vs. bevacizumab-based treatment in RAS wt left-sided mCRC are available only from the phase 2 PEAK study, in which OS trended toward improvement with panitumumab; however, the results did not reach statistical significance (96). Although ~86% of the currently published data for first-line studies of anti-EGFR agents vs. bevacizumab in left-sided tumors come from cetuximab trials, studies suggest similar results with either cetuximab or panitumumab compared with bevacizumab in patients with RAS wt, left-sided mCRC.
Although patients with right-sided tumors consistently had worse prognoses than patients with left-sided tumors, they may still derive tumor shrinkage benefits with anti-EGFR-mAb-based treatment, according to a meta-analysis by Wang et al. (including the CRYSTAL, TAILOR, PRIME, and 20050181 trials) that demonstrated that anti-EGFR-mAb-based treatment significantly improves response rates and PFS in patients with RAS wt mCRC, independent of primary tumor location (97). Additionally, a meta-analysis by Arnold et al. (including the CRYSTAL, FIRE-3, CALGB 80405, PRIME, PEAK, and 20050181 studies) confirmed the prognostic value of primary tumor location and demonstrated that patients with left-sided tumors significantly benefited from an anti-EGFR antibody plus chemotherapy vs. chemotherapy with or without bevacizumab. For patients with right-sided disease, there was no significant benefit in OS or PFS; however, an analysis of ORR showed that an anti-EGFR plus chemotherapy doublet can be a treatment option when cytoreduction is the goal (68). The findings of both meta-analyses support the preferential utilization of an anti-EGFR mAb plus chemotherapy in patients with RAS wt, left-sided mCRC, with most of the data being extracted from cetuximab-based trials. Although patients with right-sided tumors tended to derive limited benefit from available therapy, a pooled analysis of prospective trials showed that some proportion of patients with right-sided tumors could respond to cetuximab, suggesting that some patients with right-sided disease may benefit from an anti-EGFR agent plus chemotherapy as an initial treatment (98).
Although cetuximab and panitumumab have not been compared directly in first- or second-line mCRC, a limited number of phase 2 studies exist for each that had comparable trial designs. A phase 2 trial by Carrato et al. evaluated the efficacy of second-line panitumumab plus irinotecan in patients with KRAS wt mCRC who had received either 5-FU, oxaliplatin, or irinotecan in the first line. Panitumumab plus irinotecan yielded a PFS and OS of 4.5 and 15.1 months, respectively, and an ORR of 23%. The outcomes observed by Hong et al. with second-line cetuximab plus irinotecan, also in patients with KRAS wt disease, were a median PFS and OS of 8.3 and 18.3 months, respectively, and an ORR of 45% (99, 100). The only randomized, phase 3 trial to compare cetuximab and panitumumab directly was ASPECCT, which confirmed the non-inferiority of panitumumab compared with cetuximab as a monotherapy in the third- and later-line setting in patients with KRAS wt mCRC. Results of the RAS wt subset of the ASPECCT study are still pending (101). In the final analysis, median PFS and OS were 4.1 vs. 4.4 months and 10.4 vs. 10.0 months with panitumumab vs. cetuximab, respectively. The ORR was 22.0% with panitumumab and 19.8% with cetuximab. ASPECCT was a non-inferiority trial (rather than a superiority trial), but a trial powered to investigate efficacy differences between cetuximab and panitumumab in colorectal cancer had not been conducted at the time of this article. Therefore, the results from ASPECCT might not be extrapolated to earlier lines of therapy and to treatment in combination with chemotherapy. One other noteworthy study, the phase 2, randomized WJOG6510G trial, compared cetuximab plus irinotecan and panitumumab plus irinotecan in patients with KRAS wt mCRC in whom 5-FU–, oxaliplatin-, and irinotecan-based therapy had previously failed. The results suggested non-inferiority of panitumumab plus irinotecan compared with cetuximab plus irinotecan in this setting (102). Additional third- and further-line studies of cetuximab or panitumumab monotherapy or in combination with irinotecan are difficult to compare directly because many of the early trials with cetuximab were conducted prior to the discovery of the KRAS mutation biomarker, and therefore enrollment was determined by EGFR expression status only (103–110). However, the phase 3 CO.17 trial demonstrated how mutation status of the KRAS gene was associated with OS in mCRC patients treated with cetuximab after prior chemotherapy (111). More recently, a retrospective analysis of the EPIC study demonstrated that post-study cetuximab was associated with improved OS in the RAS wt population (112).
One final difference in clinical efficacy that has been observed between cetuximab and panitumumab concerns the effect of prior bevacizumab treatment on response to subsequent anti-EGFR therapy. Recent evidence has suggested that prior bevacizumab therapy, if administered within a certain time interval of initiation of anti-EGFR therapy, can compromise responsiveness to cetuximab but not to panitumumab (101, 113–117). These findings not only underline the fact that the two mAbs are non-interchangeable, but they also have implications in treatment sequencing—namely, that in order to maximize the potential number of therapeutic lines of treatment, cetuximab should be administered prior to bevacizumab.
Safety Findings With Cetuximab and Panitumumab in Colorectal Cancer
The rates of grade 3/4 adverse events (AEs) considered related to anti-EGFR therapy in patients treated with first-line anti-EGFR plus chemotherapy are presented in Table 4A. Additionally, the rates of grade 3/4 AEs from the third-line head-to-head ASPECCT trial are presented in Table 4B.
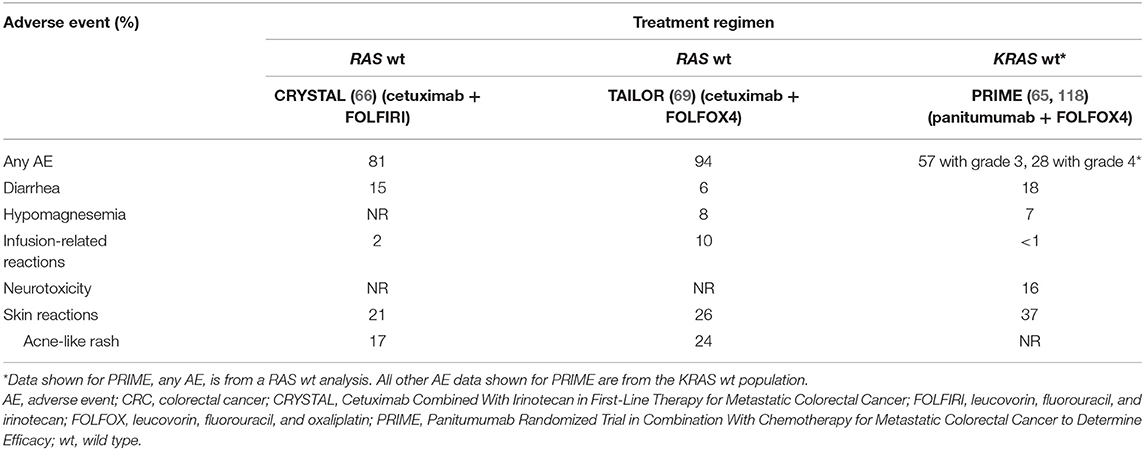
Table 4A. Comparison of cetuximab- and panitumumab-associated grade 3/4 adverse events: evidence from (A) first-line and (B) third-line phase 3 trials.
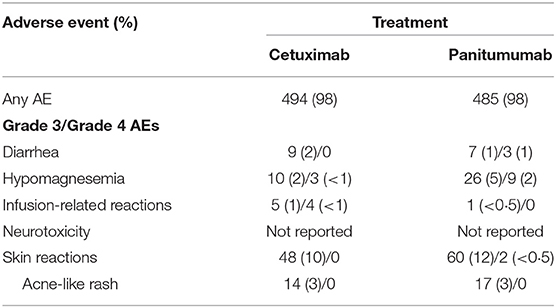
Table 4B. Evidence from the phase 3, head-to-head ASPECCT trial in 3L KRAS wt mCRC patients (101).
Although a direct comparison is confounded by the lack of AE rates for RAS wt patients in PRIME (the PRIME trial did not present rates of individual AEs for the RAS subgroup), the addition of cetuximab to chemotherapy was associated with an increased incidence of grade 3/4 infusion-related reactions, whereas the addition of panitumumab exacerbated the incidence of grade 3/4 diarrhea (65, 66, 69, 118). A meta analysis by Petrelli et al. concluded that while cetuximab and panitumumab have a similar burden of overall toxicity in terms of severe AEs, the individual safety profiles are distinct. Panitumumab was associated with a higher rates of grade 3/4 skin toxicities, hypomagnesemia, fatal AEs, and treatment discontinuations, while cetuximab was associated with a higher rates of skin rash, infusion reactions, and gastrointestinal toxicity (119). As noted in Petrelli et al., the third-line, anti-EGFR monotherapy trial ASPECCT also identified increased rates of grade 3/4 hypomagnesemia and decreased rates of infusion-related reactions with panitumumab compared with cetuximab (101). Finally, whereas the CRYSTAL and TAILOR trials reported no treatment-related grade 3/4 neurotoxicity occurring at a rate of ≥5% frequency in either arm, a rate of 16% was reported in the patient population of the PRIME trial (118). Petrelli et al. similarly identified a higher rate of grade 3/4 neurotoxicity in panitumumab trials than in cetuximab trials (119). The reasons for the increased incidence of (likely oxaliplatin-related) neurotoxicity (120) in panitumumab trials remain unknown.
Regarding chemotherapy backbones for the two mAbs, the selection of FOLFIRI vs. FOLFOX for first-line treatment can depend on which toxicity profile is likely to be more tolerable for the patient in question, because the two regimens are considered to have similar activities in mCRC (2). Therefore, differences in the toxicity profiles between the two chemotherapy backbones in combination with panitumumab vs. cetuximab are of substantial clinical relevance during treatment selection. However, it is worth noting that a meta-analysis by Teng et al. found a slight improvement in time to progression, and thus in OS, with FOLFIRI followed by FOLFOX compared with the reverse sequence (121). This finding reinforces the importance of treatment sequencing and how the differential findings with cetuximab and panitumumab can be applied, namely, that cetuximab has been shown to pair well with either FOLFOX or FOLFIRI vs. FOLFOX or FOLFIRI alone, whereas all available phase 3 data for panitumumab efficacy in first-line mCRC are in combination with FOLFOX. Notably, there are several small studies, although without comparator arms, that have provided evidence for the activity of panitumumab in combination with FOLFIRI in mCRC (87, 90).
Efficacy With Cetuximab and Panitumumab in Head and Neck Cancer
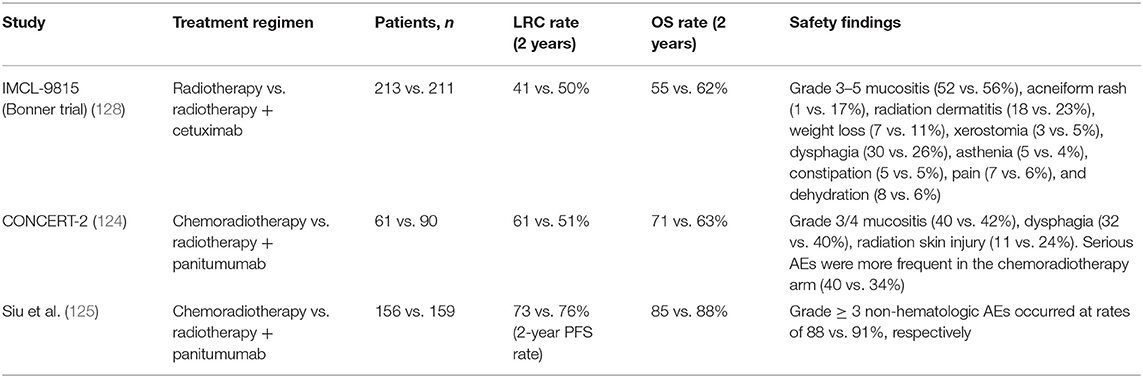
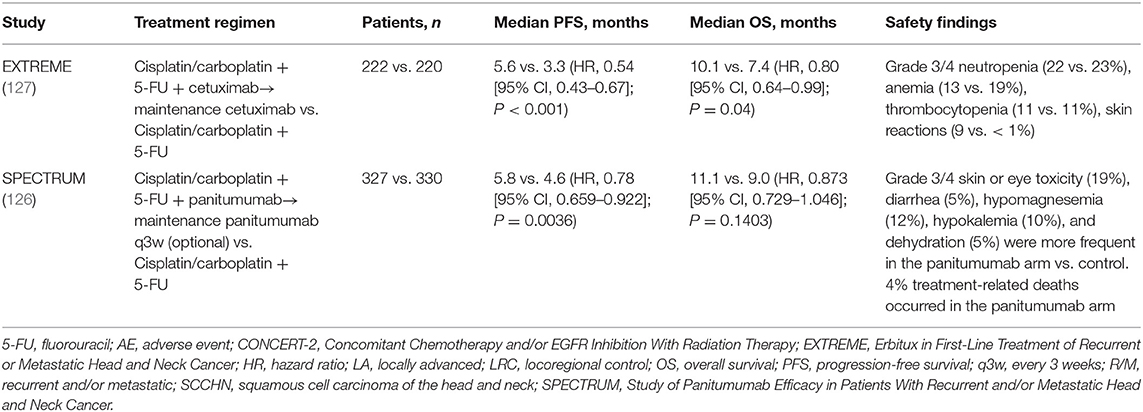
As previously mentioned, cetuximab has been approved for use in combination with radiotherapy in locally advanced SCCHN (LA SCCHN) and in combination with platinum and 5-FU, followed by cetuximab maintenance, for recurrent and/or metastatic SCCHN (R/M SCCHN) (122, 123). Panitumumab has been investigated in combination with radiotherapy in LA SCCHN but has failed to improve upon the current standard-of-care chemoradiotherapy treatment (124, 125), and it did not demonstrate a significant improvement in OS when added to platinum plus 5-FU chemotherapy in the R/M setting (126). A caveat is that panitumumab maintenance was optional in the SPECTRUM trial, following panitumumab plus platinum and 5-FU in patients with first-line R/M SCCHN, whereas cetuximab maintenance therapy in the EXTREME trial was given to all patients who achieved stable disease or a response during combination treatment (126, 127). Therefore, we are unable to directly compare the two agents in the SCCHN setting (Tables 5A,B). What can be said with certainty is that cetuximab is highly active in SCCHN, and proposed explanations include the increased potential contribution of cetuximab's immune actions in this tumor type, given the predominance of EGFR-overexpressing cells and immunologic sensitivity in head and neck tumors (25). Specifically, cetuximab's stimulation of ADCC and other immunostimulatory activities (DC maturation, T-cell recruitment to the tumor, increased antigen presentation, and cytotoxic T-cell priming) are dependent on cetuximab's simultaneous binding of the EGFR and the CD16 receptor on NK cells (25). Indeed, evidence has suggested the link between high baseline ADCC and EGFR overexpression and better outcomes with cetuximab plus radiotherapy but not with chemoradiotherapy (55). Thus, while it is difficult to prove the clinical impact of cetuximab-driven immunostimulation on tumor cell death, tumor shrinkage, and disease control, a wealth of evidence suggests that it is, in fact, a contributing factor to cetuximab's antitumor activity in SCCHN (25), and it may be the key differentiating aspect between cetuximab and panitumumab in head and neck cancer.
Potential of Anti-EGFR mAbs in Combination With Immunotherapy Regimens
Cetuximab and panitumumab behave differently, despite their therapeutic targeting of the same receptor; thus, available clinical data for one should not be applied to the other. Looking to the future in mCRC treatment, emerging immunotherapies have yet to demonstrate paradigm-shifting clinical activity in mismatch repair–proficient mCRC (129), suggesting that the way forward will continue to be combinatorial, including chemotherapy elements. In this respect, irinotecan's and oxaliplatin's synergistic effects with cetuximab (130–132) and possible differences from a treatment-sequencing standpoint suggest that cetuximab plus either FOLFIRI or FOLFOX is a suitable combination partner for checkpoint inhibitors and other immunotherapies. For example, cetuximab induces NK cell–mediated ADCC, resulting in increased immunogenic cell death, and cetuximab-treated cells have been shown to be more susceptible to phagocytosis by DCs. In the same study by Pozzi et al., even measurable immunogenic cell death occurred when CRC cell lines and mouse CRC models were co-treated with cetuximab plus FOLFIRI (31). Similarly, oxaliplatin has been shown to have some immunostimulatory properties, including immunogenic cell death (133–136) and the ability to prime tumors for checkpoint blockade in preclinical models (11, 136, 137). Cetuximab's known immune actions, including increasing immune infiltration and immune visibility of the tumor, suggest that it will be the more potent combination partner for either irinotecan- or oxaliplatin-based therapy, to which checkpoint inhibitors may theoretically be added to increase the immune antitumor response.
Conclusions and Future Directions for Cetuximab and Panitumumab
Cetuximab and panitumumab are both currently used to treat RAS wt mCRC. Clinical data for panitumumab in combination with chemotherapy is mostly limited to FOLFOX in the first-line setting, whereas cetuximab has demonstrated efficacy and safety in phase 3 first-line trials with both FOLFOX and FOLFIRI. Additionally, their combinability with FOLFIRI and known activity following prior bevacizumab treatment may have implications for optimal treatment sequencing in the continuum of care for mCRC.
Aside from the fact that panitumumab is a human mAb and cetuximab is a mouse/human chimeric mAb, the two anti-EGFR agents are composed of different IgG isotypes. Because cetuximab is an IgG1 mAb, it has additional immunogenic activity not demonstrated by panitumumab (IgG2). Cetuximab, unlike panitumumab, can prime the tumor microenvironment for an immune attack by enabling multiple processes, including ADCC and activation of innate and adaptive immune effector cells. Interestingly, both cetuximab and panitumumab improve outcomes in CRC. Despite extensive immune system activation induced by cetuximab, residual tumor-associated cells can prevent the final attack of cytotoxic T cells on the tumor by upregulation of PD-1, PD-L1, and CTLA-4 on their surface or by releasing cytokines such as TGF-β or chemokines such as CXCL12, which inactivate effector cells (25). Whether cetuximab will be clinically superior to panitumumab in the immunotherapy era remains to be determined by future clinical trials employing immune checkpoint inhibitors, which may complement the “immune priming” activity of cetuximab (and chemotherapy). We eagerly anticipate upcoming results from future and ongoing clinical trials.
Author Contributions
All authors contributed equally to the conception of the intellectual content, interpretation of the data, and writing of the manuscript. All authors also reviewed any revisions that were made and provided their final approval of the manuscript.
Conflict of Interest Statement
JG-F has had an advisory role and has received honoraria for talks from Amgen, Bayer, Sanofi, Merck Healthcare KGaA, Roche, Servier, Eli Lilly, Novartis, Pfizer, BMS, MSD, and AstraZeneca. YS has received honoraria for talks from Taiho Pharmaceutical, Chugai Pharmaceutical, Yakult Honsha, Takeda, Merck Serono, Bayer Yakuhin, and Sanofi. DA has had an advisory role and has received honoraria from Merck Healthcare KGaA, Teva, and Bayer. ZW has received consultation fees and honoraria from EMD Serono, Lilly, Genentech, and Novartis. PR and PW are employees of Merck Healthcare KGaA, Darmstadt, Germany. SS has had an advisory role and has received honoraria for talks from Amgen, Bayer, Eli Lilly, Merck Healthcare KGaA, Roche, Sanofi, and Takeda.
Acknowledgments
Medical writing assistance was provided by Ina Nikolaeva, Ph.D., of ClinicalThinking, Inc., Hamilton, NJ, USA, and funded by Merck Healthcare KGaA, Darmstadt, Germany.
References
1. Shim H. One target, different effects: a comparison of distinct therapeutic antibodies against the same targets. Exp Mol Med. (2011) 43:539–49. doi: 10.3858/emm.2011.43.10.063
2. Van Cutsem E, Cervantes A, Adam R, Sobrero A, Van Krieken JH, Aderka D, et al. ESMO consensus guidelines for the management of patients with metastatic colorectal cancer. Ann Oncol. (2016) 27:1386–422. doi: 10.1093/annonc/mdw235
3. NCCN Clinical Practice Guidelines in Oncology. Colon Cancer. V2.2018. Fort Washington, PA: National Comprehensive Cancer Network (2018).
4. Kim GP, Grothey A. Targeting colorectal cancer with human anti-EGFR monoclonocal antibodies: focus on panitumumab. Biologics. (2008) 2:223–8. doi: 10.2147/BTT.S1980
5. Misale S, Di Nicolantonio F, Sartore-Bianchi A, Siena S, Bardelli A. Resistance to anti-EGFR therapy in colorectal cancer: from heterogeneity to convergent evolution. Cancer Discov. (2014) 4:1269–80. doi: 10.1158/2159-8290.CD-14-0462
6. Erbitux (cetuximab). Summary of Product Characteristics. Available online at: https://www.ema.europa.eu/en/documents/product-information/erbitux-epar-product-information_en.pdf (accessed 22 July, 2019).
7. Vectibix (panitumumab). Summary of Product Characteristics. Available online at: https://www.ema.europa.eu/en/documents/product-information/vectibix-epar-product-information_en.pdf (accessed 22 July, 2019).
8. Amgen Press Release. (2016). Available online at: https://www.amgen.com/media/news-releases/2016/10/new-retrospective-analyses-confirm-vectibix-panitumumab-treatment-provided-survival-benefit-over-chemotherapy-with-or-without-bevacizumab-in-metastatic-colorectal-cancer-patients-with-tumors-of-leftsided-origin/ (accessed March 2018).
9. Merck aA Press Release. (2017). Available online at: https://www.ots.at/presseaussendung/OTE_20170302_OTE0001/nice-expands-positive-recommendation-for-erbitux-as-first-line-treatment-for-ras-wild-type-mcrc (accessed March 2018).
10. Park NJ, Wang X, Diaz A, Goos-Root DM, Bock C, Vaught JD, et al. Measurement of cetuximab and panitumumab-unbound serum EGFR extracellular domain using an assay based on slow off-rate modified aptamer (SOMAmer) reagents. PLoS ONE. (2013) 8:e71703. doi: 10.1371/journal.pone.0071703
11. Zhou Y, Goenaga AL, Harms BD, Zou H, Lou J, Conrad F, et al. Impact of intrinsic affinity on functional binding and biological activity of EGFR antibodies. Mol Cancer Ther. (2012) 11:1467–76. doi: 10.1158/1535-7163.MCT-11-1038
12. Lu Y, Li X, Liang K, Luwor R, Siddik ZH, Mills GB, et al. Epidermal growth factor receptor (EGFR) ubiquitination as a mechanism of acquired resistance escaping treatment by the anti-EGFR monoclonal antibody cetuximab. Cancer Res. (2007) 67:8240–7. doi: 10.1158/0008-5472.CAN-07-0589
13. Sickmier EA, Kurzeja RJ, Michelsen K, Vazir M, Yang E, Tasker AS. The panitumumab EGFR complex reveals a binding mechanism that overcomes cetuximab induced resistance. PLoS ONE. (2016) 11:e0163366. doi: 10.1371/journal.pone.0163366
14. Qin S, Xu JM, Wang L, Cheng Y, Liu TS, Chen J, et al. Impact of tumor epidermal growth factor receptor (EGFR) status on the outcomes of first-line FOLFOX-4 ± cetuximab in patients (pts) with RAS-wild-type (wt) metastatic colorectal cancer (mCRC) in the randomized phase 3 TAILOR trial. Ann Oncol. (2016) 27(suppl. 6):527. doi: 10.1093/annonc/mdw370.75
15. Voigt M, Braig F, Gothel M, Schulte A, Lamszus K, Bokemeyer C, et al. Functional dissection of the epidermal growth factor receptor epitopes targeted by panitumumab and cetuximab. Neoplasia. (2012) 14:1023–31. doi: 10.1593/neo.121242
16. Trivedi S, Concha-Benavente F, Srivastava RM, Jie HB, Gibson SP, Schmitt NC, et al. Immune biomarkers of anti-EGFR monoclonal antibody therapy. Ann Oncol. (2015) 26:40–7. doi: 10.1093/annonc/mdu156
17. Tabernero J, Ciardiello F, Rivera F, Rodriguez-Braun E, Ramos FJ, Martinelli E, et al. Cetuximab administered once every second week to patients with metastatic colorectal cancer: a two-part pharmacokinetic/pharmacodynamic phase I dose-escalation study. Ann Oncol. (2010) 21:1537–45. doi: 10.1093/annonc/mdp549
18. Krens LL, Baas JM, Guchelaar HJ, Gelderblom H. Pharmacokinetics and safety of panitumumab in a patient with chronic kidney disease. Cancer Chemother Pharmacol. (2018) 81:179–82. doi: 10.1007/s00280-017-3479-2
19. Lo L, Patel D, Townsend AR, Price TJ. Pharmacokinetic and pharmacodynamic evaluation of panitumumab in the treatment of colorectal cancer. Expert Opin Drug Metab Toxicol. (2015) 11:1907–24. doi: 10.1517/17425255.2015.1112787
20. Perrotte P, Matsumoto T, Inoue K, Kuniyasu H, Eve BY, Hicklin DJ, et al. Anti-epidermal growth factor receptor antibody C225 inhibits angiogenesis in human transitional cell carcinoma growing orthotopically in nude mice. Clin Cancer Res. (1999) 5:257–65.
21. Vincenzi B, Santini D, Russo A, Silletta M, Gavasci M, Battistoni F, et al. Angiogenesis modifications related with cetuximab plus irinotecan as anticancer treatment in advanced colorectal cancer patients. Ann Oncol. (2006) 17:835–41. doi: 10.1093/annonc/mdl031
22. Srivastava RM, Trivedi S, Concha-Benavente F, Hyun-Bae J, Wang L, Seethala RR, et al. STAT1-induced HLA class I upregulation enhances immunogenicity and clinical response to anti-EGFR mAb cetuximab therapy in HNC patients. Cancer Immunol Res. (2015) 3:936–45. doi: 10.1158/2326-6066.CIR-15-0053
23. Correale P, Botta C, Cusi MG, Del Vecchio MT, De Santi MM, Gori Savellini G, et al. Cetuximab +/- chemotherapy enhances dendritic cell-mediated phagocytosis of colon cancer cells and ignites a highly efficient colon cancer antigen-specific cytotoxic T-cell response in vitro. Int J Cancer. (2012) 130:1577–89. doi: 10.1002/ijc.26181
24. Srivastava RM, Lee SC, Andrade Filho PA, Lord CA, Jie HB, Davidson HC, et al. Cetuximab-activated natural killer and dendritic cells collaborate to trigger tumor antigen-specific T-cell immunity in head and neck cancer patients. Clin Cancer Res. (2013) 19:1858–72. doi: 10.1158/1078-0432.CCR-12-2426
25. Ferris RL, Lenz HJ, Trotta AM, Garcia-Foncillas J, Schulten J, Audhuy F, et al. Rationale for combination of therapeutic antibodies targeting tumor cells and immune checkpoint receptors: harnessing innate and adaptive immunity through IgG1 isotype immune effector stimulation. Cancer Treat Rev. (2018) 63:48–60. doi: 10.1016/j.ctrv.2017.11.008
26. Xynos ID, Karadima ML, Voutsas IF, Amptoulach S, Skopelitis E, Kosmas C, et al. Chemotherapy +/- cetuximab modulates peripheral immune responses in metastatic colorectal cancer. Oncology. (2013) 84:273–83. doi: 10.1159/000343282
27. Trivedi S, Srivastava RM, Concha-Benavente F, Ferrone S, Garcia-Bates TM, Li J, et al. Anti-EGFR targeted monoclonal antibody isotype influences anti-tumor cellular immunity in head and neck cancer patients. Clin Cancer Res. (2016) 22:5229–37. doi: 10.1158/1078-0432.CCR-15-2971
28. Trotta AM, Ottaiano A, Romano C, Nasti G, Nappi A, De Divitiis C, et al. Prospective evaluation of cetuximab-mediated antibody-dependent cell cytotoxicity in metastatic colorectal cancer patients predicts treatment efficacy. Cancer Immunol Res. (2016) 4:366–74. doi: 10.1158/2326-6066.CIR-15-0184
29. Holubec L, Polivka J Jr, Safanda M, Karas M, Liska V. The role of cetuximab in the induction of anticancer immune response in colorectal cancer treatment. Anticancer Res. (2016) 36:4421–6. doi: 10.21873/anticanres.10985
30. Chen S, Li X, Chen R, Yin M, Zheng Q. Cetuximab intensifies the ADCC activity of adoptive NK cells in a nude mouse colorectal cancer xenograft model. Oncol Lett. (2016) 12:1868–76. doi: 10.3892/ol.2016.4835
31. Pozzi C, Cuomo A, Spadoni I, Magni E, Silvola A, Conte A, et al. The EGFR-specific antibody cetuximab combined with chemotherapy triggers immunogenic cell death. Nat Med. (2016) 22:624–31. doi: 10.1038/nm.4078
32. Melero I, Rouzaut A, Motz GT, Coukos G. T-cell and NK-cell infiltration into solid tumors: a key limiting factor for efficacious cancer immunotherapy. Cancer Discov. (2014) 4:522–6. doi: 10.1158/2159-8290.CD-13-0985
33. Motz GT, Coukos G. The parallel lives of angiogenesis and immunosuppression: cancer and other tales. Nat Rev Immunol. (2011) 11:702–11. doi: 10.1038/nri3064
34. Motz GT, Santoro SP, Wang LP, Garrabrant T, Lastra RR, Hagemann IS, et al. Tumor endothelium FasL establishes a selective immune barrier promoting tolerance in tumors. Nat Med. (2014) 20:607–15. doi: 10.1038/nm.3541
35. Inoue Y, Hazama S, Suzuki N, Tokumitsu Y, Kanekiyo S, Tomochika S, et al. Cetuximab strongly enhances immune cell infiltration into liver metastatic sites in colorectal cancer. Cancer Sci. (2017) 108:455–60. doi: 10.1111/cas.13162
36. Maeda K, Hazama S, Tokuno K, Kan S, Maeda Y, Watanabe Y, et al. Impact of chemotherapy for colorectal cancer on regulatory T-cells and tumor immunity. Anticancer Res. (2011) 31:4569–74.
37. Vincent J, Mignot G, Chalmin F, Ladoire S, Bruchard M, Chevriaux A, et al. 5-Fluorouracil selectively kills tumor-associated myeloid-derived suppressor cells resulting in enhanced T cell-dependent antitumor immunity. Cancer Res. (2010) 70:3052–61. doi: 10.1158/0008-5472.CAN-09-3690
38. Lee SC, Srivastava RM, Lopez-Albaitero A, Ferrone S, Ferris RL. Natural killer (NK): dendritic cell (DC) cross talk induced by therapeutic monoclonal antibody triggers tumor antigen-specific T cell immunity. Immunol Res. (2011) 50:248–54. doi: 10.1007/s12026-011-8231-0
39. Huang SM, Li J, Harari PM. Molecular inhibition of angiogenesis and metastatic potential in human squamous cell carcinomas after epidermal growth factor receptor blockade. Mol Cancer Ther. (2002) 1:507–14.
40. Srivastava RM, Trivedi S, Concha-Benavente F, Gibson SP, Reeder C, Ferrone S, et al. CD137 Stimulation enhances cetuximab-induced natural killer: dendritic cell priming of antitumor T-cell immunity in patients with head and neck cancer. Clin Cancer Res. (2017) 23:707–16. doi: 10.1158/1078-0432.CCR-16-0879
41. Kohrt HE, Colevas AD, Houot R, Weiskopf K, Goldstein MJ, Lund P, et al. Targeting CD137 enhances the efficacy of cetuximab. J Clin Invest. (2014) 124:2668–82. doi: 10.1172/JCI73014
42. Lopez-Albaitero A, Mailliard R, Hackman T, Andrade Filho PA, Wang X, Gooding W, et al. Maturation pathways of dendritic cells determine TAP1 and TAP2 levels and cross-presenting function. J Immunother. (2009) 32:465–73. doi: 10.1097/CJI.0b013e3181a1c24e
43. Botta C, Bestoso E, Apollinari S, Cusi MG, Pastina P, Abbruzzese A, et al. Immune-modulating effects of the newest cetuximab-based chemoimmunotherapy regimen in advanced colorectal cancer patients. J Immunother. (2012) 35:440–7. doi: 10.1097/CJI.0b013e31825943aa
44. Schoppy DW, Sunwoo JB. Immunotherapy for head and neck squamous cell carcinoma. Hematol Oncol Clin North Am. (2015) 29:1033–43. doi: 10.1016/j.hoc.2015.07.009
45. Bhat R, Watzl C. Serial killing of tumor cells by human natural killer cells-enhancement by therapeutic antibodies. PLoS ONE. (2007) 2:e326. doi: 10.1371/journal.pone.0000326
46. Pahl JH, Ruslan SE, Buddingh EP, Santos SJ, Szuhai K, Serra M, et al. Anti-EGFR antibody cetuximab enhances the cytolytic activity of natural killer cells toward osteosarcoma. Clin Cancer Res. (2012) 18:432–41. doi: 10.1158/1078-0432.CCR-11-2277
47. Krawczyk PA, Kowalski DM. Genetic and immune factors underlying the efficacy of cetuximab and panitumumab in the treatment of patients with metastatic colorectal cancer. Contemp Oncol (Pozn). (2014) 18:7–16. doi: 10.5114/wo.2013.38566
48. Kubach J, Hubo M, Amendt C, Stroh C, Jonuleit H. IgG1 anti-epidermal growth factor receptor antibodies induce CD8-dependent antitumor activity. Int J Cancer. (2015) 136:821–30. doi: 10.1002/ijc.29037
49. Hodi FS, O'Day SJ, McDermott DF, Weber RW, Sosman JA, Haanen JB, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. (2010) 363:711–23. doi: 10.1056/NEJMoa1003466
50. Lopez-Albaitero A, Lee SC, Morgan S, Grandis JR, Gooding WE, Ferrone S, et al. Role of polymorphic Fc gamma receptor IIIa and EGFR expression level in cetuximab mediated, NK cell dependent in vitro cytotoxicity of head and neck squamous cell carcinoma cells. Cancer Immunol Immunother. (2009) 58:1853–64. doi: 10.1007/s00262-009-0697-4
51. McLaughlin P, Grillo-Lopez AJ, Link BK, Levy R, Czuczman MS, Williams ME, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J Clin Oncol. (1998) 16:2825–33. doi: 10.1200/JCO.1998.16.8.2825
52. Monteverde M, Milano G, Strola G, Maffi M, Lattanzio L, Vivenza D, et al. The relevance of ADCC for EGFR targeting: a review of the literature and a clinically-applicable method of assessment in patients. Crit Rev Oncol Hematol. (2015) 95:179–90. doi: 10.1016/j.critrevonc.2015.02.014
53. Hatjiharissi E, Xu L, Santos DD, Hunter ZR, Ciccarelli BT, Verselis S, et al. Increased natural killer cell expression of CD16, augmented binding and ADCC activity to rituximab among individuals expressing the Fc{gamma}RIIIa-158 V/V and V/F polymorphism. Blood. (2007) 110:2561–4. doi: 10.1182/blood-2007-01-070656
54. Jochems C, Hodge JW, Fantini M, Fujii R, Morillon YM II, Greiner JW, et al. An NK cell line (haNK) expressing high levels of granzyme and engineered to express the high affinity CD16 allele. Oncotarget. (2016) 7:86359–73. doi: 10.18632/oncotarget.13411
55. Lattanzio L, Denaro N, Vivenza D, Varamo C, Strola G, Fortunato M, et al. Elevated basal antibody-dependent cell-mediated cytotoxicity (ADCC) and high epidermal growth factor receptor (EGFR) expression predict favourable outcome in patients with locally advanced head and neck cancer treated with cetuximab and radiotherapy. Cancer Immunol Immunother. (2017) 66:573–9. doi: 10.1007/s00262-017-1960-8
56. Dechant M, Weisner W, Berger S, Peipp M, Beyer T, Schneider-Merck T, et al. Complement-dependent tumor cell lysis triggered by combinations of epidermal growth factor receptor antibodies. Cancer Res. (2008) 68:4998–5003. doi: 10.1158/0008-5472.CAN-07-6226
57. Diebolder CA, Beurskens FJ, de Jong RN, Koning RI, Strumane K, Lindorfer MA, et al. Complement is activated by IgG hexamers assembled at the cell surface. Science. (2014) 343:1260–3. doi: 10.1126/science.1248943
58. De Roock W, Claes B, Bernasconi D, De Schutter J, Biesmans B, Fountzilas G, et al. Effects of KRAS, BRAF, NRAS, and PIK3CA mutations on the efficacy of cetuximab plus chemotherapy in chemotherapy-refractory metastatic colorectal cancer: a retrospective consortium analysis. Lancet Oncol. (2010) 11:753–62. doi: 10.1016/S1470-2045(10)70130-3
59. Foltran L, De Maglio G, Pella N, Ermacora P, Aprile G, Masiero E, et al. Prognostic role of KRAS, NRAS, BRAF and PIK3CA mutations in advanced colorectal cancer. Future Oncol. (2015) 11:629–40. doi: 10.2217/fon.14.279
60. Arena S, Bellosillo B, Siravegna G, Martinez A, Canadas I, Lazzari L, et al. Emergence of multiple EGFR extracellular mutations during cetuximab treatment in colorectal cancer. Clin Cancer Res. (2015) 21:2157–66. doi: 10.1158/1078-0432.CCR-14-2821
61. Bouchahda M, Karaboue A, Saffroy R, Innominato P, Gorden L, Guettier C, et al. Acquired KRAS mutations during progression of colorectal cancer metastases: possible implications for therapy and prognosis. Cancer Chemother Pharmacol. (2010) 66:605–9. doi: 10.1007/s00280-010-1298-9
62. Siravegna G, Mussolin B, Buscarino M, Corti G, Cassingena A, Crisafulli G, et al. Clonal evolution and resistance to EGFR blockade in the blood of colorectal cancer patients. Nat Med. (2015) 21:827. doi: 10.1038/nm0715-827b
63. Schwartzberg LS, Rivera F, Karthaus M, Fasola G, Canon JL, Hecht JR, et al. PEAK: a randomized, multicenter phase II study of panitumumab plus modified fluorouracil, leucovorin, and oxaliplatin (mFOLFOX6) or bevacizumab plus mFOLFOX6 in patients with previously untreated, unresectable, wild-type KRAS exon 2 metastatic colorectal cancer. J Clin Oncol. (2014) 32:2240–7. doi: 10.1200/JCO.2013.53.2473
64. Peeters M, Oliner K, Parker A, Siena S, Van Cutsem E, Huang J, et al. Massively parallel tumor multigene sequencing to evaluate response to panitumumab in a randomized phase III study of metastatic colorectal cancer. Clin Cancer Res. (2013) 19:1902–12. doi: 10.1158/1078-0432.CCR-12-1913
65. Douillard JY, Oliner KS, Siena S, Tabernero J, Burkes R, Barugel M, et al. Panitumumab-FOLFOX4 treatment and RAS mutations in colorectal cancer. N Engl J Med. (2013) 369:1023–34. doi: 10.1056/NEJMoa1305275
66. Van Cutsem E, Lenz HJ, Köhne CH, Heinemann V, Tejpar S, Melezinek I, et al. Fluorouracil, leucovorin, and irinotecan plus cetuximab treatment and RAS mutations in colorectal cancer. J Clin Oncol. (2015) 33:692–700. doi: 10.1200/JCO.2014.59.4812
67. Stintzing S, Modest DP, Rossius L, Lerch MM, von Weikersthal LF, Decker T, et al. FOLFIRI plus cetuximab versus FOLFIRI plus bevacizumab for metastatic colorectal cancer (FIRE-3): a post-hoc analysis of tumour dynamics in the final RAS wild-type subgroup of this randomised open-label phase 3 trial. Lancet Oncol. (2016) 17:1426–34. doi: 10.1016/S1470-2045(16)30269-8
68. Arnold D, Lueza B, Douillard JY, Peeters M, Lenz HJ, Venook A, et al. Prognostic and predictive value of primary tumour side in patients with RAS wild-type metastatic colorectal cancer treated with chemotherapy and EGFR directed antibodies in six randomised trials. Ann Oncol. (2017) 28:1713–29. doi: 10.1093/annonc/mdx175
69. Qin S, Li J, Wang L, Xu J, Cheng Y, Bai Y, et al. Efficacy and tolerability of first-line cetuximab plus leucovorin, fluorouracil, and oxaliplatin (FOLFOX-4) versus FOLFOX-4 in patients with RAS wild-type metastatic colorectal cancer: the open-label, randomized, phase III TAILOR trial. J Clin Oncol. (2018) 36:3031–9. doi: 10.1200/JCO.2018.78.3183
70. Misale S, Yaeger R, Hobor S, Scala E, Janakiraman M, Liska D, et al. Emergence of KRAS mutations and acquired resistance to anti-EGFR therapy in colorectal cancer. Nature. (2012) 486:532–6. doi: 10.1038/nature11156
71. Santini D, Vincenzi B, Addeo R, Garufi C, Masi G, Scartozzi M, et al. Cetuximab rechallenge in metastatic colorectal cancer patients: how to come away from acquired resistance? Ann Oncol. (2012) 23:2313–8. doi: 10.1093/annonc/mdr623
72. Liu X, George GC, Tsimberidou AM, Naing A, Wheler JJ, Kopetz S, et al. Retreatment with anti-EGFR based therapies in metastatic colorectal cancer: impact of intervening time interval and prior anti-EGFR response. BMC Cancer. (2015) 15:713. doi: 10.1186/s12885-015-1701-3
73. Rossini D, Cremolini C, Conca E, Del Re M, Busico A, Pietrantonio F, et al. Liquid biopsy to predict benefit from rechallenge with cetuximab (cet) + irinotecan (iri) in RAS/BRAF wild-type metastatic colorectal cancer patients (pts) with acquired resistance to first-line cet+iri: final results and translational analyses of the CRICKET study by GONO. J Clin Oncol. (2018) 36(suppl):12007. doi: 10.1200/JCO.2018.36.15_suppl.12007
74. Sanchez-Martin FJ, Bellosillo B, Gelabert-Baldrich M, Dalmases A, Canadas I, Vidal J, et al. The first-in-class anti-EGFR antibody mixture Sym004 overcomes cetuximab resistance mediated by EGFR extracellular domain mutations in colorectal cancer. Clin Cancer Res. (2016) 22:3260–7. doi: 10.1158/1078-0432.CCR-15-2400
75. Montagut C, Dalmases A, Bellosillo B, Crespo M, Pairet S, Iglesias M, et al. Identification of a mutation in the extracellular domain of the Epidermal Growth Factor Receptor conferring cetuximab resistance in colorectal cancer. Nat Med. (2012) 18:221–3. doi: 10.1038/nm.2609
76. Van Emburgh BO, Arena S, Siravegna G, Lazzari L, Crisafulli G, Corti G, et al. Acquired RAS or EGFR mutations and duration of response to EGFR blockade in colorectal cancer. Nat Commun. (2016) 7:13665. doi: 10.1038/ncomms13665
77. Braig F, Marz M, Schieferdecker A, Schulte A, Voigt M, Stein A, et al. Epidermal growth factor receptor mutation mediates cross-resistance to panitumumab and cetuximab in gastrointestinal cancer. Oncotarget. (2015) 6:12035–47. doi: 10.18632/oncotarget.3574
78. Venook AP, Niedzwiecki D, Lenz HJ, Innocenti F, Fruth B, Meyerhardt JA, et al. Effect of first-line chemotherapy combined with cetuximab or bevacizumab on overall survival in patients with KRAS wild-type advanced or metastatic colorectal cancer: a randomized clinical trial. JAMA. (2017) 317:2392–401. doi: 10.1001/jama.2017.7105
79. Khattak MA, Martin H, Davidson A, Phillips M. Role of first-line anti-epidermal growth factor receptor therapy compared with anti-vascular endothelial growth factor therapy in advanced colorectal cancer: a meta-analysis of randomized clinical trials. Clin Colorectal Cancer. (2015) 14:81–90. doi: 10.1016/j.clcc.2014.12.011
80. Venook AP, Neidzwiecki D, Innocenti F, Fruth B, Greene C, O'Neil BH, et al. Impact of primary (1°) tumor location on overall survival (OS) and progression-free survival (PFS) in patients (pts) with metastatic colorectal cancer (mCRC): analysis of CALGB/SWOG 80405 (Alliance). In: American Society of Clinical Oncology 2016 Annual Meeting; June 03–07. Chicago, IL (2016). doi: 10.1200/JCO.2016.34.15_suppl.3504
81. Maughan TS, Adams RA, Smith CG, Meade AM, Seymour MT, Wilson RH, et al. Addition of cetuximab to oxaliplatin-based first-line combination chemotherapy for treatment of advanced colorectal cancer: results of the randomised phase 3 MRC COIN trial. Lancet. (2011) 377:2103–14. doi: 10.1016/j.yonc.2011.08.011
82. Bokemeyer C, Bondarenko I, Makhson A, Hartmann JT, Aparicio J, de Braud F, et al. Fluorouracil, leucovorin, and oxaliplatin with and without cetuximab in the first-line treatment of metastatic colorectal cancer. J Clin Oncol. (2009) 27:663–71. doi: 10.1200/JCO.2008.20.8397
83. Bokemeyer C, Köhne CH, Ciardiello F, Lenz HJ, Heinemann V, Klinkhardt U, et al. FOLFOX4 plus cetuximab treatment and RAS mutations in colorectal cancer. Eur J Cancer. (2015) 51:1243–52. doi: 10.1016/j.ejca.2015.04.007
84. Xu JM, Ren L, Wei Y, Zheng P, Ye LC, Feng QY, et al. Effects of beyond KRAS mutations on the efficacy of cetuximab plus chemotherapy for patients with unresectable colorectal liver-limited metastases (BELIEF): a prospective-retrospective biomarker analysis of a Chinese trial. Eur J Cancer. (2016) 51:S369. doi: 10.1016/S0959-8049(16)31039-5
85. Antoniotti C, Cremolini C, Loupakis F, Bergamo F, Grande R, Tonini G. Modified FOLFOXIRI (mFOLFOXIRI) plus cetuximab (cet), followed by cet or bevacizumab (bev) maintenance, in RAS/BRAF wild-type (wt) metastatic colorectal cancer (mCRC): results of the phase II randomized MACBETH trial by GONO. J Clin Oncol. (2016) 34(suppl):3543. doi: 10.1200/JCO.2016.34.15_suppl.3543
86. Cremolini C, Antoniotti C, Lonardi S, Aprile G, Bergamo F, Masi G, et al. Activity and safety of cetuximab plus modified FOLFOXIRI followed by maintenance with cetuximab or bevacizumab for RAS and BRAF wild-type metastatic colorectal cancer: a randomized phase 2 clinical trial. JAMA Oncol. (2018) 4:529–36. doi: 10.1001/jamaoncol.2017.5314
87. Carrato A, Abad A, Massuti B, Gravalos C, Escudero P, Longo-Munoz F, et al. First-line panitumumab plus FOLFOX4 or FOLFIRI in colorectal cancer with multiple or unresectable liver metastases: a randomised, phase II trial (PLANET-TTD). Eur J Cancer. (2017) 81:191–202. doi: 10.1016/j.ejca.2017.04.024
88. Geissler M, Martens UM, Knorrenschield R, Greeve J, Florschuetz A, Tannapfel A, et al. mFOLFOXIRI + panitumumab versus FOLFOXIRI as first-line treatment in patients with RAS wild-type metastatic colorectal cancer m(CRC): a randomized phase II VOLFI trial of the AIO (AIO-KRK0109). Ann Oncol. (2017) 28(suppl. 5):475O. doi: 10.1093/annonc/mdx393.002
89. Saridaki Z, Androulakis N, Vardakis N, Vamvakas L, Kabouraki E, Kalbakis K, et al. A triplet combination with irinotecan (CPT-11), oxaliplatin (LOHP), continuous infusion 5-fluorouracil and leucovorin (FOLFOXIRI) plus cetuximab as first-line treatment in KRAS wt, metastatic colorectal cancer: a pilot phase II trial. Br J Cancer. (2012) 107:1932–7. doi: 10.1038/bjc.2012.509
90. Köhne CH, Hofheinz R, Mineur L, Letocha H, Greil R, Thaler J, et al. First-line panitumumab plus irinotecan/5-fluorouracil/leucovorin treatment in patients with metastatic colorectal cancer. J Cancer Res Clin Oncol. (2012) 138:65–72. doi: 10.1007/s00432-011-1061-6
91. Peeters M, Price TJ, Cervantes A, Sobrero AF, Ducreux M, Hotko Y, et al. Final results from a randomized phase 3 study of FOLFIRI {+/−} panitumumab for second-line treatment of metastatic colorectal cancer. Ann Oncol. (2014) 25:107–16. doi: 10.1093/annonc/mdt523
92. Fornaro L, Lonardi S, Masi G, Loupakis F, Bergamo F, Salvatore L, et al. FOLFOXIRI in combination with panitumumab as first-line treatment in quadruple wild-type (KRAS, NRAS, HRAS, BRAF) metastatic colorectal cancer patients: a phase II trial by the Gruppo Oncologico Nord Ovest (GONO). Ann Oncol. (2013) 24:2062–7. doi: 10.1093/annonc/mdt165
93. Benedix F, Kube R, Meyer F, Schmidt U, Gastinger I, Lippert H, et al. Comparison of 17,641 patients with right- and left-sided colon cancer: differences in epidemiology, perioperative course, histology, and survival. Dis Colon Rectum. (2010) 53:57–64. doi: 10.1007/DCR.0b013e3181c703a4
94. Tejpar S, Stintzing S, Ciardiello F, Tabernero J, Van Cutsem E, Beier F, et al. Prognostic and predictive relevance of primary tumor location in patients with RAS wild-type metastatic colorectal cancer: retrospective analyses of the CRYSTAL and FIRE-3 trials. JAMA Oncol. (2017) 3:194–201. doi: 10.1001/jamaoncol.2016.3797
95. Sagawa T, Hamaguchi K, Sakurada A, Tamura F, Hayashi T, Fujikawa K, et al. Primary tumor location as a prognostic and predictive factor in metastatic colorectal cancer (mCRC) treated with chemotherapy plus cetuximab: a retrospective analysis. J Clin Oncol. (2017) 35(suppl):711. doi: 10.1200/JCO.2017.35.4_suppl.711
96. Boeckx N, Koukakis R, Op de Beeck K, Rolfo C, Van Camp G, Siena S, et al. Primary tumor sidedness has an impact on prognosis and treatment outcome in metastatic colorectal cancer: results from two randomized first-line panitumumab studies. Ann Oncol. (2017) 28:1862–8. doi: 10.1093/annonc/mdx119
97. Wang Z-X, He M-M, Wang Y-N, Wang F, Xu R-H. Predictive value of primary tumor location (TL) in patients (pts) with pan-RAS wild-type (wt) metastatic colorectal cancer (mCRC) receiving chemotherapy (CTX) ± cetuximab or panitumumab (C/P): an updated meta-analysis. J Clin Oncol. (2018) 36(suppl):830. doi: 10.1200/JCO.2018.36.4_suppl.830
98. Sunakawa Y, Tsuji A, Fujii M, Ichikawa W. No benefit from the addition of anti-EGFR antibody in all right-sided metastatic colorectal cancer? Ann Oncol. (2017) 28:2030–1. doi: 10.1093/annonc/mdx231
99. Hong YS, Kim HJ, Park SJ, Kim KP, Lee JL, Park JH, et al. Second-line cetuximab/irinotecan versus oxaliplatin/fluoropyrimidines for metastatic colorectal cancer with wild-type KRAS. Cancer Sci. (2013) 104:473–80. doi: 10.1111/cas.12098
100. Carrato A, Gomez A, Escudero P, Chaves M, Rivera F, Marcuello E, et al. Panitumumab and irinotecan every 3 weeks is an active and convenient regimen for second-line treatment of patients with wild-type K-RAS metastatic colorectal cancer. Clin Transl Oncol. (2013) 15:705–11. doi: 10.1007/s12094-012-0993-x
101. Price T, Kim TW, Li J, Cascinu S, Ruff P, Suresh AS, et al. Final results and outcomes by prior bevacizumab exposure, skin toxicity, and hypomagnesaemia from ASPECCT: randomized phase 3 non-inferiority study of panitumumab versus cetuximab in chemorefractory wild-type KRAS exon 2 metastatic colorectal cancer. Eur J Cancer. (2016) 68:51–9. doi: 10.1016/j.ejca.2016.08.010
102. Sugimoto N, Sakai D, Tamura T, Hara H, Nishina T, Esaki T, et al. Randomized phase II study of panitumumab (Pmab) + irinotecan (CPT-11) versus cetuximab (Cmab) + CPT-11 in patients (pts) with KRAS wild-type (WT) metastatic colorectal cancer (mCRC) after fluoropyrimidine (FU), CPT-11, and oxaliplatin (L-OHP) failure: WJOG6510G. J Clin Oncol. (2017) 35(suppl):661. doi: 10.1200/JCO.2017.35.4_suppl.661
103. Andre T, Blons H, Mabro M, Chibaudel B, Bachet JB, Tournigand C, et al. Panitumumab combined with irinotecan for patients with KRAS wild-type metastatic colorectal cancer refractory to standard chemotherapy: a GERCOR efficacy, tolerance, and translational molecular study. Ann Oncol. (2013) 24:412–9. doi: 10.1093/annonc/mds465
104. Nishi T, Hamamoto Y, Nagase M, Denda T, Yamaguchi K, Amagai K, et al. Phase II trial of panitumumab with irinotecan as salvage therapy for patients with advanced or recurrent colorectal cancer (TOPIC study). Oncol Lett. (2016) 11:4049–54. doi: 10.3892/ol.2016.4532
105. Gil Delgado M, Spano JP, Khayat D. Cetuximab plus irinotecan in refractory colorectal cancer patients. Expert Rev Anticancer Ther. (2007) 7:407–13. doi: 10.1586/14737140.7.4.407
106. Lim R, Sun Y, Im SA, Hsieh RK, Yau TK, Bonaventura A, et al. Cetuximab plus irinotecan in pretreated metastatic colorectal cancer patients: the ELSIE study. World J Gastroenterol. (2011) 17:1879–88. doi: 10.3748/wjg.v17.i14.1879
107. Pantelic A, Markovic M, Pavlovic M, Jancic S. Cetuximab in third-line therapy of patients with metastatic colorectal cancer: a single institution experience. J BUON. (2016) 21:70–9.
108. Buzaid AC, Mathias Cde C, Perazzo F, Simon SD, Fein L, Hidalgo J, et al. Cetuximab Plus irinotecan in pretreated metastatic colorectal cancer progressing on irinotecan: the LABEL study. Clin Colorectal Cancer. (2010) 9:282–9. doi: 10.3816/CCC.2010.n.041
109. Van Cutsem E, Tejpar S, Vanbeckevoort D, Peeters M, Humblet Y, Gelderblom H, et al. Intrapatient cetuximab dose escalation in metastatic colorectal cancer according to the grade of early skin reactions: the randomized EVEREST study. J Clin Oncol. (2012) 30. doi: 10.1200/JCO.2011.40.9243
110. Wilke H, Glynne-Jones R, Thaler J, Adenis A, Preusser P, Aguilar EA, et al. Cetuximab plus irinotecan in heavily pretreated metastatic colorectal cancer progressing on irinotecan: MABEL Study. J Clin Oncol. (2008) 26:5335–43. doi: 10.1200/JCO.2008.16.3758
111. Karapetis CS, Khambata-Ford S, Jonker DJ, O'Callaghan CJ, Tu D, Tebbutt NC, et al. K-ras mutations and benefit from cetuximab in Advanced Colorectal Cancer. N Engl J Med. (2008) 359:1757–65. doi: 10.1056/NEJMoa0804385
112. Sobrero AF, Lenz H-J, Eng C, Scheithauer W, Middleton GW, Chen W-F, et al. Retrospective analysis of overall survival (OS) by subsequent therapy in patients (pts) with RAS wild-type (wt) metastatic colorectal cancer (mCRC) receiving irinotecan ± cetuximab in the EPIC study. J Clin Oncol. (2019) 37(suppl):3580. doi: 10.1200/JCO.2019.37.15_suppl.3580
113. Modest D, Stintzing S, Fischer von Weikersthal L, Decker T, Kiani A, Vehling-Kaiser U, et al. 2nd-line therapies after 1st-line therapy with FOLFIRI in combination with cetuximab or bevacizumab in patients with KRAS wild-type metastatic colorectal cancer (mCRC) - analysis of the AIO KRK 0306 (FIRE 3) - trial. Ann Oncol. (2014) 25(suppl 2):0018. doi: 10.1093/annonc/mdu193.18
114. Taniguchi H, Komori A, Narita Y, Kadowaki S, Ura T, Andoh M, et al. A short interval between bevacizumab and anti-epithelial growth factor receptor therapy interferes with efficacy of subsequent anti-EGFR therapy for refractory colorectal cancer. Jpn J Clin Oncol. (2016) 46:228–33. doi: 10.1093/jjco/hyv193
115. Nishina T, Takano Y, Denda T, Yasui H, Takeda K, Ura T, et al. A phase II clinical study of mFOLFOX6 plus bevacizumab as first-line therapy for Japanese advanced/recurrent colorectal cancer patients. Jpn J Clin Oncol. (2013) 43:1080–6. doi: 10.1093/jjco/hyt127
116. Nishina T, Taniguchi H, Sakai D, Kawakami H, Sugimoto N, Hara H, et al. Analysis of RAS/BRAF mutations in a randomized phase II WJOG6510G study of panitumumab plus irinotecan versus cetuximab plus irinotecan in chemorefractory metastatic colorectal cancer. J Clin Oncol. (2018) 36(suppl):624. doi: 10.1200/JCO.2018.36.4_suppl.624
117. Taniguchi H, Yamanaka T, Sakai D, Yamazaki K, Muro K, Peeters M, et al. Panitumumab versus cetuximab in patients with wild-type KRAS exon 2 metastatic colorectal cancer who received prior bevacizumab therapy: a combined analysis of individual patient data from ASPECCT and WJOG6510G. J Clin Oncol. (2018) 36(suppl):745. doi: 10.1200/JCO.2018.36.4_suppl.745
118. Douillard JY, Siena S, Cassidy J, Tabernero J, Burkes R, Barugel M, et al. Final results from PRIME: randomized phase III study of panitumumab with FOLFOX4 for first-line treatment of metastatic colorectal cancer. Ann Oncol. (2014) 25:1346–55. doi: 10.1093/annonc/mdu141
119. Petrelli F, Ardito R, Ghidini A, Zaniboni A, Ghidini M, Barni S, et al. Different toxicity of cetuximab and panitumumab in metastatic colorectal cancer treatment: a systematic review and meta-analysis. Oncology. (2018) 94:191–9. doi: 10.1159/000486338
120. Avan A, Postma TJ, Ceresa C, Avan A, Cavaletti G, Giovannetti E, et al. Platinum-induced neurotoxicity and preventive strategies: past, present, and future. Oncologist. (2015) 20:411–32. doi: 10.1634/theoncologist.2014-0044
121. Teng CL, Wang CY, Chen YH, Lin CH, Hwang WL. Optimal sequence of irinotecan and oxaliplatin-based regimens in metastatic colorectal cancer: a population-based observational study. PLoS ONE. (2015) 10:e0135673. doi: 10.1371/journal.pone.0135673
122. NCCN Clinical Practice Guidelines in Oncology. Head and Neck Cancers. V2.2017. Fort Washington, PA: National Comprehensive Cancer Network (2017).
123. Gregoire V, Lefebvre JL, Licitra L, Felip E, EHNS-ESMO-ESTRO Guidelines Working Group. Squamous cell carcinoma of the head and neck: EHNS-ESMO-ESTRO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol. (2010) 21(Suppl 5):v184–6. doi: 10.1093/annonc/mdq185
124. Giralt T, Trigo J, Nuyts S. A phase 2, randomized trial (CONCERT-2) of panitumumab plus radiotherapy compared with chemoradiotherapy in patients with unresected, locally advanced squamous cell carcinoma of the head and neck. Lancet Oncol. (2014) 16:221–32. doi: 10.1016/S1470-2045(14)71200-8
125. Siu LL, Waldron JN, Chen BE, Winquist E, Wright JR, Nabid A, et al. Phase III randomized trial of standard fractionation radiotherapy (SFX) with concurrent cisplatin (CIS) versus accelerated fractionation radiotherapy (AFX) with panitumumab (PMab) in patients (pts) with locoregionally advanced squamous cell carcinoma of the head and neck (LA-SCCHN): NCIC Clinical Trials Group HN.6 trial. J Clin Oncol. (2015) 33(suppl):6000. doi: 10.1200/jco.2015.33.15_suppl.6000
126. Vermorken JB, Stohlmacher-Williams J, Davidenko I, Licitra L, Winquist E, Villanueva C, et al. Cisplatin and fluorouracil with or without panitumumab in patients with recurrent or metastatic squamous-cell carcinoma of the head and neck (SPECTRUM): an open-label phase 3 randomised trial. Lancet Oncol. (2013) 14:697–710. doi: 10.1016/S1470-2045(13)70181-5
127. Vermorken JB, Mesia R, Rivera F, Remenar E, Kawecki A, Rottey S, et al. Platinum-based chemotherapy plus cetuximab in head and neck cancer. N Engl J Med. (2008) 359:1116–27. doi: 10.1056/NEJMoa0802656
128. Bonner JA, Harari PM, Giralt J, Azarnia N, Shin DM, Cohen RB, et al. Radiotherapy plus cetuximab for squamous-cell carcinoma of the head and neck. N Engl J Med. (2006) 354:567–78. doi: 10.1056/NEJMoa053422
129. Toh JW, de Souza P, Lim SH, Singh P, Chua W, Ng W, et al. The potential value of immunotherapy in colorectal cancers: review of the evidence for programmed death-1 inhibitor therapy. Clin Colorectal Cancer. (2016) 15:285–91. doi: 10.1016/j.clcc.2016.07.007
130. Liu X, Guo WJ, Zhang XW, Cai X, Tian S, Li J. Cetuximab enhances the activities of irinotecan on gastric cancer cell lines through downregulating the EGFR pathway upregulated by irinotecan. Cancer Chemother Pharmacol. (2011) 68:871–8. doi: 10.1007/s00280-011-1559-2
131. Mahtani RL, Macdonald JS. Synergy between cetuximab and chemotherapy in tumors of the gastrointestinal tract. Oncologist. (2008) 13:39–50. doi: 10.1634/theoncologist.2006-0049
132. Morelli MP, Cascone T, Troiani T, De Vita F, Orditura M, Laus G, et al. Sequence-dependent antiproliferative effects of cytotoxic drugs and epidermal growth factor receptor inhibitors. Ann Oncol. (2005) 16(Suppl 4):iv61–8. doi: 10.1093/annonc/mdi910
133. Garg AD, More S, Rufo N, Mece O, Sassano ML, Agostinis P, et al. Trial watch: immunogenic cell death induction by anticancer chemotherapeutics. Oncoimmunology. (2017) 6:e1386829. doi: 10.1080/2162402X.2017.1386829
134. Di Blasio S, Wortel IM, van Bladel DA, de Vries LE, Duiveman-de Boer T, Worah K, et al. Human CD1c(+) DCs are critical cellular mediators of immune responses induced by immunogenic cell death. Oncoimmunology. (2016) 5:e1192739. doi: 10.1080/2162402X.2016.1192739
135. Hato SV, Khong A, de Vries IJ, Lesterhuis WJ. Molecular pathways: the immunogenic effects of platinum-based chemotherapeutics. Clin Cancer Res. (2014) 20:2831–7. doi: 10.1158/1078-0432.CCR-13-3141
136. Pfirschke C, Engblom C, Rickelt S, Cortez-Retamozo V, Garris C, Pucci F, et al. Immunogenic chemotherapy sensitizes tumors to checkpoint blockade therapy. Immunity. (2016) 44:343–54. doi: 10.1016/j.immuni.2015.11.024
Keywords: colorectal cancer, cetuximab, panitumumab, FOLFOX, FOLFIRI, antibody-dependent cell-mediated cytotoxicity
Citation: García-Foncillas J, Sunakawa Y, Aderka D, Wainberg Z, Ronga P, Witzler P and Stintzing S (2019) Distinguishing Features of Cetuximab and Panitumumab in Colorectal Cancer and Other Solid Tumors. Front. Oncol. 9:849. doi: 10.3389/fonc.2019.00849
Received: 25 April 2019; Accepted: 19 August 2019;
Published: 20 September 2019.
Edited by:
Arndt Vogel, Hannover Medical School, GermanyReviewed by:
Feng Wei, Tianjin Medical University Cancer Institute and Hospital, ChinaTimothy Jay Price, University of Adelaide, Australia
Copyright © 2019 García-Foncillas, Sunakawa, Aderka, Wainberg, Ronga, Witzler and Stintzing. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Jesús García-Foncillas, jesus.garciafoncillas@oncohealth.eu
 Jesús García-Foncillas
Jesús García-Foncillas Yu Sunakawa
Yu Sunakawa Dan Aderka
Dan Aderka Zev Wainberg4
Zev Wainberg4 Sebastian Stintzing
Sebastian Stintzing